Abstract
The majority of Americans are overweight, and the incidence of obesity continues to increase. This trend predisposes people to a number of deleterious consequences, including the metabolic syndrome and other conditions that lead to a greater number of hospital admissions. Invasive candidiasis is an important nosocomial infection that results from these admissions. Echinocandins such as micafungin are indicated for treatment. We have previously demonstrated that overweight patients exhibit higher micafungin systemic clearance (SCL) than leaner patients. We hypothesized that obese and extremely obese people would show even higher SCL than merely overweight patients. To test this, we performed a prospective study of 36 adult volunteers randomized to receive a single dose of either 100 mg or 300 mg of micafungin whose body mass index fell within one of the following categories: <25, 25 to 40, and >40 kg/m2. The male-to-female ratio was 1:1. The minimum weight was 43 kg, the median 97 kg, and the maximum weight 155 kg. A two-compartment model was examined using the maximum likelihood solution via the expectation-maximization algorithm. Men had a higher median SCL of 1.53 liters/h versus 1.29 liters/h (P = 0.01) in the Mann-Whitney U-test. The typical SCL was 1.04 liters/h but increased by a factor of (weight/66)0.75 as weight increased above 66 kg. Thus, the relationship between micafungin SCL and weight in adults is best described by fractal-geometry-based laws. Furthermore, micafungin SCL continues to increase as weight increases, with no obvious plateau. This leads to a requirement for strategies to determine individualized dosing levels for obese and extremely obese patients.
INTRODUCTION
The mass of an object is determined according to the amount of substance the object contains and for humans is measured in kilograms in accordance with the International System (SI) of units. In SI units, the term “weight” refers to force and is measured in Newtons. In colloquial English usage, however, the term “weight” is often used as equivalent to mass. In this report, we retain that colloquial use of the term “weight.” In 1960s America, the average weight of men 20 to 74 years old was 76 kg and that of women was 64 kg (22), close to the oft-quoted average for men of 73 kg and average for women of 63 kg. Over the intervening decades, it has become increasingly difficult to find these people of “average” weight. By 2002, the average weight for men was 86 kg and that for women 74 was kg (22). By 2008, 68% of Americans were overweight (body mass index [BMI] ≥ 25 kg/m2), 34% were obese (BMI ≥ 30 kg/m2), and 6% were morbidly obese (BMI ≥ 40 kg/m2) (6). Unfortunately, the increasing rates of obesity are not confined to Americans or even the American continent; obesity is now a worldwide problem. Beyond the obvious and unfortunate large increase in patients with the metabolic syndrome, the problem of weight also has a direct impact on dosing practices. Kleiber's law states that the whole organism metabolic rate is proportional to an index that corresponds to its mass value raised to the power of 0.75 (13, 14, 28). This statement is a direct consequence of fractal geometry constraints (19, 27–29). Since systemic clearance (SCL) of drugs is due to xenobiotic metabolism, it is likely that drug clearance could also correlate with patient weight.
Micafungin is currently licensed for the treatment of candidiasis at doses of either 100 or 150 mg a day. The efficacy of micafungin is linked to the area under the concentration-time curve over 24 h in the steady state divided by the MIC (AUC0–24/MIC ratio) (2, 3, 8). The AUC is inversely proportional to SCL; thus, as SCL increases, AUC0–24/MIC declines, which should affect drug efficacy in a negative fashion. In previous studies, we found that for some antifungal agents such as micafungin, SCL increased once weight rose above 66 kg (9). However, the variance of patient weight in that study was relatively low, making it difficult to explore the relationship between weight and clearance with any degree of certainty. On the basis of those previous results, we hypothesized that clearance would increase in relation to the body weight of subjects and that the clearance would progressively increase as weight categories increased from the merely overweight to the morbidly obese. Further, we predicted that weight could, in theory, have an impact on the clinical efficacy of micafungin. Here, we evaluated micafungin pharmacokinetics as a function of weight in a prospective study in which subject weight ranged across a wide span of BMIs. Specifically, we hypothesized that above the threshold of 66 kg, micafungin SCL would increase as predicted by Kleiber's law.
MATERIALS AND METHODS
Regulatory compliance.
