Abstract
Nasal colonization of Staphylococcus aureus is a risk factor for pathogenic autoinfection, particularly in postoperative patients and the immunocompromised. As such, standardized preoperative nasal decolonization of S. aureus has become a major consideration for the prevention of nosocomial infection. However, only a few treatment options for nasal decolonization are currently available, with resistance to these approaches already a concern. Here we have identified the macrocyclic θ-defensin analogue RC-101 as a promising anti-S. aureus agent for nasal decolonization. RC-101 exhibits bactericidal effects against S. aureus with the use of in vitro epithelium-free systems, while also preventing the pathogen's proliferation and attachment in an ex vivo human nasal epithelial cell adhesion model and an organotypic model of human airway epithelia. Peptide concentrations as low as 2.5 μM elicited significant reductions in S. aureus growth in epithelium-free systems, with 10 μM concentrations being completely bactericidal for all strains tested, including USA300. In ex vivo nasal colonization models, RC-101 significantly reduced adherence, survival, and proliferation of S. aureus on human nasal epithelia. Reductions in S. aureus viability were evident in these assays, with as little as 1 μg of peptide per tissue, while 10 μg of RC-101 completely prevented adhesion of all strains tested. Furthermore, RC-101 did not exhibit cellular toxicity to human nasal epithelia at concentrations up to 200 μM, nor did it induce a proinflammatory response in these cells. Collectively, the findings of this study identify RC-101 as a potential preventative of S. aureus nasal colonization.
INTRODUCTION
Nasal colonization by Staphylococcus aureus occurs in approximately 20 to 30% of healthy individuals (28). The primary reservoir for S. aureus is the anterior nares, but the occurrence of nasal colonization also increases the prevalence of this bacterium on other surfaces of the body (14). As such, nasal carriage of S. aureus is a major risk factor for endogenous infection (autoinfection), ranging from minor skin and soft tissue infections to serious bacteremia (2, 30). Multiple studies have shown that removal of S. aureus from the nasal vestibule using antimicrobial agents (referred to as nasal decolonization), prior to hospitalization, significantly reduces the incidence of nosocomial infection (1, 13, 15, 30, 31). For the past 25 years, the most common means of nasal decolonization prior to hospitalization has been the use of mupirocin ointment; however, resistance to this antibiotic is increasing (2, 11). Thus, there is an urgent need to develop novel compounds to prevent or treat S. aureus nasal carriage, particularly in preoperative patients.
Toward this goal, we discovered that the retrocyclin class of θ-defensins is potently active against a broad spectrum of microbes, including strains of S. aureus (6). Retrocyclins are 18-residue peptides that contain three intramolecular disulfide bonds, which stabilize a β-sheet conformation (26), and represent the first truly circular peptides of vertebrate origin (17, 23, 27). They are extremely stable and can resist boiling, acidic conditions, and other harsh environments. Notably, RC-101 has been recovered and found to remain bioactive after 9 days of treatment in an ex vivo model of organotypic human vaginal tissue containing vaginal mucus (3). Similarly, RC-101 was observed to be stable and bioactive after 8 days of in vivo vaginal treatment in pigtailed macaques (7), thus highlighting the stability of this peptide in mucosal environments.
Whereas both human and nonhuman primates produce α- and β-defensin peptides (23), humans do not produce endogenous θ-defensin peptides because a premature stop codon precludes translation (6, 19). As such, these peptides have been recreated by solid-phase synthesis and found to exhibit broad-spectrum antimicrobial activity against a number of bacteria, fungi, and viruses (6, 17, 23, 27, 32), without any noted cellular toxicity or inflammation in vivo, ex vivo, and in vitro (3, 7). One retrocyclin analogue, RC-101, contains a single arginine-to-lysine mutation compared to wild-type retrocyclin, exhibits heightened activity in antiviral assays (20), is nonhemolytic to human red blood cells, and is not cytotoxic to a number of human cell lines at concentrations up to 500 μg/ml (3, 6, 7, 12, 29).
In the current study, we have characterized further the antimicrobial properties of RC-101 against both nasal carriage and clinical isolates of S. aureus. Importantly, RC-101 prevents the adherence and survival of S. aureus on cultured human nasal epithelia while inducing no noticeable cytotoxicity or proinflammatory responses. These findings identify RC-101 as a potentially promising therapeutic agent for nasal decolonization of S. aureus and support further development of this peptide as an intranasal antibiotic.
MATERIALS AND METHODS
Bacterial isolates.
Nasal carriage strains of S. aureus were collected from the anterior nares of donors at the University of Central Florida (UCF; Orlando, FL) following the protocol described in reference 16. Written consent was obtained from all donors, and samples were collected under a human subjects protocol approved by the UCF Institutional Review Board. The clinical strains, USA300, N315, and COL, were obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus (Eurofins Medinet, Inc., Chantilly, VA), of which our laboratory is a member.
Peptide synthesis, storage, and utilization.
