Abstract
The Gram-negative plague bacterium, Yersinia pestis, has historically been regarded as one of the deadliest pathogens known to mankind, having caused three major pandemics. After being transmitted by the bite of an infected flea arthropod vector, Y. pestis can cause three forms of human plague: bubonic, septicemic, and pneumonic, with the latter two having very high mortality rates. With increased threats of bioterrorism, it is likely that a multidrug-resistant Y. pestis strain would be employed, and, as such, conventional antibiotics typically used to treat Y. pestis (e.g., streptomycin, tetracycline, and gentamicin) would be ineffective. In this study, cethromycin (a ketolide antibiotic which inhibits bacterial protein synthesis and is currently in clinical trials for respiratory tract infections) was evaluated for antiplague activity in a rat model of pneumonic infection and compared with levofloxacin, which operates via inhibition of bacterial topoisomerase and DNA gyrase. Following a respiratory challenge of 24 to 30 times the 50% lethal dose of the highly virulent Y. pestis CO92 strain, 70 mg of cethromycin per kg of body weight (orally administered twice daily 24 h postinfection for a period of 7 days) provided complete protection to animals against mortality without any toxic effects. Further, no detectable plague bacilli were cultured from infected animals' blood and spleens following cethromycin treatment. The antibiotic was most effective when administered to rats 24 h postinfection, as the animals succumbed to infection if treatment was further delayed. All cethromycin-treated survivors tolerated 2 subsequent exposures to even higher lethal Y. pestis doses without further antibiotic treatment, which was related, in part, to the development of specific antibodies to the capsular and low-calcium-response V antigens of Y. pestis. These data demonstrate that cethromycin is a potent antiplague drug that can be used to treat pneumonic plague.
INTRODUCTION
There are 11 known yersiniae; however, only 3 species are human pathogens, and, of the 3, Yersinia pestis is the most notorious. Y. pestis has a complicated life cycle involving growth in an arthropod vector, the flea, and a mammalian host. The mammalian host is often a rodent (e.g., rats, squirrels, and prairie dogs); however, in many instances, especially where endemic rodent populations are high, infected fleas hungry for a blood meal can feed on humans who are in close proximity to the animal reservoirs (9). Historically, Y. pestis has caused three major pandemics and is estimated to have killed over 200 million people (7, 14). Unfortunately, plague continues to cause morbidity and mortality with 1,000 to 2,000 human infection cases reported annually, primarily in parts of India and China where rodent populations are high. Furthermore, Y. pestis is ubiquitous and can be found within the United States in the Four Corners region where Arizona, Colorado, New Mexico, and Utah meet, as well as in parts of California (6, 24).
Considering plague's designation as a reemerging infectious disease by the World Health Organization (WHO) (3, 13) and the current relevance of multidrug-resistant Y. pestis strains as a bioterror threat (2, 18), it is essential that novel antiplague drugs be developed, evaluated, and marketed. This infection manifests itself in septicemic (characterized by whole-body involvement via spread of Y. pestis through the blood/or lymphatics) and pneumonic (person-to-person transmission via Y. pestis-charged respiratory droplets) forms, both of which lead to very rapid death in humans if left untreated (50 to 90% mortality). However, if the infecting strain is one that does not possess the multidrug-resistant trait, mortality can be reduced to approximately 15% if streptomycin or gentamicin is administered within 24 to 36 h of infection (http://www.cdc.gov/ncidod/dvbid/plague/info.htm). Although the most common form of plague is bubonic, which leads to swollen lymph nodes or buboes and results in a mortality rate of 30 to 75% if untreated, antibiotic intervention is usually highly effective if started in time.
Unfortunately, antibiotic-resistant Y. pestis strains have already been isolated from human cases of bubonic plague in Madagascar (8, 10). Such strains harbor genes encoding chloramphenicol acetyltransferase, streptomycin-modifying enzyme, and TEM-1 beta-lactamase, conferring resistance to chloramphenicol, streptomycin, and penicillin derivatives, respectively. These multidrug-resistant Y. pestis strains, including those resistant to tetracycline and fluroquinolone, could potentially find their way into the hands of bioterrorists (14). Therefore, the development and marketing of novel chemotherapeutic agents are of paramount importance to counteract potential Y. pestis bioweapon attacks.
Previously, we have characterized mouse, guinea pig, and rabbit models of respiratory infections caused by category A select agents such as Bacillus anthracis, Y. pestis, and Francisella tularenesis (25). However, rat models may be preferred for plague infection since, unlike mice, which do not typically develop buboes, rats develop more human-like forms of bubonic plague (15, 33). Rats have also been successfully used to determine the kinetics of bubonic infection, as well as for examining the host immune response to Y. pestis (4, 29). In addition, rats are the zoonotic animal reservoir for Y. pestis (29). We became one of the first groups to fully characterize the rat model of pneumonic plague (1). Now, we wish to report the efficacy of a novel chemotherapeutic, cethromycin (a ketolide antibiotic currently in clinical trials for respiratory tract infections), as an antiplague antimicrobial by using the rat pneumonic plague model and comparing the activity of cethromycin with that of levofloxacin. Although our earlier study demonstrated efficacy of levofloxacin in the mouse plague model (25), it was never tested in the rat model of infection.
Our detailed studies with levofloxacin and cethromycin were conducted independently in the rat model; however, we included the former in several cethromycin experiments as a positive control for direct comparison. These two antibiotics operate via different mechanisms. Levofloxacin inhibits bacterial topoisomerase IV and DNA gyrase and thus inhibits DNA replication, transcription, DNA repair, and recombination (22). In contrast, cethromycin inhibits bacterial protein synthesis by binding to the 23S ribosomal subunit portion of the 50S subunit or depleting the ribosome as a whole. This binding (through hydrophobic interactions) prevents the exit of nascent peptides from the ribosomal complex, resulting in translation arrest (26).
