Abstract
The subclass B2 metallo-β-lactamase (MBL) Sfh-I from Serratia fonticola UTAD54 was cloned and overexpressed in Escherichia coli. The recombinant protein binds one equivalent of zinc, as shown by mass spectrometry, and preferentially hydrolyzes carbapenem substrates. However, compared to other B2 MBLs, Sfh-I also shows limited hydrolytic activity against some additional substrates and is not inhibited by a second equivalent of zinc. These data confirm Sfh-I to be a subclass B2 metallo-β-lactamase with some distinctive properties.
INTRODUCTION
Metallo-β-lactamases (MBLs) are zinc-dependent enzymes of growing clinical concern (23). They hydrolyze carbapenems, which are key antibiotics for resistant Gram-negative bacteria, and are insusceptible to serine β-lactamase inhibitors (7). Sequence information divides MBLs into three subclasses, B1, B2, and B3 (13). Subclass B2, the least studied, includes CphA (16, 20), ImiS (6, 24), and AsbM1 (25) from Aeromonas spp. and Sfh-I (18) from the occasional pathogen Serratia fonticola (4, 8, 14, 17). These are monozinc enzymes that strongly prefer carbapenem substrates. Sfh-I (18) and a class A carbapenemase, SFC-1 (12, 15), are specific to S. fonticola strain UTAD54. Sfh-I diverges markedly (58% ImiS and 60% CphA sequence identity) from the Aeromonas B2 MBLs (18). Here we expressed, purified, and biochemically characterized recombinant Sfh-I.
The complete blaSfh-I gene (GenBank accession no. AF197943) (primers SfhFv4 [5′-GGATCCCATATGAATATTAAATATTTATTTACGGCTG-3′] and SfhRv2 [5′-GTGCTCGAGTTACTTAGGCGCCTTCTCAAGC-3′]) and the fragment encoding the expected mature protein (nucleotides 55 to 759; primers SfhFv3 [5′-GGATCCCATATGTCTGAAAAAAACTTAACGCTTACC-3′] and SfhRv2) were amplified by PCR; the products were ligated into vector pET-26b (Novagen), generating plasmids pSfh-Iv4 and pSfh-Iv3, respectively, and the constructs were sequenced. Protein was expressed in Escherichia coli BL21 Star (DE3) (Invitrogen) (37°C, M9Y medium, 50 μg/ml kanamycin, overnight induction [1 mM IPTG] at 25°C) and purified by anion exchange (Q-Sepharose; 50 mM Tris-HCl, 100 μM ZnCl2, pH 8.5) and size exclusion (Superdex 75; 50 mM HEPES, pH 7.0) chromatography, yielding approximately 50 mg/liter Sfh-I at >95% purity as adjudged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Sfh-I molecular mass and metal content characteristics were investigated by electrospray mass spectrometry (QStar XL qTOF system, positive-ion mode, nanoliter/minute sample delivery, Nanomate source [0.3 lb/in2, 1.4 kV]). The mass obtained for Sfh-Iv3 under denaturing conditions (26,136 Da; Fig. 1 A) agreed with the value predicted (26,134 Da) from the sequence after removal of the N-terminal methionine. A similar result for Sfh-Iv4 confirmed removal of a 21-amino-acid signal peptide (18). Additional peaks may represent oxidized (26,136 + 16 = 26,152 Da [observed]) and subsequently hydrated (26,152 + 18 = 26,170 Da [observed]) adducts. In the native spectrum (Fig. 1B), peaks at 26,201 Da (26,136 + 65 Da), 26,218 Da (26,152 + 66 Da), and 26,239 Da (26,170 + 69 Da) indicate additions of one equivalent of zinc (65 Da) to species found under denaturing conditions. Thus, as isolated, the Sfh-I was primarily mononuclear, as observed for CphA and ImiS (6, 16) and in the Sfh-I crystal structures (Protein Data Bank [PDB] accession no. 3Q6V and 3SD9) (11), and typical of a B2 MBL.
Fig. 1.
Mass spectra of recombinant Sfh-I. (A) Spectrum acquired under denaturing conditions. (B) Spectrum acquired under nondenaturing (native) conditions.
