Abstract
In this review, we summarize the current “state of the art” of carbapenem antibiotics and their role in our antimicrobial armamentarium. Among the β-lactams currently available, carbapenems are unique because they are relatively resistant to hydrolysis by most β-lactamases, in some cases act as “slow substrates” or inhibitors of β-lactamases, and still target penicillin binding proteins. This “value-added feature” of inhibiting β-lactamases serves as a major rationale for expansion of this class of β-lactams. We describe the initial discovery and development of the carbapenem family of β-lactams. Of the early carbapenems evaluated, thienamycin demonstrated the greatest antimicrobial activity and became the parent compound for all subsequent carbapenems. To date, more than 80 compounds with mostly improved antimicrobial properties, compared to those of thienamycin, are described in the literature. We also highlight important features of the carbapenems that are presently in clinical use: imipenem-cilastatin, meropenem, ertapenem, doripenem, panipenem-betamipron, and biapenem. In closing, we emphasize some major challenges and urge the medicinal chemist to continue development of these versatile and potent compounds, as they have served us well for more than 3 decades.
INTRODUCTION
Carbapenems play a critically important role in our antibiotic armamentarium. Of the many hundreds of different β-lactams, carbapenems possess the broadest spectrum of activity and greatest potency against Gram-positive and Gram-negative bacteria. As a result, they are often used as “last-line agents” or “antibiotics of last resort” when patients with infections become gravely ill or are suspected of harboring resistant bacteria (23, 174–176, 229). Unfortunately, the recent emergence of multidrug-resistant (MDR) pathogens seriously threatens this class of lifesaving drugs (189). Several recent studies clearly show that resistance to carbapenems is increasing throughout the world (35, 64, 73, 123, 151, 155, 173, 200). Despite this menacing trend, our understanding of how to best use these agents is undergoing a renaissance, especially concerning their role with regard to β-lactamase inhibition. In this context, we view the number, type, and diversity of carbapenems as compelling reasons to explore these compounds for new insights into drug development.
IN THE BEGINNING…
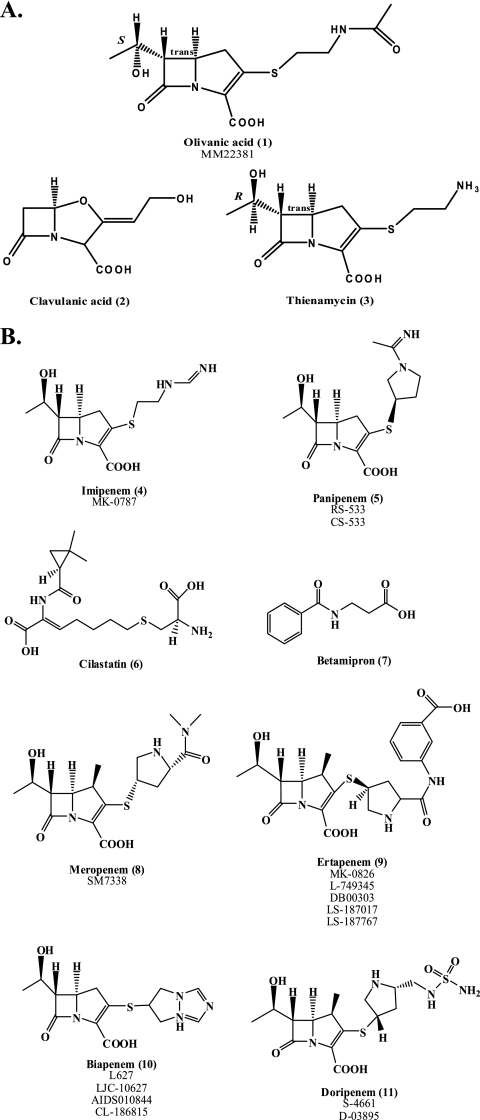
In the late 1960s, as bacterial β-lactamases emerged and threatened the use of penicillin, the search for β-lactamase inhibitors began in earnest (38, 199). By 1976, the first β-lactamase inhibitors were discovered; these olivanic acids (compound 1 in Fig. 1) were natural products produced by the Gram-positive bacterium Streptomyces clavuligerus. Olivanic acids possess a “carbapenem backbone” (a carbon at the 1 position, substituents at C-2, a C-6 ethoxy, and sp2-hybridized C-3) and act as broad-spectrum β-lactams (25, 27, 199). Due to chemical instability and poor penetration into the bacterial cell, the olivanic acids were not further pursued (192). Shortly thereafter, two superior β-lactamase inhibitors were discovered: (i) clavulanic acid (compound 2) from S. clavuligerus, the first clinically available β-lactamase inhibitor (25), and (ii) thienamycin (compound 3) from Streptomyces cattleya (Fig. 1A) (3, 112). Thienamycin was the first “carbapenem” and would eventually serve as the parent or model compound for all carbapenems. A series of other carbapenems were also identified (30, 92, 107, 147, 149, 162, 163, 172, 233); however, the discovery of thienamycin was paramount.
Fig. 1.
(A) Chemical structures of olivanic acid, clavulanic acid, and thienamycin. (B) Clinically available carbapenems, as well as cilastatin and betamipron. Previous identifiers of compounds are listed below each name.
