Abstract
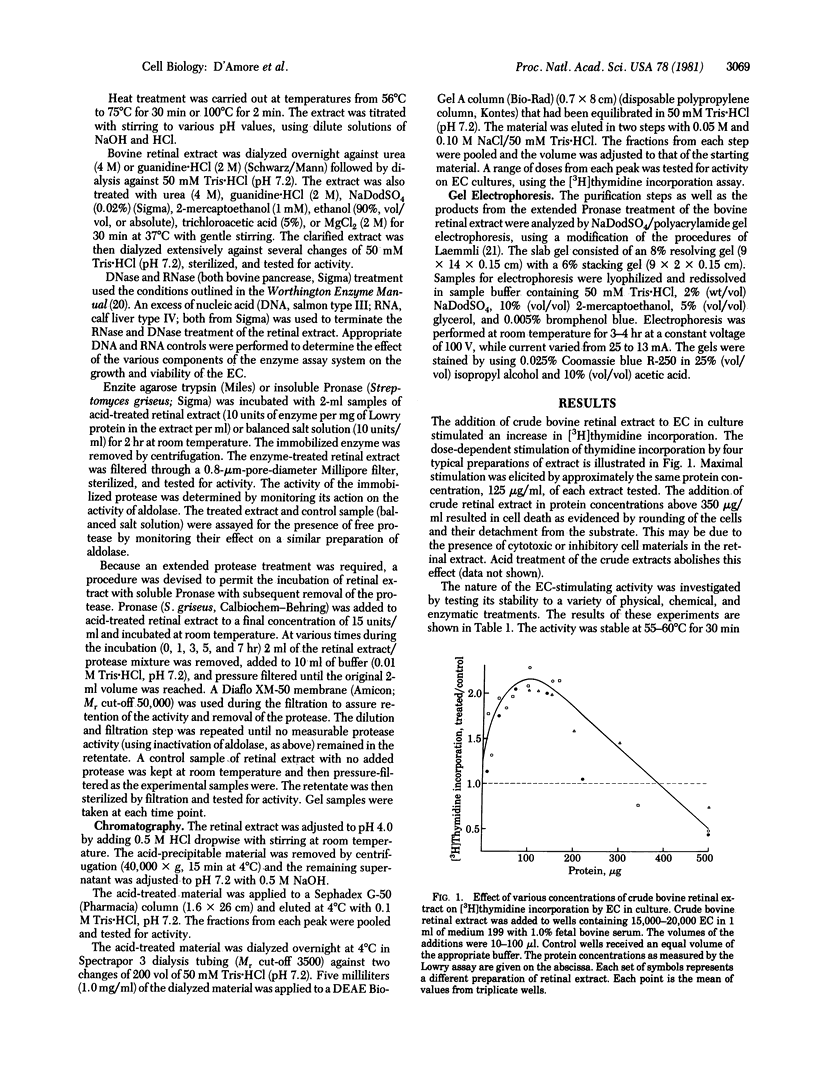
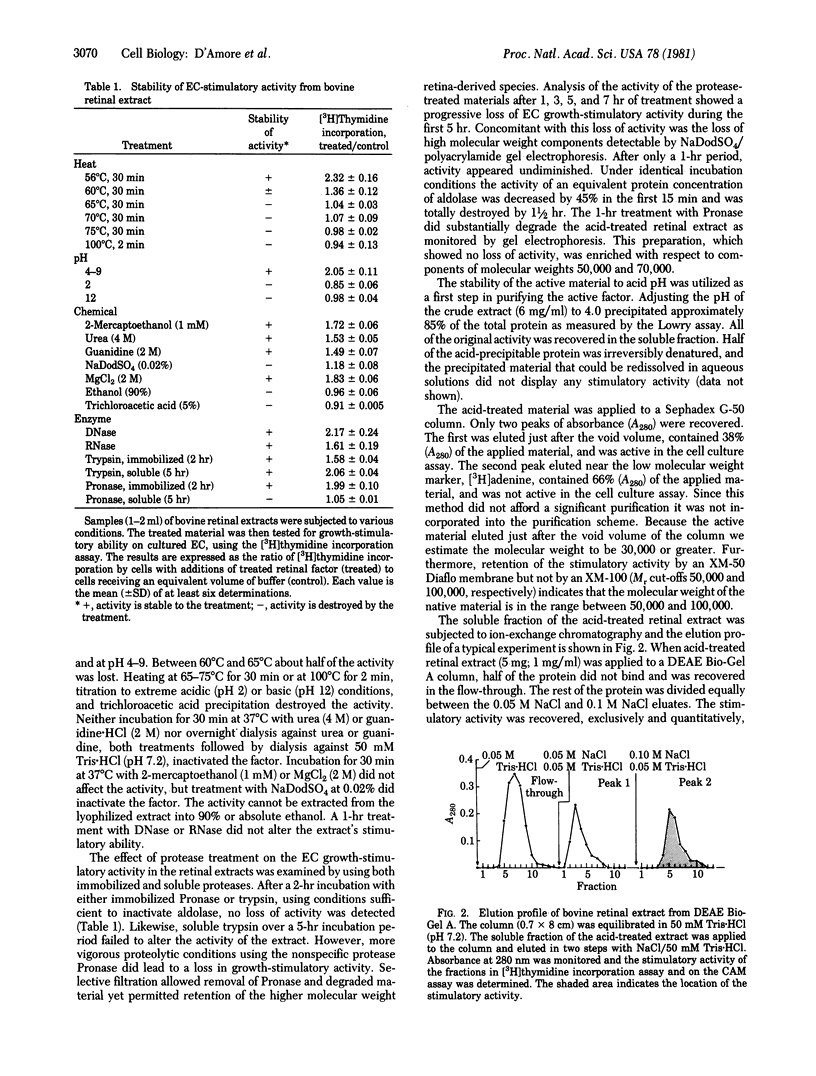
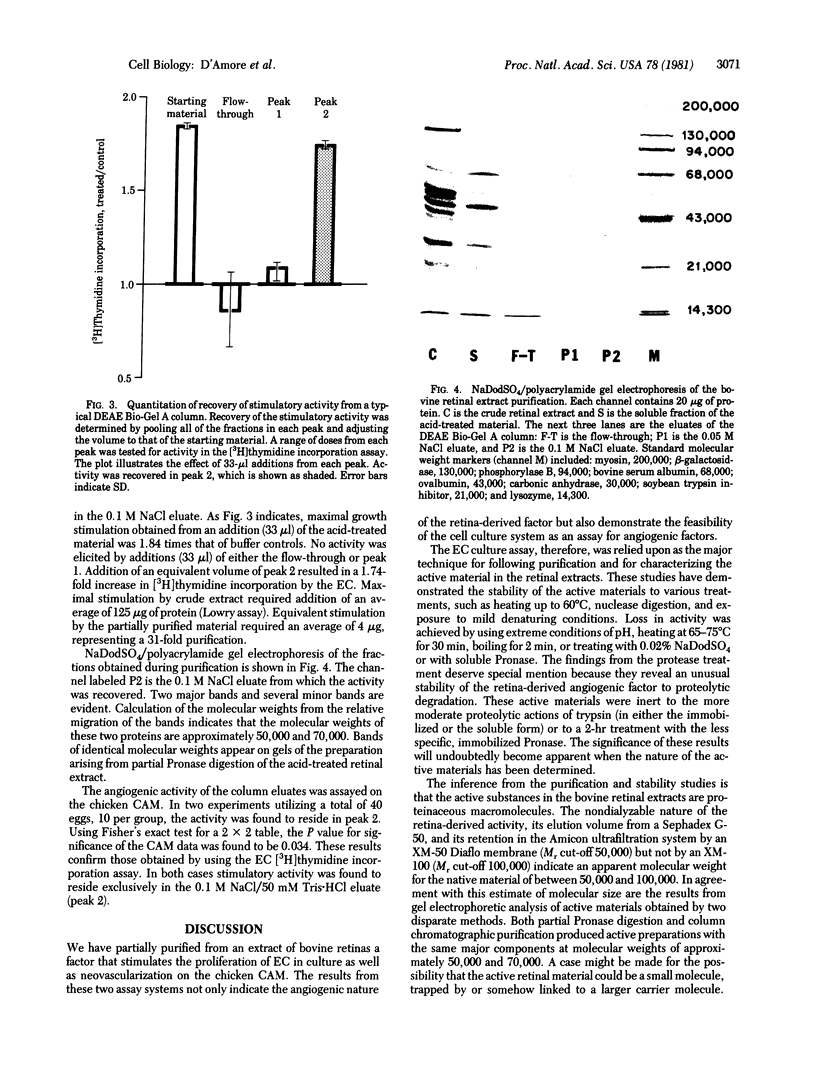
Bovine retinas contain a factor that stimulates proliferation of aortic endothelial cells in culture as well as neovascularization on the chicken chorioallantoic membrane. The stimulatory activity has been partially purified from a balanced salt solution extract of bovine retinas. The stability of the activity to acid pH was utilized as the first step in purification. The acid-treated material was subjected to ion-exchange chromatography on DEAE Bio-Gel A. The material that was eluted with 0.1 M NaCl/50 mM Tris.HCl (pH 7.6) stimulated both the proliferation of vascular endothelial cells in culture and angiogenesis in vivo. A molecular weight of between 50,000 and 100,000 for the native molecule is suggested by ultrafiltration and gel chromatography; gel electrophoresis of partially purified material under reducing and denaturing conditions revealed the presence of two major components with apparent molecular weights of 50,000 and 70,000. The endothelial cell stimulatory activity was stable to pH 4-9 and to heating at up to 60 degrees C for 30 min, to incubating for 1 hr with DNase or RNase, to incubating for 2 hr with immobilized Pronase or immobilized or soluble trypsin, and to treating with 1 mM 2-mercaptoethanol, 4 M urea, or 2 M guanidine. Heating at 65-75 degrees C for 30 min, boiling for 2 min, extreme acidic (pH 2) or basic (pH 12) conditions, treating with 0.02% NaDodSO4, or incubating (5 hr) with soluble Pronase destroyed the activity. The significance of an angiogenic factor derived from retina stems from the fact that neovascularization is a serious complication in a number of ocular diseases.