A recent study has evaluated the in vitro activity of cethromycin against the intrinsically multidrug-resistant Burkholderia pseudomallei, an etiological agent of melioidosis, and was found to be bactericidal at 4 times the MIC and bacteriostatic at the MIC (23). In addition, the immunomodulatory and anti-inflammatory effects of cethromycin have been documented. In a murine model of pneumonia caused by Mycoplasma pneumoniae, therapy with oral cethromycin at 25 mg/kg of body weight/day for 10 days demonstrated a statistically significant improvement of not only microbiological, histological, and respiratory but also immunological markers of disease severity (27). The effects of cethromycin on the release of cytokines and chemokines may not only hasten the resolution of the acute illness but also influence the type of adaptive immune response at the later stages of infection, as well as in subsequent encounters with this microorganism. The modulation of innate immunity against lung inflammation by cethromycin may confer additional benefits to the treatment of pneumonic plague. Although the MIC for levofloxacin against Y. pestis was reported to be 0.03 μg/ml (11, 25), cethromycin exhibited a MIC of 1 μg/ml based on one report presented at the 2006 ICAAC meeting (12). In this study, we reevaluated the MIC of cethromycin against Y. pestis and found it to be 2.5 μg/ml. Finally, we showed that infected rats rescued with cethromycin developed significant antibodies to the capsular (F1) and low-calcium-response V (LcrV) antigens of Y. pestis, which were partly responsible for subsequent protection against Y. pestis rechallenges.
MATERIALS AND METHODS
Bacterial strain and reagents.
For all rat infections, the fully virulent Y. pestis CO92 strain (obtained from the Biodefense and Emerging Infections [BEI] Research Resources Repository, Manassas, VA) was used. The female Brown Norway rats (75 to 100 g) were purchased from Charles River Laboratories International Inc. (Wilmington, MA). Cethromycin is a ketolide antibiotic initially discovered by Abbott, designated as ABT-773, and licensed to Advanced Life Sciences Inc. (Woodridge, IL) in 2004. The drug is currently in late-stage clinical trials for treatment against community-acquired bacterial pneumonia. Unless otherwise noted, cethromycin was administered orally either once daily (q.d.) or twice daily (b.i.d.) 24 h after Y. pestis pneumonic challenge at the indicated doses and length of time. The cethromycin solution (8 mg/ml) was prepared by initially suspending the antibiotic in sterile water followed by dropwise addition of acetic acid until it was completely dissolved before making up the volume with water. The antibiotic solution was kept in the dark at the refrigeration temperature and was stable for 7 days. A sterile, injectable formulation of levofloxacin (Janssen Pharmaceutica, N.V., Beerse, Belgium) was purchased from the UTMB Pharmacy. The animals in this study were administered levofloxacin by intraperitoneal (i.p.) injection or via the oral route at the indicated concentrations. Finally, purified LcrV (for performing enzyme-linked immunoabsorbent assay [ELISA] and Western blot analysis) was obtained from BEI, while F1 was available in our laboratory.
Animal infection and rechallenge studies.
Once we developed the rat model of pneumonic plague (1), it became obvious to us that we should evaluate the efficacy of levofloxacin in the rat model system as a natural continuation of our earlier levofloxacin efficacy studies performed in the mouse model of plague (25). The 50% lethal dose (LD50) of Y. pestis CO92 was initially determined in the rat model by intranasal (i.n.) inoculation and found to be in the range of 329 to 500 CFU. This LD50 for rats was very similar to that noted for the Swiss-Webster mice (30). For all of our subsequent studies, 500 CFU of Y. pestis CO92 was considered 1 LD50.
Since in our mouse model of pneumonic plague, we used 5 times the LD50 of Y. pestis CO92, we decided to increase the dose of bacteria to be used for challenge studies in the rat model, since we were convinced that any protective effects of levofloxacin would not be compromised by using a higher dose of 7 to 14 times the LD50. This increased dose also ensured that we would obtain 100% death of animals in the control group, which did not receive antibiotic treatment. This is an important consideration since, at times, lower doses of bacteria may not kill all control animals.
Thus, for the levofloxacin experiments, Brown Norway rats (n = 6 to 9 per group) were intranasally exposed to 7 to 14 times the 50% lethal dose (LD50) of Y. pestis CO92 in an animal biosafety level 3 (ABSL-3) facility at the Galveston National Laboratory, UTMB. The animals were anesthetized with a mixture of ketamine and xylazine by the i.p. route before infection as per the protocol approved by the Institutional Animal Care and Use Committee (IACUC). For bacterial inoculation, the desired number of organisms was delivered in a total volume of 50 μl, with 25 μl given in each naris followed by washing of both of the nostrils with 25 μl of phosphate-buffered saline (PBS). Twenty-four hours following the Y. pestis CO92 pneumonic challenge, levofloxacin treatment (25) was initiated at various doses (0.5 to 15 mg/kg/day) given by the i.p. route or a selected oral dose (20 mg/kg/day) for 6 days. The animals were then observed for clinical symptoms and mortality for 42 days postchallenge. On day 43, the survivors from each group were rechallenged with Y. pestis CO92 (7 LD50), and animals were monitored for an additional 17 days (until day 60). This study allowed evaluation of levofloxacin-enhanced protection against Y. pestis challenge following an initial pneumonic plague exposure.
For the cethromycin experiments, the infection was performed as reported above for the levofloxacin studies, with the exception that higher bacterial doses were used. Thus, Brown Norway rats (n = 9 per group) were intranasally exposed to 24 to 30 times the LD50 of Y. pestis CO92. Our rat studies conducted with levofloxacin allowed us to further increase the dose of bacteria in animal challenge experiments with cethromycin to better gauge its protective effects against pneumonic plague.
Twenty-four hours following the Y. pestis CO92 pneumonic challenge, cethromycin was administered to rats orally by using disposable animal feeding needles (20G × 1.50 in.; Thermo Fisher Scientific, Pittsburgh, PA) at the following doses: 20 mg/kg, 60 mg/kg, 100 mg/kg, 120 mg/kg, and 140 mg/kg as single daily doses (q.d.) or b.i.d. at 70 mg/kg. The aforementioned range of doses was given for periods of 7, 10, or 14 days to determine the minimal/optimal drug kinetic dosing scheme in the rat model. The animals were examined for mortality and other clinical symptoms over a period of at least 3 weeks. The survivors were intranasally rechallenged with Y. pestis CO92 at 32 LD50 on day 55 after the first challenge. Subsequently, the survivors were challenged a third time with 90 LD50 of CO92 on day 110 after the initial challenge. The subsequent second and third challenges provided an indication of protection in animals possibly afforded by the host immune response that was triggered by Y. pestis antigens (e.g., F1 and LcrV), as no antibiotic treatment was given when rats were reinfected.