β-Lactam hydrolysis (50 mM HEPES, pH 7.0 [25°C]) was investigated as described previously (19) (Table 1). Experiments were performed in triplicate, recording data points every 3 to 4 s for 20 to 30 min and adjusting concentrations to ensure measurement of the linear phase of the reaction. Sfh-I efficiently hydrolyzes carbapenems (imipenem and meropenem kcat/Km > 105 M−1 s−1) but resembles other B2 MBLs in its low affinity (Km > 300 μM) and catalytic efficiency (kcat/Km ≤ 103 M−1 s−1) for many penicillins and cephalosporins (9, 10, 20, 22, 24, 25). This is consistent with previous data showing that Sfh-I expression in E. coli substantially elevated carbapenem MICs but had little effect on those for other β-lactams (18). Sfh-I hydrolyzed benzylpenicillin, cefotaxime, ceftazidime, and sulbactam, but kinetic parameters for these substrates could not be derived due to low affinities. Unexpectedly, Sfh-I (0.01 μM) hydrolyzed cefepime (against which 0.5 μM CphA shows no detectable activity [9]) with relatively high efficiency. While differences between laboratories and experimental conditions make comparison with data from previously characterized B2 MBLs difficult, we note that the low affinity of Sfh-I for penicillins is consistent with results seen with other enzymes (possibly excepting AsbM1) and that higher kcat but weaker Km values for hydrolysis of meropenem, compared to imipenem, may be a general property of subclass B2 MBLs.
Table 1.
Kinetic parameters of purified Sfh-I β-lactamase and comparison with other B2 metallo-β-lactamasesa
| Substrate |
kcat (s−1) |
Km (μM) |
kcat/Km (M−1 s−1) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sfh-I | CphA | AsbM1 | ImiS | Sfh-I | CphA | AsbM1 | ImiS | Sfh-I | CphA | AsbM1 | ImiS | |
| Ampicillin | 9.5 ± 1.11 | <0.01b | 0.13 | 1.1 | 676 ± 187 | 2,500b | 65 | 510 | 1.41 × 104 | <4b | 7.5 × 103 | 2.2 × 104 |
| Benzylpenicillin | ND | 0.03 | 1.2 | 0.53 | >1,500 | 870 | 160 | 250 | ND | 35 | 2.0 × 103 | 2.1 × 103 |
| Carbenicillin | 9.15 ± 1.58 | 10d | 0.44 | 0.5 | 1,280 ± 363 | 500d | 28 | 260 | 7.18 × 103 | 2.0 × 104d | 1.6 × 104 | 2.1 × 103 |
| Nitrocefin | 0.06 ± 0.003 | 0.0028 | ≤0.02 | 0.024g | 106 ± 13.4 | 1,200 | ND | 51g | 5.7 × 102 | 2.5 | ND | 4.0 × 103g |
| Cephaloridine | 8.22 × 10−5 ± 1.46 × 10−5 | <0.006 | ≤0.06 | NH | 335 ± 124.0 | ∼6,000 | ND | NH | 2.46 × 10−1 | <1 | ND | NH |
| Cephalothin | NDh | NR | NR | NR | 84i | NR | NR | NR | ND | NR | NR | NR |
| Cefotaxime | ND | >0.0002b | NR | NH | >1,000 | >100b | NR | NH | ND | 2b | NR | NH |
| Ceftazidime | ND | NR | NR | NR | >500 | NR | NR | NR | ND | NR | NR | NR |
| Cefoxitin | 0.032 ± 0.00094 | 0.02e | ≤0.06 | NH | 99 ± 7 | 615e | ND | NH | 3.23 × 102 | 33e | ND | NH |
| Cefepime | 0.64 ± 0.073 | NH | NR | NR | 202 ± 43.7 | NH | NR | NR | 3.16 × 103 | NH | NR | NR |
| Moxalactam | 0.017 ± 0.0012 | NR | NR | NH | 91 ± 14 | NR | NR | NR | 1.87 × 102 | 5.6e | NR | NH |
| Sulbactam | ND | 0.12c | NR | NH | >1,000 | 37c | NR | NH | ND | 3.2 × 103c | NR | NH |
| Imipenem | 51 ± 2.8 | 1,200 | 71 | 350f | 79.2 ± 11.2 | 340 | 230 | 100f | 6.42 × 105 | 3.5 × 106 | 3.1 × 105 | 9.1 × 105f |
| Meropenem | 109 ± 9.5 | 3,100 | 220 | 296g | 215 ± 47.0 | 1,340 | 630 | 308g | 5.05 × 105 | 2.3 × 106 | 3.5 × 105 | 3.3 × 106g |
Kinetic data displayed for CphA, AsbM1, and ImiS as reported by Vanhove et al. (22), Yang and Bush (25) and Walsh et al. (24), respectively, except where otherwise indicated. ND, values could not be determined; NH, no hydrolysis detected; NR, not reported.