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHTON N., WARD B., SERPELL G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954 Jul;38(7):397–432. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHTON N., WARD B., SERPELL G. Role of oxygen in the genesis of retrolental fibroplasia; a preliminary report. Br J Ophthalmol. 1953 Sep;37(9):513–520. doi: 10.1136/bjo.37.9.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. A., Weiss J. B., Tomlinson I. W., Phillips P., Kumar S. Angiogenic factor from synovial fluid resembling that from tumours. Lancet. 1980 Mar 29;1(8170):682–685. [PubMed] [Google Scholar]
- Fenselau A., Mello R. J. Growth stimulation of cultured endothelial cells by tumor cell homogenates. Cancer Res. 1976 Sep;36(9 PT1):3269–3273. [PubMed] [Google Scholar]
- Folkman J., Merler E., Abernathy C., Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971 Feb 1;133(2):275–288. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Proceedings: Tumor angiogenesis factor. Cancer Res. 1974 Aug;34(8):2109–2113. [PubMed] [Google Scholar]
- Glaser B. M., D'Amore P. A., Michels R. G., Patz A., Fenselau A. Demonstration of vasoproliferative activity from mammalian retina. J Cell Biol. 1980 Feb;84(2):298–304. doi: 10.1083/jcb.84.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkind P. Ocular neovascularization. The Krill memorial lecture. Am J Ophthalmol. 