The 18-amino acid peptide RC-101 was synthesized as previously described (4, 6). Following synthesis, the lyophilized peptide was stored at −20°C until use. Prior to use, RC-101 was reconstituted in sterile water-0.01% (vol/vol) acetic acid and diluted accordingly to desired working concentrations. Surplus peptide was aliquoted in single-use volumes and stored at −20°C. As such, working volumes of peptide were limited to not more than one freeze-thaw cycle prior to use.
Turbidity assay.
As an initial screen of the anti-S. aureus activity of RC-101, turbidity assays were performed as adapted from reference 18. Briefly, bacteria were grown to logarithmic growth phase and diluted in Mueller-Hinton broth (Sigma-Aldrich Co., St. Louis, MO) containing 0.5% sucrose. Aliquots of approximately 104 CFU (80 μl) were added to the wells of a flat bottom 96-well plate (MidSci, St. Louis, MO) with 20 μl of either vehicle or RC-101 (2.5 to 20 μM final volume). Plates were incubated in a SpectraMax 190 microplate reader (Molecular Devices, Sunnyvale, CA) at 37°C for 16 h. Turbidity readings at 550 nm were acquired every 5 min following 15 s of agitation. Optical density data were plotted against time to generate growth curves for all samples. The time at which each sample entered logarithmic growth was considered the growth threshold (GT) for these assays. The GT for each sample was compared to the GT of the vehicle-treated sample, and the retardation in growth was represented as Delta GT.
Tissue culture.
Human nasal epithelia (RPMI 2650; American Type Culture Collection, Manassas, VA) were grown to confluence on collagen-coated transwell inserts (12-mm diameter, 0.4-μm pore size; Corning Inc., Corning, NY). Cell culture media contained Dulbecco's modified Eagle's medium (DMEM; Mediatech, Inc., Manassas, VA) with glucose (4.5 g/liter), l-glutamine (584 mg/liter), and sodium pyruvate (110 mg/liter), supplemented with 10% (vol/vol) fetal bovine serum (FBS; Gemini Bioproducts, West Sacramento, CA), penicillin (100 U/ml), and streptomycin (100 μg/ml). Antibiotic-supplemented media were changed daily until epithelia reached confluence. Following confluence, cells were cultured in antibiotic-free media at the air-liquid interface at 37°C and 5% CO2 for 4 days prior to use in adhesion assays. During culturing at the air-liquid interface, antibiotic-free media were changed daily.
Organotypic airway epithelial tissues (EpiAirway) were obtained from MatTek Corporation (Ashland, MA) and maintained according to the manufacturer's instructions. These tissues resemble closely epithelial tissue of the respiratory tract. They are representative of healthy human donors and contain pseudostratified, highly differentiated tracheal/bronchial epithelia.
CFU assay.
To study the bactericidal effects of RC-101 on S. aureus, CFU assays were carried out with a procedure adapted from references 5 and 9. Briefly, nasal carriage isolates of S. aureus were grown to logarithmic growth phase in Trypticase soy broth at 37°C and 250 rpm and diluted in minimal media containing DMEM with glucose (4.5 g/liter), l-glutamine (584 mg/liter), and sodium pyruvate (110 mg/liter), supplemented with 0.05% (vol/vol) FBS to approximately 100,000 CFU/ml. S. aureus survivability reactions were prepared by incubating 4 μl of dilute bacteria with 1 μl of either vehicle or RC-101 at the appropriate concentration (1 to 10 μM final). Cultures of 5 μl were grown in sterile 72-well polystyrene Nunc minitrays (Nalge Nunc International, Rochester, NY) with 3 μl of liquid wax overlaid to prevent evaporation. Cultures were incubated at 37°C and 5% CO2 for 0, 0.25, 0.5, 1, 2, 3, 6, and 9 h, after which time the entire sample, or dilutions thereof, were plated on Trypticase soy agar (TSA) and incubated for 16 h at 37°C. The survival of S. aureus was determined by enumerating CFU from RC-101-treated samples and comparing the numbers to those of the vehicle-treated samples.
For studies assessing the effects of bacterial starting inocula on RC-101 activity, RC-101 (10 μM final concentration) was incubated with increasing starting concentrations of S. aureus. Cultures were incubated at 37°C and 5% CO2 for 0, 0.5, 1, 2, 3, 6, and 9 h, after which time the entire sample, or dilutions thereof, were plated on TSA and incubated for 16 h at 37°C. The survival of S. aureus was determined by enumerating CFU from RC-101-treated samples and comparing those numbers to those of vehicle-treated samples.
Epithelial cell adhesion assays.