As reported by Bebrone et al. (2).
As reported by Felici et al. (10).
As reported by Segatore et al. (20).
As reported by Zervosen et al. (26).
As reported by Sharma et al. (21).
As reported by Crawford et al. (6).
Value could not be determined due to inhibition at higher concentrations.
Ki value determined from inhibition of nitrocefin hydrolysis.
CphA is inactivated by cefoxitin, moxalactam, and other cephalosporins with good 3′ leaving groups (26). Cephalosporin degradation products also inhibit the Bacillus cereus B1 MBL BcII (1). Whereas Sfh-I hydrolyzes cefoxitin, moxalactam, and cephalothin with low efficiency (Table 1), at higher concentrations each inhibited imipenem and nitrocefin hydrolysis. Interaction of Sfh-I with these compounds and their hydrolysis products was investigated. Although the mode of inhibition by the intact compounds was unclear, nitrocefin hydrolysis assays showed that preincubation with cephalothin or its hydrolysis product (purified by centrifugal filtration after hydrolysis using IMP-1 β-lactamase) led to time-dependent inactivation. Loss of Sfh-I activity correlated with cephalothin hydrolysis, indicating that the hydrolysis product likely represents the inhibitory species. Incubating Sfh-I (3.6 μM; 18 h; 25°C; 50 mM HEPES, pH 7.0) with various hydrolysis products (1 mM) reduced hydrolysis of 200 μM imipenem by 13% (cefoxitin), 36% (cephalothin), and 94% (moxalactam). The level of activity of cephalothin-treated samples remained at 54% of the control level, even after overnight dialysis, suggesting inactivation by enzyme modification. Unfortunately, mass spectrometry failed to identify the inhibitory species; while all expected Sfh-I-derived peptides were detected, modified fragments were not, suggesting a labile inhibitory complex. Previous workers suggested that the moxalactam hydrolysis product covalently binds the CphA Cys-221 thiol (26). Association of Cys-221 with the thiol of an opened dihydrothiazine ring of the hydrolyzed cephalosporin (1), or addition to the exomethylene formed upon elimination of the 3′ leaving group, could also explain the cephalothin inactivation of Sfh-I.
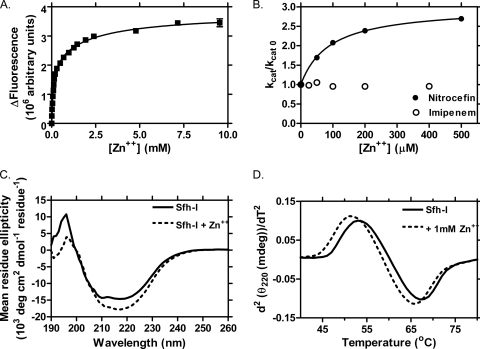
CphA is most active with a single tightly bound zinc ion (Kd1 < 20 nM) and weakly binds a second, inhibitory zinc (Kd2 = 46 μM) (16). We used fluorescence spectroscopy (Jobin Yvon FluoroLog) to investigate the Sfh-I-zinc interaction. Titration of as-isolated (monozinc) Sfh-I with zinc (Fig. 2 A) showed two distinct transitions, demonstrating binding of two further equivalents (Kd2, 95 ± 14 μM; Kd3, 2,300 ± 870 μM). The Kd2 value is comparable to that of binding of a second zinc to CphA (46 μM [16]). The third, low-affinity zinc (millimolar Kd3) is unlikely to be physiologically relevant. (CphA binds a third zinc at a surface site distinct from the active site [3].)
Fig. 2.