The study was approved by the Institutional Review Board (IRB) of The University of Texas Southwestern Medical Center at Dallas. We filed an Investigational New Drug (IND) application with the Food and Drug Administration (IND 107097) and received an exemption. The study protocol was registered at www.clinicaltrials.gov (NCT01090141) prior to enrollment of the first subject.
Study population.
We recruited volunteers to the Clinical Trials Research Center (CTRC) at the UT Southwestern Medical Center between March 2010 and November 2010. Male and female subjects (ages ≥ 18 years) of all self-identified racial and ethnic groups who were able to provide written informed consent were eligible for study participation. Exclusion criteria included pregnant women, nursing mothers, women unwilling to use a reliable contraception method during the study, a history of allergies to echinocandins, other medical contraindications to echinocandins, or abnormal liver function test results. Abnormal liver function test results were defined as detection of a transaminase level >10 times the upper limit for healthy individuals, an alkaline phosphatase level >5 times the upper limit for healthy individuals, or a total bilirubin level >5 times the upper limit for healthy individuals.
Experimental design.
The study was designed to recruit 12 people into each of the following weight categories: BMI <25, BMI 25 to 40, and BMI >40 kg/m2. Half the people within each BMI category were male. The volunteers were randomized within each BMI category to receive a single dose of either 100 mg or 300 mg of micafungin sodium.
Study and sampling procedures.
After randomization, the drug was prepared by the Aston Investigational Drug Service of UT Southwestern Medical Center. Patients received micafungin doses as a single 200-ml infusion over 60 min. At the end of infusion, the peripheral line used for the drug infusion was removed. Blood draws were performed through a second line, which was flushed between blood draws. Each patient had 10 ml of blood drawn at each of 7 time points, at t = 0 h (predose), 1, 4, 8, 12, 16, and 24 h following the start of the 1-h micafungin infusion. The blood was spun down for serum separation, and the serum was stored at −80°C until transportation for measurement of the micafungin concentration. Patients had a medical history taken, and a physical examination was performed prior to drug infusion. Blood was also collected for a complete blood count and comprehensive metabolic profile at discharge. Vital signs were also recorded after the last blood draw. All infusions and blood draws were performed by a study nurse.
Subject safety and data monitoring.
Any serious adverse events were to be reported to the IRB within 24 h. For routine data safety monitoring, the study coordinators and all three investigators met after the enrollment of the 12th, 24th, and 36th volunteers. Safety reports were then generated for the IRB.
Micafungin assay.
Drug concentrations were measured at Covance laboratories. Micafungin and an internal standard (FR195743) were extracted from human serum by protein precipitation. After the dilution of the supernatant with 10.0 mM ammonium acetate–water, the mixed solution was directly injected and analyzed using liquid chromatography (LC) with mass spectrometric detection. The standard curve range was 0.05 to 25.0 μg/ml for a serum sample volume of 0.01 ml. Percent accuracy values were 106.7, 90.5, 109.6, and 93.6, with relative standard deviations (RSD) of 9.2, 6.1, 6.3, and 3.5% for quality control (QC) levels of 0.150 μg/ml for low QC, 2.00 μg/ml for moderate QC, 18.8 μg/ml for high QC, and 50.0 μg/ml for dilute (10×) QC, respectively.
Pharmacokinetic analysis.
Micafungin concentrations were modeled using the ADAPT 5 program of D'Argenio and colleagues (5). Based on prior work, micafungin is known to follow two-compartment model pharmacokinetics in humans (2, 9, 12). Therefore, a two-compartment model with first-order input and elimination was utilized. Initial population pharmacokinetic parameter estimates were identified using the standard two-stage approach. The estimates were then used in the POPINIT subroutine to create an ADAPT 5 Fortran model file for further analysis. Population pharmacokinetic parameters for the base model were estimated by use of the maximum-likelihood solution via the expectation-maximization (MLEM) algorithm. The relationships between pharmacokinetic parameter and weight, gender, age, comorbid condition, and chronic medications were then examined in scatter plots. When a relationship was identified, the slope of the relationship between covariate and pharmacokinetic parameter was calculated. Those covariates and initial estimates of slope were then introduced into the COVMOD subroutine of the ADAPT program. The relationships between covariate parameters and pharmacokinetic parameters were then determined using the MLEM algorithm to yield the final model estimates. Specifically determinations based on weight or mass (M) in the fractal relationship SCL = SCLstd × (M/66)0.75 were made using ADAPT 5 software, with 66 kg being the breakpoint weight identified in the past (9).