Epithelial cell adhesion assays have previously been used to assess the binding of bacteria to human epithelia under a number of different conditions (10, 21, 22, 24, 25). Here, we have employed this assay to elucidate the nasal epithelial cell adhesion properties of S. aureus in the presence of RC-101. Adhesion assays were carried out as previously described in references 21 and 22 using two different models of human airway epithelia. EpiAirway tissues (MatTek tissues) or confluent layers of human nasal epithelia (RPMI 2650), exposed to the air-liquid interphase for 4 days (described above), were inoculated with 10 to 50 bacteria (100 μl) in minimal medium containing Dulbecco modified Eagle medium (DMEM) with glucose (4.5 g/liter), l-glutamine (584 mg/liter), and sodium pyruvate (110 mg/liter), supplemented with 0.05% (vol/vol) fetal bovine serum (FBS). Inoculated epithelial layers were incubated at 37°C and 5% CO2 for 15 min prior to treatment with either vehicle or RC-101 (1 to 20 μg/tissue). At 0, 3, 6, and 9 h posttreatment, wash fractions containing nonadherent bacteria were collected by rinsing the apical epithelial cell surface three times in 300 μl of minimal medium (900 μl total). Following collection of the wash fraction, adherent bacteria (adhere fraction) were isolated by scraping the cell layer, and the sample was collected in 900 μl of minimal medium. To liberate bacteria from epithelia, the adhered fraction was then sonicated (model 100 Sonic Dismembrator; Fisher Scientific, Pittsburgh, PA) using 10 0.5-s pulses on power setting three. Samples were then plated on Trypticase soy agar and incubated for 16 h at 37°C. The survival of S. aureus was determined by graphing the numbers of CFU from RC-101-treated samples and comparing them to those of the vehicle-treated samples.
Epithelial cell viability assays.
Human nasal epithelial cell viability was quantified using an MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) reduction assay, according to the manufacturer's instructions (Trevigen Inc., Gaithersburg, MD). Cytotoxicity was also measured by protease release using the CytoTox-GLO cytotoxicity assay (Promega Corp., Madison, WI) according to the manufacturer's instructions. Cell viability for organotypic airway epithelia was measured using an MTT assay from the tissue supplier (MatTek Corporation, Ashland, MA), according to the manufacturer's instructions.
Detection of proinflammatory cytokines.
To identify possible proinflammatory effects of RC-101 on human nasal epithelia or organotypic human airway epithelial tissues, multiplex enzyme-linked immunosorbent assays were performed. For these assays, conditioned underlay (basal media) was collected after 24 h and/or 72 h of incubations of human nasal epithelia, or organotypic airway epithelia, treated with RC-101 or vehicle. Following collection, basal media were subjected to multiplex suspension bead arrays assessing 27 human proinflammatory cytokines and analyzed using a Bio-Plex 200 system (Bio-Rad Laboratories, Inc., Hercules, CA) according to the manufacturer's instructions.
Statistical analyses.
Statistical analyses were conducted throughout this study using GraphPad Prism 4 software (GraphPad Software, La Jolla, CA). Bacterial counts of CFU and adhesion assays were log10 reduced, and statistical analyses were performed on the transformed data. For turbidity, CFU, and adhesion assays, one-tailed Student t tests were performed assuming a two-sample unequal variance (heteroscedastic). For epithelial cell viability assays and cytokine analyses, two-tailed Student t tests were performed assuming a two-sample unequal variance (heteroscedastic). For all analyses, a P value of <0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Growth of S. aureus is retarded by RC-101 treatment.
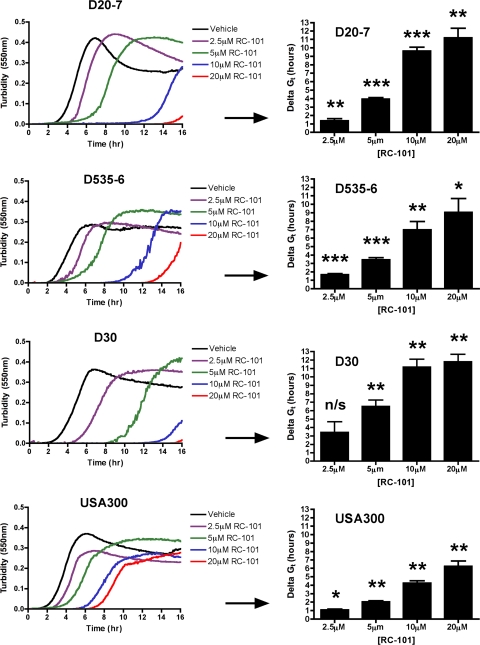
Previous studies have shown that retrocyclin exhibits antibacterial activity against a laboratory strain of S. aureus (6); however, the robustness of RC-101 activity has not been well characterized nor has the efficacy of this peptide as a potential therapeutic against S. aureus been assessed in any great detail. As an initial assessment of the robustness of RC-101's anti-S. aureus activity, turbidity assays were performed. A sampling of five nasal carriage isolates and three clinical isolates were treated with RC-101, and their growth kinetics were monitored over a 16-h period. In all assays, RC-101 retarded bacterial growth in a concentration-dependent manner. Shown in Fig. 1 are turbidity data for S. aureus nasal carriage strains D20-7, D535-6, and D30, as well as the clinical isolate USA300. Data have been transformed to reflect growth retardation time (Delta GT) for RC-101-treated samples compared to that for the vehicle-treated samples. RC-101 (2.5 μM) was sufficient to retard S. aureus growth for up to 5 h beyond that of vehicle-treated bacteria, while 20 μM concentrations were observed to retard growth beyond 10 h in nasal carriage strains and 5 h in the hypervirulent USA300 strain. Clones of D20-7 and USA300 surviving the 16-hour treatment of 20 μM RC-101 were reassessed by a CFU assay for evidence of enhanced resistance to RC-101. No indication of resistance toward RC-101 was observed after this initial round of passaging (data not shown). In addition to the four S. aureus strains shown in Fig. 1, four more strains were also examined using this assay. Included in these additional strains were two nasal carriage isolates, D547-4 and D566-5, and two clinical isolates, N315 and COL. Upon treatment with RC-101, the additional strains were found to exhibit growth kinetics similar to those shown in Fig. 1 (data not shown). Collectively, RC-101 exhibited robust anti-S. aureus activity against all strains tested in this study.