Effect of additional zinc on Sfh-I. (A) Change in Sfh-I intrinsic (tryptophan) fluorescence as a function of zinc concentration (50 mM HEPES, pH 7.0; 25°C; excitation, 280 nm; emission, 341 nm). The solid line represents a fit of nitrocefin data to a two-site hyperbola model, with Y (fluorescence) = Y2 × [Zn2+]/(Kd2 + [Zn2+]) + Y3 × [Zn2+]/(Kd3 + [Zn2+]), where Y2 and Y3 represent the maximal fluorescence changes associated with binding of a second and third zinc equivalent, corresponding to Kd2 (95 ± 14 μM) and Kd3 (2,300 ± 870 μM), respectively. (B) Effect of added zinc on kcat/kcat0 (ratio of kcat at a given zinc concentration to kcat0 determined in the absence of excess zinc) for nitrocefin and imipenem hydrolysis. The solid line represents a fit of nitrocefin data to kcat/kcat0 = (K + α × [Zn2+])/([Zn2+] + K), where α represents the value of this ratio when the zinc concentration is at a saturating level (22). (C) Far-UV circular dichroism spectra (10 mM phosphate, pH 7.0; 25°C; 1-mm cuvette) of Sfh-I (8.5 μM) in the absence (solid line) and presence (dotted line) of 1 mM Zn2+. (D) Second derivatives of plots of ellipticity (220 nm) against temperature for thermal unfolding of Sfh-I (8.5 μM) in the absence (solid line) and presence (dotted line) of 1 mM Zn2+.
Other B2 MBLs are maximally active as monozinc enzymes, with noncompetitive inhibition by a second zinc (6, 16, 25). The effect of zinc addition on Sfh-I activity was substrate dependent (Fig. 2B). Imipenem hydrolysis was unaffected by 400 μM zinc, while nitrocefinase activity increased due to an increased kcat value. The midpoint (K) for activation (90 ± 4 μM) is equivalent to Kd2, indicating that a second zinc activates nitrocefinase activity. While zinc activates nitrocefin hydrolysis for some CphA mutants (2), and the ImiS M146I mutation abolishes zinc inhibition of imipenemase activity (5), the combination of zinc-dependent nitrocefinase activity and zinc-independent imipenemase activity was not previously described. Circular dichroism spectroscopy (Jasco-J810 spectropolarimeter) showed that the presence of a second zinc induced a small increase in Sfh-I secondary structure (Fig. 2C) with little effect on thermal stability (Tm for Sfh-I unfolding, 60°C; in 1 mM zinc, 58°C; Fig. 2D).
In conclusion, Sfh-I is a monozinc enzyme with a strong preference for carbapenems, confirming it to be a B2 MBL and these to be common properties of the members of this subgroup. However, Sfh-I differs from CphA, the most studied B2 MBL, in its activity toward cefepime and in the effect of additional zinc, indicating that not all B2 enzymes are inactivated by excess zinc. Further investigations should establish the basis for these differences.
Acknowledgements
The work was supported by the Fundação para a Ciência e a Tecnologia (Portugal; Ph.D. grant BD/30490/2006 to F.F.).
We thank D. N. Woolfson (School of Chemistry, University of Bristol) for access to the spectrapolarimeter.
Footnotes
Present address: Department of Physics, Durham University, South Road, Durham DH1 3LE, United Kingdom.
Published ahead of print on 29 August 2011.
REFERENCES
- 1. Badarau A., Llinas A., Laws A. P., Damblon C., Page M. I. 2005. Inhibitors of metallo-β-lactamase generated from β-lactam antibiotics. Biochemistry 44:8578–8589 [DOI] [PubMed] [Google Scholar]
- 2. Bebrone C., et al. 2005. Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-β-lactamase by site-directed mutagenesis. J. Biol. Chem. 280:28195–28202 [DOI] [PubMed] [Google Scholar]
- 3. Bebrone C., et al. 2009. The structure of the dizinc subclass B2 metallo-β-lactamase CphA reveals that the second inhibitory zinc ion binds in the histidine site. Antimicrob. Agents Chemother. 53:4464–4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bollet C., Gainnier M., Sainty J. M., Orhesser P., De Micco P. 1991. Serratia fonticola isolated from a leg abscess. J. Clin. Microbiol. 29:834–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Costello A. L., Sharma N. P., Yang K. W., Crowder M. W., Tierney D. L. 2006. X-ray absorption spectroscopy of the zinc-binding sites in the class B2 metallo-β-lactamase ImiS from Aeromonas veronii bv. sobria. Biochemistry 45:13650–13658 [DOI] [PubMed] [Google Scholar]