RESULTS
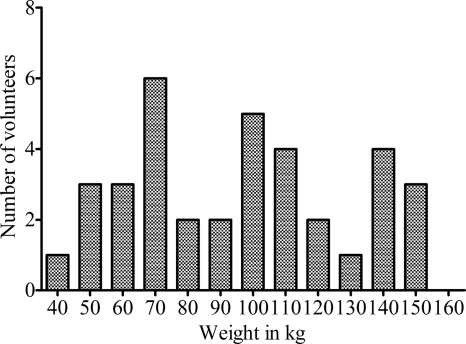
Demographic features of the study population are shown in Table 1. A large proportion of these volunteers had other chronic comorbid conditions, including metabolic syndrome. The weight distribution of the population of enrolled subjects is shown in Fig. 1. The minimum weight was 43.0 kg, the median 96.6 kg, and the maximum weight 154.8 kg; thus, weight varied ∼4-fold. Therefore, as intended, patients representing a wide range of the weight distribution were recruited into the study. The median weight for men was 96.2 kg versus 98.4 kg for women, values that were not significantly different as determined based on the nonparametric Mann-Whitney U test (P = 0.33). The most difficult category of people to recruit, and the last population to be completed, was of men with a BMI < 25 kg/m2.
Table 1.
Demographic characteristics of study participants
| Demographic parameter | Valuea |
|---|---|
| Mean age in yrs ± SD | 39.78 ± 15.05 |
| Gender (men/women) | 50/50 |
| Self-identified race (%) | |
| White | 56 |
| African American | 33 |
| Asian | 11 |
| Proportion with chronic comorbid condition | 55.56 |
| Proportion with metabolic syndrome component(s) | 36.1 |
| Patients on chronic medication(s) | 50 |
Values represent percentages except where otherwise indicated.
Fig. 1.
Weight distribution in people recruited into the micafungin study. The recruitment was meant to capture all extremes of weight; thus, the weight values are not normally distributed.
One person developed abdominal pain prior to start of micafungin infusion that continued after the infusion; this was judged unrelated to the micafungin dose. A second person developed self-limited diarrhea after infusion of micafungin; this was judged likely related to the micafungin infusion. Two patients exhibited decreases in the level of serum bicarbonate (HCO3) into the range that would be identified as representative of acidosis. One patient exhibited a decline in HCO3 level from 24 to 19 mEq/liter, and the second patient exhibited a decline from 21 mEq/liter to 15 mEq/liter; however, these abnormalities had resolved by the time the blood was redrawn in a follow-up examination after the end of the study. There were no symptoms or signs of acidosis or compensation for the presence of acidosis. Only one patient had liver function test values that increased at least 2-fold. The tested levels for the patient (who had a history of chronic heart failure) included a serum aspartate aminotransferase increase from 22 to 42 U/liter, an alanine aminotransferase increase from 14 to 28 U/liter, and an alkaline phosphatase increase from 133 to 194 U/liter. These also had resolved by the time that follow-up laboratory examinations were performed at end of the study. None of the adverse events were considered serious.
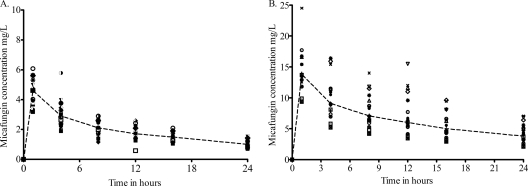
There were 252 serum samples analyzed, revealing the concentrations shown in Fig. 2. The ratio of the highest peak concentration to the lowest peak concentration was 1.91 after infusion of the 100-mg dose and 2.62 after infusion of the 300-mg dose. The drug concentrations were analyzed using the standard two-stage approach in order to generate the initial estimates. Pharmacokinetic parameter estimates generated using this method are shown in Table 2.
Fig. 2.
Concentrations of micafungin achieved after administration of a single dose of micafungin. (A) Concentrations in patients treated with a 100-mg dose. (B) Concentrations in patients treated with a 300-mg dose. The dashed lines represent the median concentrations determined using naïve pooling and demonstrate a biphasic decline consistent with a two-compartment model.
Table 2.