Fig. 1.
RC-101 retards the growth of nasal carriage and clinical strains of S. aureus. Shown are growth curves for three representative nasal carriage strains (D20-7, D535-6, and D30) and one representative clinical strain (USA300) treated with RC-101. Delta GT represents the time difference between RC-101- and vehicle-treated samples to reach their respective GT values (onset of logarithmic growth phase). (Left) Representative growth curves for one of three assays; (right) data representative of three assays. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n/s, nonsignificant. P values indicate statistical significance compared to vehicle-treated samples.
RC-101 exhibits bactericidal effects against S. aureus.
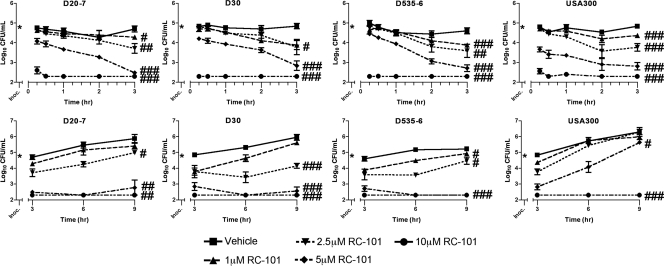
To elucidate further the effects of RC-101 on S. aureus growth, CFU assays were performed. A total of eight S. aureus strains were tested, five of which were nasal carriage isolates and three of which were strains of clinical origin. As shown in Fig. 2 (top), RC-101 exhibited a concentration-dependent inhibition of growth in nasal carriage strains of S. aureus (D20-7, D535-6, and D30) and the clinical isolate, USA300. Importantly, bactericidal effects were observed within 15 min of RC-101 treatment. RC-101 concentrations as low as 5 μM resulted in significant reductions (P = 0.017 to 0.0002 for all strains) in bacterial growth within 15 min compared to that of vehicle-treated bacteria. Peptide concentrations of 10 μM were observed to be almost completely bactericidal over the same 15-min time frame (P ≤ 0.0014 for all strains compared to their respective vehicle treatments), with complete growth inhibition observed after 30 min. Within 3 h of treatment with RC-101 significant reductions in bacterial growth were observed for all peptide concentrations (Fig. 2, top).
Fig. 2.
RC-101 is bactericidal toward both nasal carriage and clinical isolates of S. aureus. Shown are growth rate data from three nasal carriage isolates (D20-7, D30, and D535-6) and one clinical isolate (USA300) of S. aureus in the presence and absence of RC-101. (Top) RC-101 treatment for 0 to 3 h; (bottom) RC-101 treatment for 3 to 9 h. Limit of detection equals 200 (i.e., log10 = 2.3) CFU/ml. n ≥ 3 for all strains. Values are means ± standard errors of the means (SEM) (error bars). * indicates the starting inoculum. #, P < 0.05; ##, P < 0.01; ###, P < 0.001. P values indicate statistical significance compared to vehicle-treated samples. For clarity of presentation, P values are shown for 3- and 9-h treatments only.
To monitor the propensity for recovery among RC-101-treated strains, growth was monitored over an extended time course of 9 h. An RC-101 concentration of 5 μM significantly inhibited bacterial growth in all nasal carriage strains after 9 h, while a peptide concentration of 10 μM completely prevented bacterial growth from being observed with these same strains over 9 h (Fig. 2, bottom). The clinical isolate USA300 also experienced a significant reduction in growth after 9 h of 5 μM RC-101 treatment, with a 10 μM concentration completely preventing growth from being observed over the same 9-h time frame. In addition to the four S. aureus strains shown in Fig. 2, four more strains were also treated with RC-101, all of which revealed similar growth kinetics (data not shown). Among the additional strains were two nasal carriage isolates, D547-4 and D566-5, and two clinical isolates, N315 and COL.
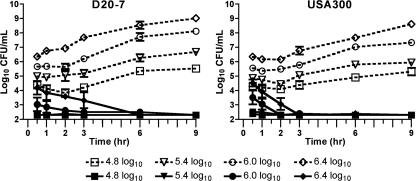
The starting inocula used for CFU assays (Fig. 2) approximate the physiological concentrations of S. aureus in nasal fluid from carriers (5, 8, 14); however, the effect of increased starting inocula on RC-101 activity was also assessed. As shown in Fig. 3, 10 μM RC-101 remained bactericidal to S. aureus with starting bacterial concentrations of approximately 2.5 million CFU/ml. Within 30 min of treating the 2.5 million CFU/ml starting concentration of D20-7 and USA300 with 10 μM RC-101, significant reductions (P = 0.04 and P = 0.01, respectively) in viable bacteria were observed (Fig. 3). Regardless of the starting bacteria concentration, a continued reduction in the number of CFU/ml was observed until undetectable levels of bacteria remained. No recovery was observed among RC-101-treated bacteria for up to 9 h.