The control animals were infected with Y. pestis but not given any antibiotic. Instead, the rats were provided with the vehicle. For drug toxicity controls, the noninfected rats (n = 9 per group) were given cethromycin for the same duration as the experimental groups and observed for clinical symptoms and mortality. For some experiments (see Fig. 2 and 3), we included levofloxacin (given at a dose of 20 mg/kg/day) as a positive control in Y. pestis-infected rats for our cethromycin studies, since this was the lowest dose of the ketolide antibiotic that we tested.
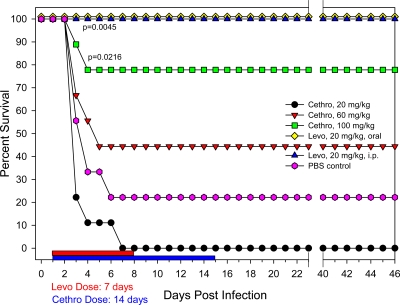
Fig. 2.
Evaluation of dose-response effect of cethromycin in a pneumonic rat model of infection. Groups of 9 rats were exposed to a 25 LD50 of Y. pestis CO92 and administered cethromycin at the indicated doses. A negative control consisted of rats administered PBS instead of cethromycin (Cethro), and as a positive control rats received 20 mg/kg/day of levofloxacin (Levo) either orally or intraperitoneally. Drug treatments were administered for either 7 or 14 days, and administration lengths are indicated by red and blue bars. P values indicate statistical significance.
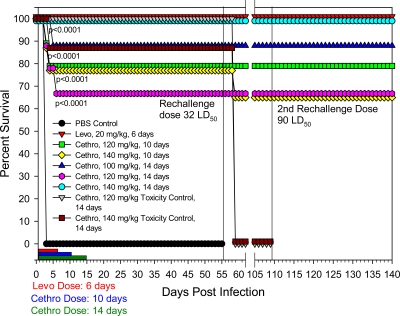
Fig. 3.
Evaluation of higher oral doses of cethromycin in a pneumonic rat model of infection and subsequent protection from 2 Y. pestis rechallenges. Groups of rats were exposed to a 30 LD50 of Y. pestis CO92 and administered cethromycin (Cethro) at the indicated doses. As a negative control, rats were administered PBS instead of cethromycin, while as a positive control, rats received 20 mg/kg/day of levofloxacin (Levo) orally. Drug treatments were administered for 6, 10, or 14 days, and administration lengths are indicated by different colored bars. Survivors were rechallenged twice following the first Y. pestis infection. As toxicity controls, uninfected rats were administered either 120 mg/kg or 140 mg/kg/day for 14 days. P values indicate statistical significance.
Bacterial load determination.
After intranasal challenge with 24 times the LD50 of Y. pestis CO92 and initiating cethromycin treatment 24 h after Y. pestis exposure at a dose of 70 mg/kg b.i.d. for 7 days, 3 representative rats out of the 30 animals were bled via cardiac puncture under anesthetic in the ABSL-3 facility each day for up to 7 days during the cethromycin treatment. Blood was evaluated for bacterial load via by plating on 5% sheep blood agar plates (Becton Dickinson and Company, Franklin Lakes, NJ) and subsequent counting of viable colonies. Another 9 rats were infected and given only the vehicle and observed for mortality. In addition to blood, the spleens of infected animals were also harvested on day 2 from the group that did not receive cethromycin and on days 2, 4, and 6 from groups that received the antibiotic treatment for evaluating bacterial clearance.
Delayed antibiotic treatment.
To evaluate the efficacy of levofloxacin when the treatment was delayed, rats were infected (n = 9 per group) with 14 times the LD50 of CO92. At time points 24, 36, 42, and 48 h postinfection, the animals were given two different doses of levofloxacin (5 and 10 mg/kg/day via the i.p. route) for 6 days. Animal survival was monitored and recorded for 33 days postchallenge. On day 34, survivors from all groups were rechallenged with 12 times the LD50 of Y. pestis CO92 and monitored through day 65.
In another set of experiments, rats (n = 9 per group) were infected with Y. pestis at 24 times the LD50 of Y. pestis CO92 and received cethromycin treatment at 70 mg/kg b.i.d. for a period of 7 days starting at either 24, 36, 48, or 60 h after infection. The animals were observed for mortality for 64 days, and all of the surviving animals were then rechallenged with 30 times the LD50 of Y. pestis CO92 and observed for another 41 days for mortality. The control group of rats (n = 9) were infected and treated only with the vehicle.
MIC determination.
The MIC is the lowest concentration of an antimicrobial agent that completely inhibits the growth of an organism. For cethromycin, it was determined by using the broth macrodilution method as published by the Clinical and Laboratory Standards Institute (5). Briefly, the Y. pestis CO92 culture was adjusted to a 0.5 McFarland standard before adding the same volume of the culture to a 2-fold serial dilution of the antibiotic (starting concentration of cethromycin was 1 mg/ml) in 3 ml of cation-adjusted Mueller-Hinton broth. Cultures were grown for 24 h with shaking at 35°C, and absorbance was read at an optical density of 600 nm (OD600). Bacterial growth without the antibiotic or with levofloxacin served as a negative or positive control, respectively, for the MIC evaluation.
ELISA and Western blot analysis.
For ELISA, 96-well microtiter plates (Evergreen Scientific, Los Angeles, CA) were coated with 10 ng/well of LcrV or F1 antigen at 4°C overnight. Following blocking and washing (32), sera from naïve and infected rats were serially diluted and incubated with the affixed antigens in the microtiter plates for 1 h at room temperature. Following several washes, horseradish peroxidase (HRP)-conjugated goat anti-rat secondary antibodies were added to the wells at a dilution of 1:10,000 for 1 h at room temperature, followed by several washes and the addition of the substrate TMB (3,3′,5,5′-tetramethylbenzidine). Following a 20-min colorimetric development period, the reaction was quenched by the addition of 2 N H2SO4, and plate/well absorbance was read at 450 nm by using an ELISA reader.