Pharmacokinetic parameter estimates based on the standard two-stage approach
| Parameter | Median | Minimum | Maximum |
|---|---|---|---|
| Ke (h−1) | 0.173 | 0.001 | 0.440 |
| Vc (liters) | 8.115 | 3.028 | 39.54 |
| K12 (h−1) | 4.792 | 0.114 | 11.38 |
| K21 (h−1) | 1.579 | 0.1554 | 4.356 |
Analysis of the concentrations in a two-compartment model based on the MLEM algorithm resulted in the plot of observed concentration versus predicted concentration shown in Fig. 3. Table 3 shows the micafungin pharmacokinetic parameter estimates from the base model. The α half-life was 0.11 ± 0.03 h, and the β half-life was 14.1 ± 1.1 h, as is consistent with prior studies (9, 11, 26).
Fig. 3.
Observed versus predicted concentrations of micafungin in the base model. Concentrations are indicated in milligrams per liter.
Table 3.
Micafungin pharmacokinetic parameter estimates in base model
| Parametera | Estimate | SD |
|---|---|---|
| CLt (liters·h−1) | 1.39 | 0.419 |
| CLd (liters·h−1) | 35.0 | 9.92 |
| Vc (liters) | 8.05 | 2.94 |
| Vp (liters) | 19.7 | 7.06 |
| K12 (h−1) | 4.34 | 1.34 |
| K21 (h−1) | 1.78 | 0.341 |
CLt, total clearance; CLd, clearance from peripheral compartment; Vc, volume of central compartment; Vp, volume of peripheral compartment; K12, intercompartmental transfer constant from compartment 1 to 2; K21, intercompartmental transfer constant from compartment 2 to 1.
An examination of scatter plots for each of the pharmacokinetic parameters versus weight, age, and gender revealed several relationships. First, the volume of central compartment (Vc) values differed significantly between the genders as determined using the Mann-Whitney U test; the median volume was 9.08 liters in men versus 7.51 liters in women (P = 0.01). Those results were unrelated to subject weight. SCL also differed between men and women, with median values of 1.53 liters/h for men and 1.29 liters/h for women (P = 0.011); thus, the difference was a clinically insignificant 16%. When SCL was analyzed as a function of BMI, there was poor correlation between the two (see Fig. 5; r2 = 0.27), as there was between BMI and other pharmacokinetic parameters. In addition, when the patients with BMI < 25 kg/m2 (shown in Fig. 4) are excluded, the relationship between total clearance and BMI is even poorer. On the other hand, when weight alone was examined, a definite relationship could be clearly observed (Fig. 5). Figure 5A shows that there was no relationship between weight and SCL in those subjects between 43 kg and 66 kg in weight. However, in those weighing more than 66 kg, SCL increased as a function of weight. When the data for those with weight above 66 kg were plotted as log-log plots (Fig. 5B), the slope of the relationship between SCL and weight showed a strong linear relationship, with slopes of 0.79 ± 0.20 for men and 0.70 ± 0.47 for women, thus encompassing the 3/4 mass ratio predicted by Kleiber's law. The relationship was independent of gender (as shown in Fig. 5B). There was no relationship between chronic comorbid conditions or chronic medications and pharmacokinetic parameters beyond those explained by weight.
Fig. 5.
Weight versus systemic clearance. (A) Subjects with weight < 66 kg are represented by open squares; those with weight > 66 kg are represented by solid circles. There was no change in clearance below 66 kg, whereas clearance increased with weight above 66 kg. (B) A log-log plot of weight and clearance in subjects with weight > 66 kg is shown. The slope of the line for women and men encompasses the predicted 3/4 (or 0.75) relationship. Solid line, slope for males; dashed line, slope for females.
Fig. 4.
Scatter-plot of BMI versus systemic clearance. Normal BMI (<25 kg/m2) is represented by open circles, whereas solid squares correspond to people with a BMI of >25 kg/m2. BMI did not correlate closely with micafungin clearance.
Next, we performed another population pharmacokinetic analysis for people with weight greater than 66 kg in MLEM, with weight as a covariate. Pharmacokinetic parameter estimates in the final model are as follows: for Vc, 11.7 liters (55.7% relative standard error [RSE]); for CLd, 26.5 liters·h−1 (70.9% RSE); and for Vp, 18.3 liters (42.4% RSE). The relationship seen between clearance and weight above 66 kg is given by the equation SCL = 1.04 × (weight/66)0.75, with an RSE of 6.99% for the slope.