Fig. 3.
RC-101 exhibits robust anti-S. aureus activity in CFU assays with increasing starting inocula. Shown are bacterial growth data for S. aureus strains D20-7 and USA300 treated with 10 μM RC-101 under increasing bacterial starting concentrations. The starting inoculum concentrations are indicated in the symbol key. Open symbols with dotted lines represent vehicle-treated samples, while filled symbols with solid lines represent RC-101-treated samples. Limit of detection equals 200 (i.e., log10 = 2.3) CFU/ml. n = 3. Values are means ± SEM (error bars).
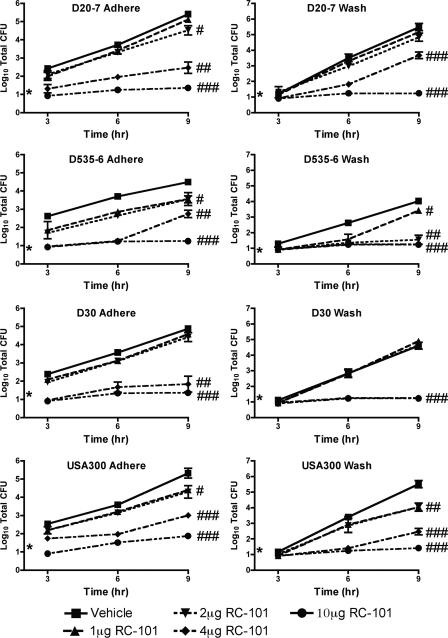
RC-101 prevents adherence of S. aureus to human nasal epithelia.
To test the capacity for RC-101 to prevent adherence of S. aureus to human nasal epithelia, ex vivo adhesion assays were performed. In all strains analyzed, 1 μg to 2 μg of RC-101 per tissue was sufficient to reduce S. aureus adherence to nasal epithelia. RC-101 concentrations of 4 μg per tissue yielded significant reductions in S. aureus adherence to nasal epithelial cells, while 10 μg completely prevented attachment (Fig. 4, left). For these assays, three nasal carriage strains were tested along with the hypervirulent USA300 strain. USA300 was analyzed as a measure of the effectiveness of RC-101 in preventing adhesion of strains frequently encountered in the clinical setting. To analyze whether RC-101 was inhibiting S. aureus growth on human nasal epithelial cells or was merely preventing adherence, the wash fraction was also analyzed. As can be seen in Fig. 4 (right), a significant reduction in S. aureus growth was apparent in all isolates, similar in trend to that observed with the adhered fraction. Collectively, treatment with RC-101 exhibited a robust inhibition of human nasal epithelial cell attachment and survival of all strains of S. aureus tested. The simultaneous reductions in the total number of CFU in both the adhere and wash fractions suggest that RC-101 is exhibiting anti-S. aureus activity as opposed to an antiadhesive property. An antiadhesive property would have been expected to result in an increase in the number of wash fraction CFU with a simultaneous reduction in the number of adhere fraction CFU. Therefore, the reduction in the total numbers of CFU of both fractions suggests a more antibacterial activity by RC-101.
Fig. 4.
RC-101 prevents adherence and survival of S. aureus on human nasal epithelia. Shown are growth curve data for adhered and wash fractions from human nasal epithelia cocultured with S. aureus in the presence and absence of RC-101. Nasal carriage strains include D20-7, D535-6, and D30. USA300 represents a clinical isolate. Limit of detection for the 3-h time point is nine (i.e., log10 = 0.95) total CFU, while the limit of detection for 6- and 9-h time points equals 18 (i.e., log10 = 1.26) total CFU. n ≥ 3 for all strains. Values are means ± SEM. * indicates the starting inoculum. #, P < 0.05; ##, P < 0.01; ###, P < 0.001. P values indicate statistical significance compared to vehicle-treated samples. For clarity of presentation, P values are shown for 9-h treatments only.
RC-101 does not exhibit cytotoxic effects toward human nasal epithelia or induce inflammation.