1978 Mar;85(3):287–301. [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McAuslan B. R., Hoffman H. Endothelium stimulating factor from Walker carcinoma cells. Relation to tumor angiogenic factor. Exp Cell Res. 1979 Mar 1;119(1):181–190. doi: 10.1016/0014-4827(79)90347-1. [DOI] [PubMed] [Google Scholar]
- Nishioka K., Katayama I. Angiogenic activity in culture supernatant of antigen-stimulated lymph node cells. J Pathol. 1978 Oct;126(2):63–69. doi: 10.1002/path.1711260202. [DOI] [PubMed] [Google Scholar]
- PATZ A., EASTHAM A., HIGGINBOTHAM D. H., KLEH T. Oxygen studies in retrolental fibroplasia. II. The production of the microscopic changes of retrolental fibroplasia in experimental animals. Am J Ophthalmol. 1953 Nov;36(11):1511–1522. [PubMed] [Google Scholar]
- Rhine W. D., Hsieh D. S., Langer R. Polymers for sustained macromolecule release: procedures to fabricate reproducible delivery systems and control release kinetics. J Pharm Sci. 1980 May;69(3):265–270. doi: 10.1002/jps.2600690305. [DOI] [PubMed] [Google Scholar]
- Sidky Y. A., Auerbach R. Lymphocyte-induced angiogenesis: a quantitative and sensitive assay of the graft-vs.-host reaction. J Exp Med. 1975 May 1;141(5):1084–1100. doi: 10.1084/jem.141.5.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISE G. N. Retinal neovascularization. Trans Am Ophthalmol Soc. 1956;54:729–826. [PMC free article] [PubMed] [Google Scholar]
- Weiss J. B., Brown R. A., Kumar S., Phillips P. An angiogenic factor isolated from tumours: a potent low-molecular-weight compound. Br J Cancer. 1979 Sep;40(3):493–496. doi: 10.1038/bjc.1979.206. [DOI] [PMC free article] [PubMed] [Google Scholar]