DISCUSSION
In the current study, we examined the single-dose pharmacokinetics of micafungin administration for “healthy” volunteers. However, an examination of comorbid conditions revealed that many of our “healthy” people had chronic medical conditions, most notably components of metabolic syndrome. This is not a surprise, given the BMI categories we examined (1), in which the proportions of overweight and obese people mirror those of the American public. The population pharmacokinetic parameter estimates for our “healthy” volunteers were virtually identical to those determined for patients with fungal infections in studies published in the past (2, 9, 11, 26), which suggests that our findings likely have relevance even for patients with fungemia.
The main finding in the current study is that micafungin SCL increases as a function of weight beyond a threshold of 66 kg. We showed that, above this weight, the clearance increases continually as weight increases, at least up to 155 kg, which was the weight of our heaviest individual. The relationship is very accurately expressed by the same 3/4-power law that has been used to describe the relationship between the mass of a whole organism and the average metabolic clearance levels seen in comparisons between different species. Evolution, via natural selection of the most fit, also leads to selection of the most optimal whole-organism metabolic capacity (28). That capacity depends on maximizing efficient movement of metabolites and biochemical reactions, such that various components of body size that are important in movement of the metabolites as well as biochemical reactions scale to the most efficient shapes and sizes (27–29). The optimal body sizes and shapes themselves are dependent on and constrained by fractal-geometry considerations; those considerations, in turn, are governed by standard dictums of chaos theory (18–21, 28). These rules have been used for interspecies scaling of comparisons of metabolisms, given the large orders of magnitude of differences in mass from microbes to large mammals. Within humans, one would expect the rules to hold true for children as well, given that body size changes by many orders of magnitude during growth. For example, the average weight of a child at birth is 3.4 ± 0.6 kg, while that of a 16 year old is 68 ± 12 kg (16), a 20-fold change. Thus, we have shown in the past that micafungin clearance is related to body mass of children (12). Here, we show that micafungin clearance differences between adults in the study, among whom there were only 4-fold differences in weight, followed the same rule. We speculate that this relationship would hold true for the other echinocandins such as caspofungin and anidulafungin as well and likely for many other drug classes. On the other hand, it is surprising that the widely used index of BMI was a less useful correlate of micafungin SCL. This suggests that height and body area may have less effect on micafungin metabolism. This was a surprise, given that the optimal surface area of organisms is also constrained by fractal-geometry laws and is an important determinant of metabolite transfer (28).
The practical consequence of the relationship between weight and micafungin SCL is in dosing the drug, in the context of a population in which 2/3 of the members are overweight. It was recently demonstrated that 98% of invasive candidiasis patients with a micafungin AUC/MIC ratio of 3,000 to 12,000 achieve microbiological clearance, as opposed to only 85% of those with an AUC/MIC ratio ≤ 3,000 (2). In the case of infections by Candida parapsilosis, which exhibits drug MICs that are 50- to100-fold higher than those seen with other species, 100% of patients with an AUC/MIC ratio ≥ 285 achieve microbiological clearance, as opposed to 82% of those below that exposure level. Our current findings of high SCL suggest that a large portion of patients who are obese, and an even larger proportion of those who are extremely obese, could fail to achieve these optimal AUC/MIC ratio thresholds at standard doses. Moreover, a susceptibility breakpoint of 0.5 mg/liter, as opposed to the current breakpoint of 2.0 mg/liter, was identified in the candidiasis study (2). This means that a larger portion of clinical isolates exhibit drug MICs that fall above the newer breakpoint compared to the old one, especially in the case of C. parapsilosis infections. Given that observation and the lower AUCs achieved with obese and extremely obese patients treated with current standard doses, a considerable portion of such patients are at risk of therapy failure. This means that novel individualized-dosing strategies need to be derived for micafungin dosing of a large proportion of patients in the United States. Further work, such as Monte Carlo simulations that take into account weight as a covariate of SCL, is needed to derive a dosing formula that can easily be used by clinicians at the bedside. Nevertheless, this is a great opportunity to further improve the efficacy of a drug whose efficacy is already 70 to 80% with the current dosing of 100 mg per day for treatment of candidemia (7, 17, 23).