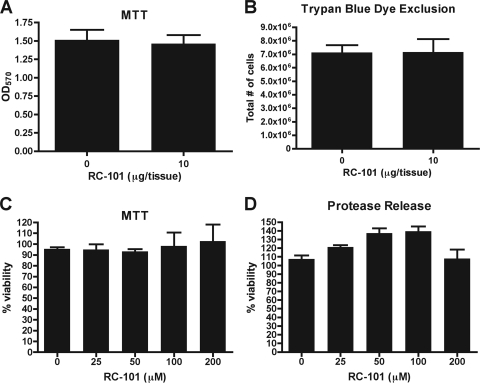
Though many agents exhibit potent antimicrobial activity, it is important that high levels of cytotoxicity do not accompany this activity. While RC-101 is effective at preventing S. aureus adherence and survival on human nasal epithelia, we also analyzed the cytotoxicity inflicted upon the nasal epithelia by RC-101 treatment. Nasal epithelia, under conditions identical to those used during adhesion assays, were subjected to either vehicle or 10 μg of RC-101 for a 24-hour period, after which time MTT reduction assays were performed. As shown in Fig. 5A, no significant reduction in nasal epithelial cell viability was observed. Trypan blue dye exclusion assays were also carried out to visualize the number of viable epithelial cells after 24 h of RC-101 treatment. As with MTT reduction assays, no cellular toxicity was observed as a result of RC-101 treatment (Fig. 5B).
Fig. 5.
RC-101 is not cytotoxic to human nasal epithelia. Transwell inserts containing human nasal epithelia were incubated in the presence of RC-101 or vehicle for 24 h and assayed for cellular viability using MTT reduction (OD570, optical density at 570 nm) (A) and trypan blue dye exclusion (B). Nasal epithelia (in 96-well plate format) were treated with increasing concentrations of RC-101 for 24 h and assayed for cellular viability using MTT reduction (C) and protease release (D) (CytoTox-GLO cytotoxicity assay). Note that RC-101 does not exhibit cytotoxicity to nasal epithelia under any of the tested conditions. n = 3 for all assays. Error bars represent the SEM.
To analyze further the possibility of RC-101 cytotoxicity to nasal epithelia, increasing peptide concentrations were incubated with human nasal epithelial cells for 24 h, after which time the viability of nasal epithelia was measured as a function of metabolic activity (indicated by MTT reduction) along with cellular apoptosis (indicated by protease release by use of the CytoTox-GLO assay). As shown in Fig. 5C and D, RC-101 concentrations of up to 200 μM were not cytotoxic to nasal epithelia. The notable lack of cytotoxicity to nasal epithelia may be due to the fact that RC-101 is an analogue of a once-functional primate gene.
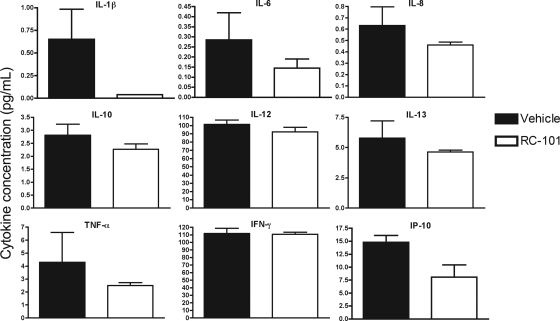
To assess whether RC-101 promotes an inflammatory response in human nasal epithelia, expression levels of 27 proinflammatory cytokines were analyzed. Nasal epithelia treated for 24 h with either vehicle or RC-101 (10 μg/tissue) revealed no significant difference in cytokine expression compared to vehicle-treated samples. Expression profiles for nine representative cytokines are shown in Fig. 6. The remaining 18 cytokines tested also exhibited trends similar to those shown in Fig. 6, with no significant difference in expression profile observed as the result of RC-101 treatment (data not shown). Collectively, the lack of both cytotoxicity and an inflammatory response by RC-101 underscores the safety of this peptide in human nasal epithelia.
Fig. 6.
RC-101 does not stimulate a proinflammatory response in human nasal epithelial cells. Nasal epithelia were incubated with either vehicle or RC-101 (10 μg/tissue) for 24 h and assayed for the production of human proinflammatory cytokines. Shown are nine representative plots from 27 cytokines analyzed. IL, interleukin; TNF-α, tumor necrosis factor alpha; IFN-γ, gamma interferon; IP-10, gamma interferon-induced protein 10. Note that no significant difference in cytokine expression was observed as the result of RC-101 treatment. n = 3. Error bars represent SEM.
RC-101 prevents adherence of S. aureus to organotypic human airway epithelial tissues.
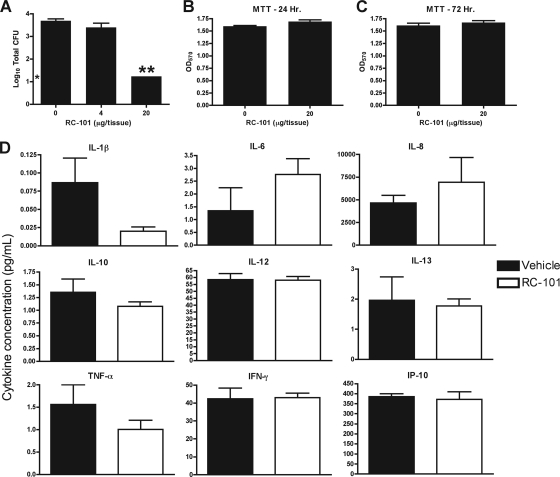
To mimic better the physiologic state, anti-S. aureus assays with RC-101 were also carried out using an organotypic model of human airway epithelia. Bacterial adhesion assays using two carrier strains of S. aureus (D20-7 and D30) were performed on these tissues for 9 h in the presence of 4 μg and 20 μg of RC-101 per tissue. As shown in Fig. 7A, 20 μg of peptide per tissue completely prevented the attachment of carrier strain D20-7, reducing adhesion by more than 2.5 log10 compared to vehicle-treated tissues alone. Similar data were also observed for strain D30 for which 20 μg of peptide per tissue completely prevented attachment to airway epithelia, again reducing attachment by approximately 2.5 log10 compared to vehicle-treated tissues (data not shown).