- 6. Crawford P. A., et al. 2004. Over-expression, purification, and characterization of metallo-β-lactamase ImiS from Aeromonas veronii bv. sobria. Protein Expr. Purif. 36:272–279 [DOI] [PubMed] [Google Scholar]
- 7. Crowder M. W., Spencer J., Vila A. J. 2006. Metallo-β-lactamases: novel weaponry for antibiotic resistance in bacteria. Acc. Chem. Res. 39:721–728 [DOI] [PubMed] [Google Scholar]
- 8. Farmer J. J., et al. 1985. Biochemical identification of new species and biogroups of Enterobacteriaceae isolated from clinical specimens. J. Clin. Microbiol. 21:46–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Felici A., Amicosante G. 1995. Kinetic analysis of extension of substrate specificity with Xanthomonas maltophilia, Aeromonas hydrophila, and Bacillus cereus metallo-β-lactamases. Antimicrob. Agents Chemother. 39:192–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Felici A., et al. 1993. An overview of the kinetic parameters of class B β-lactamases. Biochem. J. 291:151–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fonseca F., Bromley E. H. C., Saavedra M. J., Correia A., Spencer J. 2011. Crystal structure of Serratia fonticola Sfh-I: activation of the nucleophile in mono-zinc metallo-β-lactamases. J. Mol. Biol. 411:951–959 [DOI] [PubMed] [Google Scholar]
- 12. Fonseca F., et al. 2007. Biochemical characterization of SFC-1, a class A carbapenem-hydrolyzing β-lactamase. Antimicrob. Agents Chemother. 51:4512–4514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galleni M., et al. 2001. Standard numbering scheme for class B β-lactamases. Antimicrob. Agents Chemother. 45:660–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gorret J., et al. 2009. Childhood delayed septic arthritis of the knee caused by Serratia fonticola. Knee 16:512–514 [DOI] [PubMed] [Google Scholar]
- 15. Henriques I., Moura A., Alves A., Saavedra M. J., Correia A. 2004. Molecular characterization of a carbapenem-hydrolyzing class A β-lactamase, SFC-1, from Serratia fonticola UTAD54. Antimicrob. Agents Chemother. 48:2321–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernandez Valladares M., et al. 1997. Zn(II) dependence of the Aeromonas hydrophila AE036 metallo-β-lactamase activity and stability. Biochemistry 36:11534–11541 [DOI] [PubMed] [Google Scholar]
- 17. Pfyffer G. E. 1992. Serratia fonticola as an infectious agent. Eur. J. Clin. Microbiol. Infect. Dis. 11:199–200 [DOI] [PubMed] [Google Scholar]
- 18. Saavedra M. J., et al. 2003. Sfh-I, a subclass B2 metallo-β-lactamase from a Serratia fonticola environmental isolate. Antimicrob. Agents Chemother. 47:2330–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samuelsen O., Castanheira M., Walsh T. R., Spencer J. 2008. Kinetic characterization of VIM-7, a divergent member of the VIM metallo-β-lactamase family. Antimicrob. Agents Chemother. 52:2905–2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Segatore B., Massidda O., Satta G., Setacci D., Amicosante G. 1993. High specificity of cphA-encoded metallo-β-lactamase from Aeromonas hydrophila AE036 for carbapenems and its contribution to β-lactam resistance. Antimicrob. Agents Chemother. 37:1324–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharma N. P., et al. 2006. Mechanistic studies on the mononuclear ZnII-containing metallo-β-lactamase ImiS from Aeromonas sobria. Biochemistry 45:10729–10738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vanhove M., et al. 2003. Role of Cys221 and Asn116 in the zinc-binding sites of the Aeromonas hydrophila metallo-β-lactamase. Cell. Mol. Life Sci. 60:2501–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walsh T. R. 2010. Emerging carbapenemases: a global perspective. Int. J. Antimicrob. Agents 36:S8–S14 [DOI] [PubMed] [Google Scholar]
- 24. Walsh T. R., Gamblin S., Emery D. C., MacGowan A. P., Bennett P. M. 1996. Enzyme kinetics and biochemical analysis of ImiS, the metallo-β-lactamase from Aeromonas sobria 163a. J. Antimicrob. Chemother. 37:423–431 [DOI] [PubMed] [Google Scholar]
- 25. Yang Y., Bush K. 1996. Biochemical characterization of the carbapenem-hydrolyzing b-lactamase AsbM1 from Aeromonas sobria AER 14M: a member of a novel subgroup of metallo-β-lactamases. FEMS Microbiol. Lett. 137:193–200 [DOI] [PubMed] [Google Scholar]
- 26. Zervosen A., et al. 2001. Inactivation of Aeromonas hydrophila metallo-β-lactamase by cephamycins and moxalactam. Eur. J. Biochem. 268:3840–3850 [DOI] [PubMed] [Google Scholar]