While the relationship between micafungin SCL and fractal geometry is clear, the mathematical relationship nevertheless remains to be explained in terms of human physiology. The physiological events leading to increases in micafungin SCL with weight are unclear. Micafungin is metabolized by arylsulfatase and secondarily by COMT (catechol-O-methyltransferase). Since SCL increases as weight increases, it may be that there is a concomitant increase in expression of one or both of these enzymes. It is interesting that some members in the arylsulfatase family are involved in the metabolism of lipids and that deficiencies in those enzymes lead to lipidosis. Regarding COMT, which is ordinarily involved in the metabolism of catecholamines and estrogen, there is a strong association between certain single-nucleotide polymorphisms and obesity as well as type 2 diabetes mellitus (15), which may be a potential explanation for the results showing increased micafungin metabolism in obese subjects. On the other hand, it has previously been reported that 90% of the micafungin in the blood is cleared via hepatobiliary elimination of the parent compound and metabolites (10). Hepatic drug uptake transporters such as the organic-anion-transporting polypeptide (OATP) Na+-taurocholate-cotransporting polypeptide (NTCP) and the bile salt export pump have been implicated in clearance (30). OATP mechanisms have also been implicated in caspofungin clearance (25). However, expression of NTCP and OATP has been shown to be reduced in animal models of obesity and diabetes (4, 24), which would not explain the increased clearance seen in the present study. Another possible mechanism may be the consequence of differences between lean and obese patients with respect to protein binding, analogous to the differences between neonates and adult patients in protein binding (31). It has been demonstrated that there is an 8-fold difference between neonates and adults in micafungin protein binding levels that accounts for differences between these 2 age groups in micafungin clearance. In the current study, however, there was only a weak relationship between serum albumin and micafungin clearance (r2 = 0.22). Nevertheless, it would be interesting to examine the changes seen in micafungin protein binding capacity in patient plasma as weight increases.
To conclude, we have shown that, in obese and extremely obese adults, micafungin clearance continues to increase as body weight increases, based on the 3/4-power law. We would predict, based upon pharmacodynamic considerations, that current one-size-fits-all dosing is in all likelihood inadequate for patients weighing >66 kg. Furthermore, we predict that in the future we will be able to adjust the doses of these medications on the basis of weight in a fashion individualized for each particular patient. This may, in fact, be the case for many classes of antimicrobial medications in addition to the echinocandins.
ACKNOWLEDGMENTS
T.G. and the study were sponsored by a UT Southwestern Medical Center grant (BJ-07-003) from Astellas Pharma USA. R.G.H. was supported by grant KL2RR024983 to the UT Southwestern Medical Center from the NCRR/NIH. Recruitment, admission of volunteers for the study, and collection of blood samples was performed by Clinical and Translational Research Center personnel within the UT Southwestern Medical Center, which is supported by the NIH CTSA (grant UL1 RR024982).
Footnotes
Published ahead of print on 29 August 2011.