For Western blot analysis, purified LcrV and F1 antigens (3.5 μg) were subjected to SDS-8-to-16% gradient polyacrylamide gel electrophoresis and immunoblotting. The blots were probed with sera from Y. pestis-infected and cethromycin-rescued as well as rechallenged rats at a dilution of 1:1,000, followed by HRP-labeled goat anti-rat secondary antibodies at a dilution of 1:10,000. As described in our earlier study (31), blots were developed by using a Super-Signal West Pico chemiluminescent substrate (Pierce, Rockford, IL).
Statistical analysis.
For all animal studies, we used log rank (chi-square test) Kaplan-Meier survival estimates (by using the Prism program) to determine statistical significance, and a P value of ≤0.05 was considered significant. The ELISA data were analyzed statistically by using a 2-way analysis of variance (ANOVA).
RESULTS
Protective effects of levofloxacin and cethromycin against pneumonic plague in a rat model. (i) Dose-response study with levofloxacin.
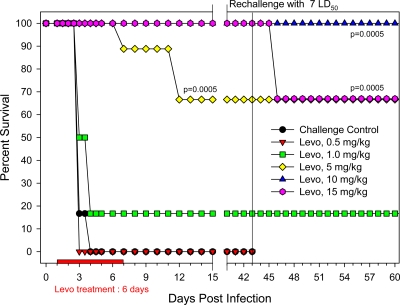
Knowing that levofloxacin is a potent anti-pneumonic plague chemotherapeutic in a murine model of infection (11, 25), it was further evaluated in rat dose-response infection studies. As shown in Fig. 1, survival of the untreated control rats (given 12 times the LD50 of Y. pestis CO92) reached 0% by day 4. Animals in other groups were administered levofloxacin at 24 h postinfection and continued to receive it for 6 consecutive days. The 0.5-mg/kg/day levofloxacin group of animals showed a mortality rate similar to that of the challenge control animals. The 1-mg/kg/day levofloxacin-treated rats exhibited 17% survival, while animals treated with 5 mg/kg/day had a 67% survival rate. Both the 10- and 15-mg/kg/day groups of levofloxacin-treated rats were 100% protected by day 42 of the study. Therefore, a 10- to 20-mg/kg/day dose of levofloxacin can be used as a positive control in this model. In fact, the 20-mg/kg/day dose of levofloxacin was employed in some of our subsequent experiments to mimic the lowest dose of cethromycin for comparison purposes. In terms of clinical symptoms at 48 h postinfection, animals in the control challenge group and those dying in the levofloxacin-treated groups were lethargic and dehydrated and had ruffled fur, hunched backs, and sunken eyes.
Fig. 1.
Evaluation of various doses of levofloxacin (Levo) in a pneumonic rat model of infection. Groups of 6 rats were exposed to a 12 LD50 of Y. pestis CO92 and administered levofloxacin at the indicated doses. Additionally, 9 rats were infected and received PBS instead of levofloxacin as a negative control. The duration of the drug treatment is indicated by the red bar. The animals in the levofloxacin-treated groups were rechallenged and monitored for lethality. P values indicate statistical significance.
On day 43 postchallenge, survivors in each group were rechallenged with Y. pestis CO92, as indicated with a vertical line on the survival graph (Fig. 1). All groups of animals maintained their original levels of survival, except for the 15-mg/kg/day group, in which the survival rate declined from 100% to 67%, which was still statistically significant when compared to that in the challenge control group. These data may indicate that rats were capable of mounting an immune response against Y. pestis that conferred resistance to subsequent infections. Indeed, sera obtained from animals following the primary challenge had specific antibodies to F1 and LcrV of Y. pestis (based on ELISA), which were likely responsible, in part, for this protective effect (data not shown).
(ii) Dose-response study with cethromycin.
In determining the in vitro MIC of cethromycin for Y. pestis CO92, the demonstrated 2.5 μg/ml seemed somewhat higher than the previously reported MIC (12). Despite the aforementioned disparity, we initially tested various doses of orally administered cethromycin, ranging from 20 to 100 mg/kg/day, in rats for either 10 or 14 days 24 h following a 25-times LD50 Y. pestis CO92 challenge. Cethromycin administered at 100 mg/kg/day protected 78% of Y. pestis-challenged rats following either a 10- or 14-day dosing regimen, in contrast to only 44% survival of rats receiving 60 mg/kg/day of cethromycin (Fig. 2). All animals dosed with 20 mg/kg/day of cethromycin died by day 7. Thus, the highest dose tested of cethromycin was not as effective in promoting rat survival as was a 20-mg/kg/day dose of levofloxacin administered either orally or via the i.p. route, which resulted in 100% survival of animals (Fig. 2). By including levofloxacin as a control in the aforementioned study, we clearly demonstrated differences in the efficacies of levofloxacin and cethromycin in providing protection to rats against Y. pestis. Importantly, protection provided by 100-mg/kg q.d. dosing of cethromycin was statistically significant compared to that of the control challenge group.
Based on these results, it appeared that in order to achieve levofloxacin-like levels of rat survival, a higher dose of cethromycin would be required. In addition, it was prudent to investigate if dosing duration would affect animal survivability. As shown in Fig. 3, a 140-mg/kg/day oral cethromycin dose for 14 days resulted in 100% protection of Y. pestis-infected rats. At a dose of 120 mg/kg/day for 14 days, the animals were 67% protected, which was statistically significant when compared to the control challenge group. In contrast, the differences between cethromycin doses ranging from 100 to 140 mg/kg/day were not statistically significant, nor were the differences between 10 and 14 days of drug administration.
Even though the 140-mg/kg/day cethromycin dose (administered for 14 days) resulted in optimal protection from an i.n. Y. pestis CO92 challenge, this dose appeared somewhat toxic, resulting in the death of 1/9 (11%) animals. However, the lower 120-mg/kg/day cethromycin dose appeared well tolerated by all animals, resulting in no toxicity-induced death (Fig. 3). Interestingly, cethromycin-treated toxicity control animals (at doses of 100 to 140 mg/kg q.d.) were asymptomatic up to 48 h, but, after 72 h, the animals were cool to the touch, had ruffled fur, sunken eyes, and dried mucus or blood in their mouths and noses, and were dehydrated. By day 4, the animals began recovering, and by day 8, animals were no longer symptomatic. These data indicated that cethromycin is somewhat toxic to rats at higher doses ranging from 100 to 140 mg/kg.