Fig. 7.
RC-101 prevents bacterial adherence to organotypic airway epithelial tissue but does not exhibit cytotoxicity or induce a proinflammatory response. (A) Total CFU counts when nasal carriage strain D20-7 was inoculated for 9 h on organotypic human airway epithelia in the presence of RC-101. Organotypic airway epithelia were also treated with RC-101 or vehicle for 24 (B) and 72 (C) h and assayed for cellular viability using MTT reduction. (D) Following treatment of airway epithelia with RC-101 (20 μg/tissue) for 72 h, cytokine production was assessed. Shown are nine representative plots of the 27 cytokines assayed. Note that RC-101 does not significantly alter expression profiles for any of the tested cytokines. Limit of detection for data shown in panel A is 18 (i.e., log10 = 1.26) total CFU. n ≥ 3. Error bars represent SEM. * indicates the starting inoculum. **, P < 0.001. P values indicate statistical significance compared to vehicle-treated samples.
Organotypic airway epithelia were also treated with RC-101 (20 μg/tissue) for 24- and 72-hour periods, after which time cell viability was measured by MTT reduction. As with the nasal epithelia, no reduction in cell viability was observed in the organotypic model as a result of RC-101 treatment (Fig. 7B and C). In addition to measuring the cytotoxic potential of RC-101 in organotypic airway epithelia, a panel of 27 proinflammatory cytokines were also analyzed after 24 and 72 h of treatment to assess whether this peptide promotes an inflammatory response. As shown in Fig. 7D, RC-101 (20 μg/tissue) did not promote inflammation in these tissues after 72 h of treatment, reinforcing the safety of this peptide to human epithelia. Similar expression profiles were also observed for the additional 18 cytokines tested, as well as for samples treated for 24 h with RC-101 (data not shown). The lack of both cytotoxicity and an inflammatory response imparted by RC-101 on these tissues, as well as the ability of RC-101 to exhibit antibacterial activities in these tissues, supports the potential of RC-101 as a therapeutic to combat respiratory infections. Toward this end, additional research detailing the antimicrobial activity of RC-101 on a wide range of microbes will first be necessary. Previous studies have shown that retrocyclin is effective against a multitude of bacteria, including Pseudomonas aeruginosa (6), which supports the evaluation of RC-101 in antibacterial applications other than nasal decolonization of S. aureus (e.g., treatment of cystic fibrosis).
Collectively, our studies have shown that the retrocyclin analogue RC-101 is a potential treatment option for the prevention and decolonization of S. aureus nasal carriage and warrants further investigation in this capacity. Future studies will be instrumental in identifying the mechanism of the anti-S. aureus action of RC-101, as well as the efficacy of this peptide as a treatment option, or preventative measure, for S. aureus nasal colonization.
ACKNOWLEDGMENTS
This work was supported in part by grant AI060753 (to A.M.C.) from the National Institutes of Health and a doctoral postgraduate scholarship (PGS-D) from the Natural Sciences and Engineering Research Council of Canada (to R.P.L.).
Footnotes
Published ahead of print on 8 August 2011.