REFERENCES
- 1. Alberti K. G., et al. 2009. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 2. Andes D., et al. 2011. Use of pharmacokinetic-pharmacodynamic analyses to optimize therapy with the systemic antifungal micafungin for invasive candidiasis or candidemia. Antimicrob. Agents Chemother. 55:2113–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andes D., et al. 2010. In vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob. Agents Chemother. 54:2497–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng Q., et al. 2008. Drug-metabolizing enzyme and transporter expression in a mouse model of diabetes and obesity. Mol. Pharm. 5:77–91 [DOI] [PubMed] [Google Scholar]
- 5. D'Argenio D. Z., Schumitzky A., Wang X. 2009. ADAPT 5 User's guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, University of Southern California, Los Angeles, Los Angeles, CA [Google Scholar]
- 6. Flegal K. M., Carroll M. D., Ogden C. L., Curtin L. R. 2010. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303:235–241 [DOI] [PubMed] [Google Scholar]
- 7. Goto N., et al. 2010. Efficacy and safety of micafungin for treating febrile neutropenia in hematological malignancies. Am. J. Hematol. 85:872–876 [DOI] [PubMed] [Google Scholar]
- 8. Gumbo T., et al. 2007. Once-weekly micafungin therapy is as effective as daily therapy for disseminated candidiasis in mice with persistent neutropenia. Antimicrob. Agents Chemother. 51:968–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gumbo T., et al. 2008. Population pharmacokinetics of micafungin in adult patients. Diagn. Microbiol. Infect. Dis. 60:329–331 [DOI] [PubMed] [Google Scholar]
- 10. Hebert M. F., et al. 2005. Concomitant cyclosporine and micafungin pharmacokinetics in healthy volunteers. J. Clin. Pharmacol. 45:954–960 [DOI] [PubMed] [Google Scholar]
- 11. Hiemenz J., et al. 2005. Pharmacokinetic and maximum tolerated dose study of micafungin in combination with fluconazole versus fluconazole alone for prophylaxis of fungal infections in adult patients undergoing a bone marrow or peripheral stem cell transplant. Antimicrob. Agents Chemother. 49:1331–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hope W. W., et al. 2007. Population pharmacokinetics of micafungin in pediatric patients and implications for antifungal dosing. Antimicrob. Agents Chemother. 51:3714–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kleiber M. 1932. Body size and metabolism. Hilgardia 6:315–353 [Google Scholar]
- 14. Kleiber M. 1947. Body size and metabolic rate. Physiol. Rev. 27:511–541 [DOI] [PubMed] [Google Scholar]
- 15. Kring S. I., et al. 2009. Polymorphisms of serotonin receptor 2A and 2C genes and COMT in relation to obesity and type 2 diabetes. PLoS One 4:e6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuczmarski R. J., et al. 2002. 2000 CDC growth charts for the United States: Methods and development. Vital Health Stat. 11:1–190 [PubMed] [Google Scholar]
- 17. Kuse E. R., et al. 2007. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 369:1519–1527 [DOI] [PubMed] [Google Scholar]
- 18. Lorenz E. N. 2011. Deterministic non-periodic flow. J. Atmospher. Sci. 20:130–141 [Google Scholar]
- 19. Mandelbrot B. B. 1982. The fractal geometry of nature. W. H. Freeman and Company, San Francisco, CA [Google Scholar]
- 20. May R. M. 1974. Biological populations with nonoverlapping generations: stable points, stable cycles, and chaos. Science 186:645–647 [DOI] [PubMed] [Google Scholar]
- 21. May R. M. 1976. Simple mathematical models with very complicated dynamics. Nature 261:459–467 [DOI] [PubMed] [Google Scholar]
- 22. Ogden C. L., Fryar C. D., Carroll M. D., Flegal K. M. 2004. Mean body weight, height, and body mass index, United States 1960–2002, no. 347. Adv. Data 347:1–17 [PubMed] [Google Scholar]
- 23. Pappas P. G., et al. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 45:883–893 [DOI] [PubMed] [Google Scholar]
- 24. Pizarro M., et al. 2004. Bile secretory function in the obese Zucker rat: evidence of cholestasis and altered canalicular transport function. Gut 53:1837–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sandhu P., et al. 2005. Hepatic uptake of the novel antifungal agent caspofungin. Drug Metab. Dispos. 33:676–682 [DOI] [PubMed] [Google Scholar]
- 26. Walsh T. J., et al. 2010. Intrapulmonary pharmacokinetics and pharmacodynamics of micafungin in adult lung transplant patients. Antimicrob. Agents Chemother. 54:3451–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. West G. B., Brown J. H., Enquist B. J. 1997. A general model for the origin of allometric scaling laws in biology. Science 276:122–126 [DOI] [PubMed] [Google Scholar]
- 28. West G. B., Brown J. H., Enquist B. J. 1999. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science 284:1677–1679 [DOI] [PubMed] [Google Scholar]
- 29. West G. B., et al. 2003. Physiology: why does metabolic rate scale with body size? Nature 421:713. [DOI] [PubMed] [Google Scholar]
- 30. Yanni S. B., et al. 2010. In vitro investigation of the hepatobiliary disposition mechanisms of the antifungal agent micafungin in humans and rats. Drug Metab. Dispos. 38:1848–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yanni S. B., et al. 2011. Higher clearance of micafungin in neonates compared with adults: role of age-dependent micafungin serum binding. Biopharm. Drug Dispos. 32:222–232 [DOI] [PMC free article] [PubMed] [Google Scholar]