More importantly, however, surviving rats (treated with either 100, 120, or 140 mg/kg/day of cethromycin) were 100% protected from both second and third i.n. Y. pestis CO92 rechallenges (Fig. 3). The animals were monitored for 30 days after the third and final challenge. These data clearly demonstrated that cethromycin was not only effective as an antiplague chemotherapeutic that could be administered to control the plague bacilli but also helped promote a lasting immunity (in part by inducing antibodies to F1 and LcrV antigen of Y. pestis; see Fig. 8 and Discussion) that was protective upon secondary and tertiary exposures to Y. pestis.
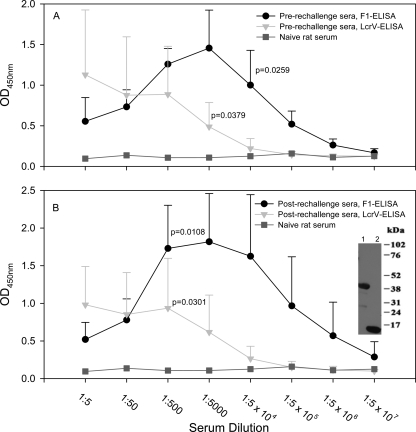
Fig. 8.
Y. pestis-infected and cethromycin-rescued rats developed antibodies to F1 and LcrV. Significant antibody titers to both F1 and LcrV were developed in rats after the first Y. pestis infection (top) and after rechallenge (bottom) based on ELISA. We used sera from infected rats (those described above for Fig. 7) that received cethromycin 24 h postinfection and were rechallenged (n = 9). The ELISA titers (shown as average means ± standard deviations [SD]) were statistically significant compared to naïve rat sera. The inset in this figure shows that rechallenge sera contained specific antibodies to LcrV (lane 1; 37 to 38 kDa) and F1 (lane 2; 15 to 16 kDa) based on Western blot analysis.
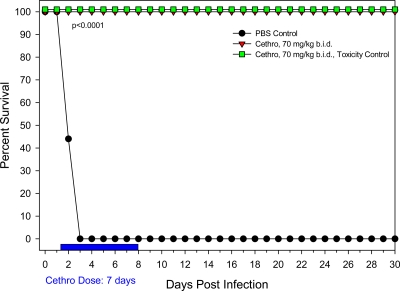
We then decided to evaluate the protective role of a total 140 mg/kg/day of cethromycin administered b.i.d. as 70-mg/kg increments for a period of 7 days (Fig. 4). The choice of a 7-day treatment period was based on the observation from our previous study that no additional animals died after 7 days (Fig. 3). We also reasoned that administering a halved dose b.i.d. might be less toxic (or not toxic) to the animals compared to the single 140-mg/kg/day dose. Indeed, by using the aforementioned 70-mg/kg b.i.d. dosing regimen for 7 days, 100% protection of rats intranasally challenged with 24 LD50 Y. pestis CO92 was achieved with no drug toxicity-induced animal deaths (Fig. 4). Importantly, when whole blood from experimental animals was evaluated for bacterial loads, not a single CFU was detected from three representative animals tested for the 7 days of treatment. This was not the case for three representative PBS control animals which did not receive any cethromycin; all three of those animals contained between 4.1 × 108 and 5.9 × 108 CFU/ml of blood, as evidenced by viable blood agar plate counts (Table 1). Similarly, spleens from Y. pestis-infected animals contained 2.9 × 107 to 1.0 × 109 CFU of bacteria 2 days following infection. However, these organisms were rapidly cleared from rats after cethromycin treatment (Table 1). Moreover, animals treated with this dose regimen of cethromycin did not show any symptoms of disease at any time.
Fig. 4.
Evaluation of 70 mg/kg b.i.d. of cethromycin in a pneumonic rat model of infection. Groups of rats were exposed to a 24 LD50 of Y. pestis CO92 and administered cethromycin (Cethro) at 70 mg/kg b.i.d., resulting in a total daily dose of 140 mg/kg. As a negative control, rats were administered PBS instead of cethromycin. A total daily dose of 140 mg/kg for 7 days was also carried out to evaluate any drug-induced animal toxicity.
Table 1.
Ability of cethromycin to kill Y. pestis CO92 (24 LD50s) in the blood and spleens of ratsa
| Day | CFU/ml in: |
|||||
|---|---|---|---|---|---|---|
| Blood |
Spleen |
|||||
| Rat 1 | Rat 2 | Rat 3 | Rat 1 | Rat 2 | Rat 3 | |
| Control | ||||||
| Day 1 | 0 | 0 | 0 | |||
| Day 2 | 4.1 × 108 | 5.9 × 108 | 5.5 × 108 | 1.2 × 108 | 1.0 × 109 | 2.9 × 107 |
| Cethro | ||||||
| Day 1 | 0 | 0 | 0 | |||
| Day 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Day 3 | 0 | 0 | 0 | |||
| Day 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Day 5 | 0 | 0 | 0 | |||
| Day 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| Day 7 | 0 | 0 | 0 | |||
Three control and cethromycin (Cethro)-treated and Y. pestis-infected rats were evaluated for either 2 or up to 7 days, respectively, for CFU in the blood. All of the control animals died by day 3. The spleens from Y. pestis-infected and cethromycin-treated and infected rats were examined for CFU on days 2, 4, and 6 postinfection.