REFERENCES
- 1. Boelaert J. R., et al. 1993. Nasal mupirocin ointment decreases the incidence of Staphylococcus aureus bacteraemias in haemodialysis patients. Nephrol. Dial. Transplant. 8:235–239 [PubMed] [Google Scholar]
- 2. Coates T., Bax R., Coates A. 2009. Nasal decolonization of Staphylococcus aureus with mupirocin: strengths, weaknesses and future prospects. J. Antimicrob. Chemother. 64:9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cole A. L., et al. 2007. The retrocyclin analogue RC-101 prevents human immunodeficiency virus type 1 infection of a model human cervicovaginal tissue construct. Immunology 121:140–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cole A. L., et al. 2006. HIV-1 adapts to a retrocyclin with cationic amino acid substitutions that reduce fusion efficiency of gp41. J. Immunol. 176:6900–6905 [DOI] [PubMed] [Google Scholar]
- 5. Cole A. M., Dewan P., Ganz T. 1999. Innate antimicrobial activity of nasal secretions. Infect. Immun. 67:3267–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cole A. M., et al. 2002. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. U. S. A. 99:1813–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cole A. M., et al. 2010. The formulated microbicide RC-101 was safe and antivirally active following intravaginal application in pigtailed macaques. PLoS One 5:e15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cole A. M., et al. 2001. Determinants of Staphylococcus aureus nasal carriage. Clin. Diagn. Lab. Immunol. 8:1064–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cole A. M., Wu M., Kim Y. H., Ganz T. 2000. Microanalysis of antimicrobial properties of human fluids. J. Microbiol. Methods 41:135–143 [DOI] [PubMed] [Google Scholar]
- 10. Conover M. S., Sloan G. P., Love C. F., Sukumar N., Deora R. 2010. The Bps polysaccharide of Bordetella pertussis promotes colonization and biofilm formation in the nose by functioning as an adhesin. Mol. Microbiol. 77:1439–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cookson B. D. 1998. The emergence of mupirocin resistance: a challenge to infection control and antibiotic prescribing practice. J. Antimicrob. Chemother. 41:11–18 [DOI] [PubMed] [Google Scholar]
- 12. Fuhrman C. A., et al. 2007. Retrocyclin RC-101 overcomes cationic mutations on the heptad repeat 2 region of HIV-1 gp41. FEBS J. 274:6477–6487 [DOI] [PubMed] [Google Scholar]
- 13. Holton D. L., Nicolle L. E., Diley D., Bernstein K. 1991. Efficacy of mupirocin nasal ointment in eradicating Staphylococcus aureus nasal carriage in chronic haemodialysis patients. J. Hosp. Infect. 17:133–137 [DOI] [PubMed] [Google Scholar]
- 14. Kluytmans J., van Belkum A., Verbrugh H. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kluytmans J. A., et al. 1996. Reduction of surgical-site infections in cardiothoracic surgery by elimination of nasal carriage of Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 17:780–785 [DOI] [PubMed] [Google Scholar]
- 16. Lamers R. P., Stinnett J. W., Muthukrishnan G., Parkinson C. L., Cole A. M. 2011. Evolutionary analyses of Staphylococcus aureus identify genetic relationships between nasal carriage and clinical isolates. PLoS One 6:e16426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leonova L., et al. 2001. Circular minidefensins and posttranslational generation of molecular diversity. J. Leukoc. Biol. 70:461–464 [PubMed] [Google Scholar]
- 18. Nekhotiaeva N., Awasthi S. K., Nielsen P. E., Good L. 2004. Inhibition of Staphylococcus aureus gene expression and growth using antisense peptide nucleic acids. Mol. Ther. 10:652–659 [DOI] [PubMed] [Google Scholar]
- 19. Nguyen T. X., Cole A. M., Lehrer R. I. 2003. Evolution of primate theta-defensins: a serpentine path to a sweet tooth. Peptides 24:1647–1654 [DOI] [PubMed] [Google Scholar]
- 20. Owen S. M., et al. 2004. RC-101, a retrocyclin-1 analogue with enhanced activity against primary HIV type 1 isolates. AIDS Res. Hum. Retroviruses 20:1157–1165 [DOI] [PubMed] [Google Scholar]
- 21. Quinn G. A., Cole A. M. 2007. Suppression of innate immunity by a nasal carriage strain of Staphylococcus aureus increases its colonization on nasal epithelium. Immunology 122:80–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quinn G. A., Tarwater P. M., Cole A. M. 2009. Subversion of interleukin-1-mediated host defence by a nasal carrier strain of Staphylococcus aureus. Immunology 128:e222–e229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang Y. Q., et al. 1999. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science 286:498–502 [DOI] [PubMed] [Google Scholar]
- 24. Thomas R., Brooks T. 2006. Attachment of Yersinia pestis to human respiratory cell lines is inhibited by certain oligosaccharides. J. Med. Microbiol. 55:309–315 [DOI] [PubMed] [Google Scholar]
- 25. Thomas R. J. 2010. Receptor mimicry as novel therapeutic treatment for biothreat agents. Bioeng. Bugs 1:17–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trabi M., Schirra H. J., Craik D. J. 2001. Three-dimensional structure of RTD-1, a cyclic antimicrobial defensin from rhesus macaque leukocytes. Biochemistry 40:4211–4221 [DOI] [PubMed] [Google Scholar]
- 27. Tran D., et al. 2002. Homodimeric theta-defensins from rhesus macaque leukocytes: isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J. Biol. Chem. 277:3079–3084 [DOI] [PubMed] [Google Scholar]
- 28. van Belkum A., et al. 2009. Reclassification of Staphylococcus aureus nasal carriage types. J. Infect. Dis. 199:1820–1826 [DOI] [PubMed] [Google Scholar]
- 29. Venkataraman N., Cole A. L., Svoboda P., Pohl J., Cole A. M. 2005. Cationic polypeptides are required for anti-HIV-1 activity of human vaginal fluid. J. Immunol. 175:7560–7567 [DOI] [PubMed] [Google Scholar]
- 30. von Eiff C., Becker K., Machka K., Stammer H., Peters G. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344:11–16 [DOI] [PubMed] [Google Scholar]
- 31. Wenzel R. P., Perl T. M. 1995. The significance of nasal carriage of Staphylococcus aureus and the incidence of postoperative wound infection. J. Hosp. Infect. 31:13–24 [DOI] [PubMed] [Google Scholar]
- 32. Yasin B., et al. 2004. Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 78:5147–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]