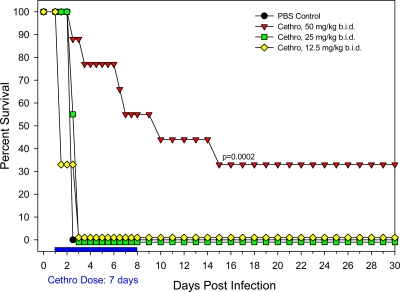
Finally, to establish whether the b.i.d. schedule could be effective by using even lower doses of cethromycin for a 7-day treatment period, we evaluated the following doses: 100 mg/kg/day (50 mg/kg b.i.d.), 50 mg/kg/day (25 mg/kg b.i.d.), and 25 mg/kg/day (12.5 mg/kg b.i.d.). Unlike the 70-mg/kg b.i.d. (for 7 days) cethromycin dosing, which provided 100% protection to rats, all of the above-mentioned lower b.i.d. doses failed to protect animals from an i.n. Y. pestis CO92 challenge (Fig. 5). In fact, 1 day after the initiation of the b.i.d. dosing, there were no surviving rats that had received either 50 mg/kg/day (25 mg/kg b.i.d.) or 25 mg/kg/day (12.5 mg/kg b.i.d.) of cethromycin, mirroring the outcome of PBS control animals that received no drug (Fig. 5). Animals that received the test dose of cethromycin at 100 mg/kg/day (50 mg/kg b.i.d.) did not fare much better. By day 15, only 33% of Y. pestis CO92-challenged animals survived (Fig. 5). Taken together, it appeared that 70-mg/kg b.i.d. cethromycin treatment for a 7-day period was well tolerated (resulting in no drug-induced animal toxicity) and 100% protective in rats challenged with Y. pestis CO92 (Fig. 5).
Fig. 5.
Evaluation of lower oral b.i.d. doses of cethromycin in a pneumonic rat model of infection. Groups 9 rats were exposed to a 30 LD50 of Y. pestis CO92 and administered cethromycin (Cethro) at the indicated doses. As a negative control, rats were administered PBS instead of cethromycin. The duration of the drug treatment is indicated by the red bar. P values indicate statistical significance.
(iii) Delayed levofloxacin treatment study.
As expected, all of the rats (n = 9) belonging to the untreated challenge control group expired due to Y. pestis CO92 infection by day 6. The remaining groups of animals were dosed (i.p.) with 5 or 10 mg/kg/day of levofloxacin, but the initiation of treatment ranged from 24 to 48 h postinfection. As the survival graph indicates (Fig. 6), delaying levofloxacin therapy up to 42 h postchallenge did not affect the survival of the animals, with all rats remaining 100% protected. These levels were statistically significant compared to those of the challenge control group beginning on day 4 postchallenge. However, the 48-h delay made a difference because animals dosed with 5 or 10 mg/kg/day each exhibited only 44% survival, a level still statistically significant relative to that for the challenge control group.
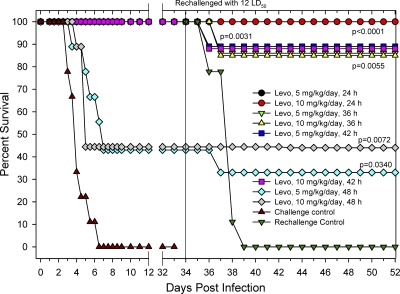
Fig. 6.
Evaluation of delayed administration of various doses of levofloxacin in a pneumonic rat model of infection. Groups of rats exposed to a 12 LD50 of Y. pestis CO92 and 24 to 48 h following Y. pestis infection were i.p. administered levofloxacin (Levo) at the indicated doses. As a negative control, 9 rats were infected and administered PBS. The solid black vertical line indicates rechallenging of the surviving animals. A group of 9 naïve rats was infected and served as rechallenge control. P values indicate statistical significance.
Thirty-four days following the initial infection, survivors were rechallenged with Y. pestis CO92 and monitored for an additional 31 days (Fig. 6 [data shown only up to 52 days]). Impressively, levofloxacin-treated rats from the 24-h post-initial infection groups remained 100% protected from the rechallenge. Survival of the 36-h delay groups dropped to 89% for both levofloxacin doses tested. Similarly, the two 42-h delay groups experienced 89% survival. Finally, the group that received 5 mg/kg/day levofloxacin beginning at 48 h post-initial infection demonstrated only a 33% survival rate following rechallenge, while the 10-mg/kg/day 48-h delay group of animals showed 44% survival. Additionally, a group of 9 naïve rats was included in the study, serving as the rechallenge control group that did not receive levofloxacin treatment but consisted of rats of the same age, group, and batch. Survival for this group plummeted to 0% by day 5, ensuring that a successful intranasal infection was achieved during the rechallenge phase.
Results from this study indicated that there was a narrow time frame (approximately 42 h) in which levofloxacin should be administered in order to achieve high levels of protection in this model. Delaying administration of the drug beyond 42 h dramatically reduced rat survival from a plague infection. The animals belonging to the groups that were treated with levofloxacin 42 h or earlier postinfection also remained protected (89 to 100% survival) upon rechallenge, again seeming to indicate that rats were capable of generating protective immunity specific for Y. pestis. These data also indicated that delays in levofloxacin treatment led to Y. pestis-induced damage in various animal organs resulting in mortality despite antibiotic treatment.
(iv) Delayed cethromycin treatment study.
Similar to the levofloxacin study, rats were treated with 70 mg/kg of cethromycin b.i.d. at 24, 36, 48, and 60 h after Y. pestis CO92 infection. All of the animals in the control infected group died by day 3 (Fig. 7). Only in the group of animals treated with cethromycin 24 h postinfection was 100% protection achieved. As antibiotic treatment was delayed to 36 and 48 h postinfection, a greatly reduced 44% protection of the animals was observed. When the drug treatment was further delayed to 60 h, no rats survived. Unlike what was found for levofloxacin, in which treatment delay up to 42 h did not negatively impact rat survival, cethromycin treatment must be initiated within 24 h of infection to achieve the same protection against plague. However, similar to our results with levofloxacin treatment, cethromycin-treated animals were also completely protected from a Y. pestis rechallenge, indicating development of Y. pestis-specific immunity.
Fig. 7.
Evaluation of delayed administration of cethromycin in a pneumonic rat model of infection. Groups of rats were exposed to a 30 LD50 of Y. pestis CO92 and administered cethromycin (Cethro) at 70 mg/kg/b.i.d. at 24 to 60 h following Y. pestis infection. As a negative control, rats were infected and administered water. The solid black vertical line indicates when the surviving animals were rechallenged with Y. pestis. P values indicate statistical significance.
(v) Y. pestis-specific immunity in rats following cethromycin treatment.
To characterize the humoral immune response generated in Y. pestis-infected animals rescued by cethromycin treatment, we determined IgG titers to F1 and LcrV antigen of Y. pestis in rat sera both before rechallenge and after the final rechallenge before the animals were sacrificed. As shown in Fig. 8, significant antibody titers to both Y. pestis antigens were detected in animal sera. These antibodies also reacted with the correct size of LcrV and F1 antigens as evidenced by immunoblotting (Fig. 8, inset).
DISCUSSION
With Y. pestis being recently reclassified as a reemerging pathogen by the WHO (3, 13) and the increasingly likely chance of a weaponized Y. pestis bioterrorist attack, the scientific community is being forced to once again contend with an etiological agent that has significantly shaped human history (7, 14, 18). Since there is currently no available Y. pestis vaccine (28), the only alternative option is to develop novel chemotherapeutics to help manage this bacterial pathogen since drug-resistant Y. pestis strains have already been isolated from human clinical infections (8, 10). Furthermore, in the event of a bioterrorist attack, it is likely that a weaponized multidrug-resistant strain of Y. pestis will be used. As a result, it has become imperative to develop and test novel chemotherapeutic agents for their potential antiplague activity in various animal models of infection.
We have extensive experience in using a rat model of plague infection and were one of the first groups to characterize the kinetics of pneumonic plague infection in rats (1). We chose to employ the rat as a study model because of its ability to develop an infection closely emulating that in humans and by virtue of the rat being the more typical zoonotic reservoir. In this body of work, we evaluated whether cethromycin, a novel ketolide antibiotic that is currently in late-stage clinical trials for the treatment of community-acquired bacterial pneumonia, was able to protect rats from a pneumonic plague challenge. We included levofloxacin within our studies as a positive control and determined that it was not quite as potent in the rat model as it in the mouse model. For instance, 5 mg/kg/day of levofloxacin provided 90 to 100% protection in mice (25), while only 67% of the rats were protected. However, the 10- and 15-mg/kg/day dosing provided 100% protection in the rats, which was similar to the results in the mouse model (Fig. 1). Additionally, orally administered cethromycin at a dose of 70 mg/kg b.i.d. 24 h following a respiratory challenge of Y. pestis was able to protect 100% of the infected animals with no drug-induced host cytotoxicity. Additionally, all cethromycin-treated survivors were resistant to 2 subsequent exposures to even higher lethal Y. pestis doses without further antibiotic treatment, which may indicate that cethromycin-treated and Y. pestis-rescued rats developed lasting immunity by generating, in part, F1 and LcrV-specific antibodies. Indeed the current subunit vaccines under clinical trials are based on these two antigens (28). These findings are very significant in developing countermeasures against plague.
Thus, cethromycin produced promising results, and our data strongly suggest that cethromycin could be manufactured as a novel antiplague chemotherapeutic agent. However, the drug kinetics and efficacy still need to be worked out in a nonhuman primate model of infection; an appropriate model to utilize could be the cynomolgus macaque (Macaca fascicularis), which has often been used as an animal model of respiratory plague infection in the past (17). Ultimately, cethromycin alone will likely be insufficient as a “silver bullet” antimicrobial to combat the formidable plague pathogen. Development and testing of additional chemotherapeutics are needed to thwart a multidrug-resistant plague offensive. Without a currently available plague vaccine (28), all avenues of antiplague treatments should be evaluated for their merit as putative antiplague treatments. It will take aggressive research efforts into novel chemotherapeutic development, phage therapy, and continued vaccine development to nullify the Y. pestis threat.
Finally, the MIC of cethromycin against Y. pestis CO92 was found to be 2.5 μg/ml in contrast to the previously observed MIC of 1 μg/ml (12). This difference could possibly be attributed either to strain variation or to the method of organism cultivation. Interestingly, the MIC of cethromycin for B. pseudomallei strains was found to be in the range of 4 to 64 μg/ml (23). Even though there have been no detailed pharmacokinetics/pharmacodynamic (PD) studies of cethromycin against Y. pestis, the murine pneumococcal pneumonia model demonstrated that all of the three PD parameters of cethromycin, i.e., T>MIC, maximum concentration in serum (Cmax)/MIC and area under the curve (AUC)/MIC, significantly correlated with changes in log10 CFU/lung, with Cmax/MIC and AUC/MIC better correlating with viable Streptococcus pneumoniae counts (16). In the current study, the fact that both 140-mg/kg/day q.d. and 70-mg/kg b.i.d. doses of cethromycin were equally effective in providing optimal protection to rats against Y. pestis suggested that an AUC/MIC ratio may be the key PD predictor, consistent with the general observation that macrolides and ketolides are usually AUC-driven drugs. Finally, in a clinical setting, one must analyze carefully the option of q.d. versus b.i.d. dosing of cethromycin in humans. This is because of the fact that q.d. treatment may lead to the development of antibiotic-resistant variants of Y. pestis, specifically if the half-life of the antibiotic is short. This will allow Y. pestis to be exposed for a longer duration of time to below the MIC dose of the drug, which may result in the emergence of antibiotic resistance and failure of the therapy (19–21). Although the proposed clinical dose of cethromycin for community-acquired bacterial pneumonia is 300 mg q.d. for 7 days (FDA briefing document for anti-infective drugs advisory committee meeting, June 2, 2009; http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM161847.pdf) the effective dose of cethromycin in context of pneumonic plague in humans still needs to be evaluated in other animal models such as the nonhuman primate model of infection.
ACKNOWLEDGMENTS
Studies conducted for this work were supported by NIH/NIAID AI064389 and N01 AI30065 grants (A.K.C.). J.A.R.'s salary effort was supported by National Aeronautics and Space Administration (NASA) cooperative agreement NNX08B4A47A. We also acknowledge a UC7 grant, which facilitated our research in the Galveston National Laboratory.
We thank Tracy MacGill, NIH/NIAID, for her advice during the course of these studies and Mardelle Susman for editing the manuscript. Our thanks are also to Elena Kozlova, Christina van Lier, and Abidat Lawal for their assistance during the revision of the manuscript.
Footnotes
Published ahead of print on 22 August 2011.
REFERENCES
- 1. Agar S. L., et al. 2009. Characterization of the rat pneumonic plague model: infection kinetics following aerosolization of Yersinia pestis CO92. Microbes Infect. 11:205–214 [DOI] [PubMed] [Google Scholar]
- 2. Alibek K., Handelman S. 1999. Biohazard: the chilling true story of the largest covert biological weapons program in the world - told from inside by the man who ran it. Random House Inc., New York, NY [Google Scholar]
- 3. Alvarez M. L., Cardineau G. A. 2010. Prevention of bubonic and pneumonic plague using plant-derived vaccines. Biotechnol. Adv. 28:184–196 [DOI] [PubMed] [Google Scholar]
- 4. Chen T. H., Foster L. E., Meyer K. F. 1974. Comparison of the immune response to three different Yersinia pestis vaccines in guinea pigs and langurs. J. Infect. Dis. 129(Suppl.):S53–S61 [DOI] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—8th edition. M07-A8. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Cully J. F., Williams E. S. 2001. Interspecific comparisons of sylvatic plague in prairie dogs. J. Mammal. 82:894–905 [Google Scholar]
- 7. Evans R. G., Crutcher J. M., Shadel B., Clements B., Bronze M. S. 2002. Terrorism from a public health perspective. Am. J. Med. Sci. 323:291–298 [DOI] [PubMed] [Google Scholar]
- 8. Galimand M., et al. 1997. Multidrug resistance in Yersinia pestis mediated by a transferable plasmid. N. Engl. J. Med. 337:677–680 [DOI] [PubMed] [Google Scholar]
- 9. Greenfield R. A., et al. 2002. Bacterial pathogens as biological weapons and agents of bioterrorism. Am. J. Med. Sci. 323:299–315 [DOI] [PubMed] [Google Scholar]
- 10. Guiyoule A., et al. 2001. Transferable plasmid-mediated resistance to streptomycin in a clinical isolate of Yersinia pestis. Emerg. Infect. Dis. 7:43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heine H. S., et al. 2007. Comparison of 2 antibiotics that inhibit protein synthesis for the treatment of infection with Yersinia pestis delivered by aerosol in a mouse model of pneumonic plague. J. Infect. Dis. 196:782–787 [DOI] [PubMed] [Google Scholar]
- 12. Heine H. S., et al. 2006. 46th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 27 to 30 September 2006, poster E-1853.
- 13. Heymann D. L. 2005. Emerging and re-emerging infectious diseases from plague and cholera to Ebola and AIDS: a potential for international spread that transcends the defenses of any single country. J. Contig. Crisis Manag. 13:29–31 [Google Scholar]
- 14. Inglesby T. V., et al. 2000. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA 283:2281–2290 [DOI] [PubMed] [Google Scholar]
- 15. Jawetz E., Meyer K. F. 1944. The behavior of virulent and avirulent P. pestis in normal and immune experimental animals. J. Infect. Dis. 74:1–13 [Google Scholar]
- 16. Kim M. K., et al. 2002. Bactericidal effect and pharmacodynamics of cethromycin (ABT-773) in a murine pneumococcal pneumonia model. Antimicrob. Agents Chemother. 46:3185–3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koster F., et al. 2010. Milestones in progression of primary pneumonic plague in cynomolgus macaques. Infect. Immun. 78:2946–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ligon B. L. 2006. Plague: a review of its history and potential as a biological weapon. Semin. Pediatr. Infect. Dis. 17:161–170 [DOI] [PubMed] [Google Scholar]
- 19. Louie A., Deziel M. R., Liu W., Drusano G. L. 2007. Impact of resistance selection and mutant growth fitness on the relative efficacies of streptomycin and levofloxacin for plague therapy. Antimicrob. Agents Chemother. 51:2661–2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Louie A., et al. 2011. Use of an in vitro pharmacodynamic model to derive a moxifloxacin regimen that optimizes kill of Yersinia pestis and prevents emergence of resistance. Antimicrob. Agents Chemother. 55:822–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Louie A., et al. 2011. Comparative efficacies of candidate antibiotics against Yersinia pestis in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 55:2623–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luzzaro F. 2008. Fluoroquinolones and Gram-negative bacteria: antimicrobial activity and mechanisms of resistance. Infez. Med. 16(Suppl. 2):5–11(In Italian.) [PubMed] [Google Scholar]
- 23. Mima T., Schweizer H. P., Xu Z. Q. 2011. In vitro activity of cethromycin against Burkholderia pseudomallei and investigation of mechanism of resistance. J. Antimicrob. Chemother. 66:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perry R. D., Fetherston J. D. 1997. Yersinia pestis—etiologic agent of plague. Clin. Microbiol. Rev. 10:35–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peterson J. W., et al. 2010. Protection afforded by fluoroquinolones in animal models of respiratory infections with Bacillus anthracis, Yersinia pestis, and Francisella tularensis. Open Microbiol. J. 4:34–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rafie S., MacDougall C., James C. L. 2010. Cethromycin: a promising new ketolide antibiotic for respiratory infections. Pharmacotherapy 30:290–303 [DOI] [PubMed] [Google Scholar]
- 27. Rios A. M., et al. 2004. Impact of cethromycin (ABT-773) therapy on microbiological, histologic, immunologic, and respiratory indices in a murine model of Mycoplasma pneumoniae lower respiratory infection. Antimicrob. Agents Chemother. 48:2897–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosenzweig J. A., et al. 2011. Progress on plague vaccine development. Appl. Microbiol. Biotechnol. 91:265–286 [DOI] [PubMed] [Google Scholar]
- 29. Sebbane F., Gardner D., Long D., Gowen B. B., Hinnebusch B. J. 2005. Kinetics of disease progression and host response in a rat model of bubonic plague. Am. J. Pathol. 166:1427–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sha J., et al. 2008. Braun lipoprotein (Lpp) contributes to virulence of yersiniae: potential role of Lpp in inducing bubonic and pneumonic plague. Infect. Immun. 76:1390–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sha J., et al. 2011. Characterization of an F1 deletion mutant of Yersinia pestis CO92, pathogenic role of F1 antigen in bubonic and pneumonic plague, and evaluation of sensitivity and specificity of F1 antigen capture-based dipsticks. J. Clin. Microbiol. 49:1708–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sha J., et al. 2005. The type III secretion system and cytotoxic enterotoxin alter the virulence of Aeromonas hydrophila. Infect. Immun. 73:6446–6457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wayson N. E., McMahon M. C., Prince F. M. 1946. An evaluation of three plague vaccines against infection in guinea pigs induced by natural and artificial methods. Public Health Rep. 61:1511–1518 [PubMed] [Google Scholar]