The term “carbapenem” is defined as the 4:5 fused ring lactam of penicillins with a double bond between C-2 and C-3 but with the substitution of carbon for sulfur at C-1. The hydroxyethyl side chain of thienamycin is a radical departure from the structure of conventional penicillins and cephalosporins, all of which have an acylamino substituent on the β-lactam ring; the stereochemistry of this hydroxyethyl side chain is a key attribute of carbapenems and is important for activity (95). Remarkably, thienamycin demonstrated potent broad-spectrum antibacterial and β-lactamase inhibitory activity (112, 113). This notable discovery was first reported at the 16th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) meeting in 1976 (3, 112). Although thienamycin is a “natural product” and the biosynthetic pathway was determined (160), yields from the purification process were low. With time, the synthetic preparation of thienamycin assumed greater importance, especially as a key derivative, imipenem (compound 4), was discovered (Fig. 1B).
Like other β-lactams, thienamycin bound to penicillin binding proteins (PBPs) (216). With time, enthusiasm for this compound grew rapidly, since thienamycin displayed inhibitory microbiological activity against Gram-negative bacteria, including isolates of Pseudomonas aeruginosa, as well as anaerobes, like Bacteroides fragilis, and Gram-positive bacteria, such as methicillin- or oxacillin-susceptible Staphylococcus aureus and streptococci (55, 225, 246).
Unfortunately, thienamycin was found to be unstable in aqueous solution, sensitive to mild base hydrolysis (above pH 8.0), and highly reactive to nucleophiles, such as hydroxylamine, cysteine, and even thienamycin's own primary amine (95). The chemical instability of thienamycin stimulated the search for analogous derivatives with increased stability. Due to the continued evolution of cephalosporin-resistant Gram-negative and Gram-positive pathogens, compounds derived from thienamycin were anticipated to have even greater value with time (8).
The first developed was the N-formimidoyl derivative, imipenem (141). Imipenem and a closely related carbapenem, panipenem (compound 5), identified later, were more-stable derivatives of thienamycin and less sensitive to base hydrolysis in solution (Fig. 1B). In 1985, imipenem (originally called MK0787) became the first carbapenem available for the treatment of complex microbial infections. Imipenem, like its parent, thienamycin, demonstrated high affinity for PBPs and stability against β-lactamases (81). However, both imipenem and panipenem were susceptible to deactivation by dehydropeptidase I (DHP-I), found in the human renal brush border (74, 83, 114). Therefore, coadministration with an inhibitor, cilastatin (compound 6) or betamipron (compound 7), was necessary (Fig. 1B) (157).
Along the journey to the discovery of more-stable carbapenems with a broader spectrum, the other currently available compounds, meropenem, biapenem, ertapenem, and doripenem (compounds 8 to 11) (Fig. 1B), were developed, and several novel carbapenems were also identified (24, 79, 80, 98, 99, 102, 117, 118, 133, 207, 221, 236). A major advance in this “synthetic journey” was the addition of a methyl group to the 1-β position (Fig. 2 A). This modification was found to be protective against DHP-I hydrolysis (62). Several carbapenems were identified with this modification in the subsequent 2 decades; many were similar to the currently available carbapenems, having a 1-β-methyl and a pyrrolidine ring at C-2 (Fig. 2B). These novel carbapenems included antipseudomonal carbapenems, anti-methicillin-resistant S. aureus (MRSA) carbapenems (i.e., cationic and dithiocarbamate carbapenems), orally available carbapenems, trinem carbapenems, a dual quinolonyl-carbapenem, and others.
Fig. 2.
(A) A 1-β-methyl (red) increases resistance to DHP-I. (B) The pyrrolidine ring (red) increases stability and spectrum. (C) Penicillin, cephalosporin, and carbapenem backbones. Carbapenem has a five-membered ring, as does penicillin, but it has a carbon at C-1 instead of sulfur. (D) Most carbapenems have a hydroxyethyl off C-7. (E) The R configuration of the hydroxyethyl increases the β-lactam's potency. (F) The trans configuration of carbapenems at the C-5—C-6 bond increases their potency compared to penicillins and cephalosporins.
CARBAPENEMS: CHEMISTRY AND BIOLOGY
Chemistry.
From the studies conducted on the early carbapenems, the carbon atom at the C-1 position was found to play a major role in the potency and spectrum of carbapenems and in their stability against β-lactamases (Fig. 2C). We have also since learned that a strategically positioned hydroxyethyl R2 side chain aids in resistance to hydrolysis by β-lactamases (Fig. 2D) (144). In addition, carbapenems with an R configuration at C-8 are also very potent (Fig. 2E). The trans configuration of the β-lactam ring at C-5 and C-6 results in stability against β-lactamases (Fig. 2F) (10). Like thienamycin, the clinically available carbapenems are R at C-8 and trans about the C-5—C-6 bond. Carbapenems with a pyrrolidine moiety (panipenem, meropenem, ertapenem, and doripenem) among various cyclic amines as a side chain have a broader antimicrobial spectrum (219).
Synthesis.
As mentioned above, several chemical approaches were developed for the synthesis of carbapenems since fermentation was not an efficient method for production (9, 28, 86, 142, 183, 201). Natural products (l-Cys, l-Val, l-α-amino adipic acid, and S-adenosyl-Met) were often used as starting material for production of carbapenems, and the synthetic approach was largely influenced by the desired stereochemistry of the final compound. In addition, once a carbapenem is developed which has an R configuration at C-8, is trans about the C-5—C-6 bond, and has a methyl at C-1 and a hydroxyethyl at C-6, most modifications are at the R1 side chain (at position C-2). Thus, carbapenems are unique compared to other β-lactams, which tend differ in both R1 and R2 side chains. The reader is referred to R. B. Woodward's classical discourse on this matter (249).
Mechanism of action.
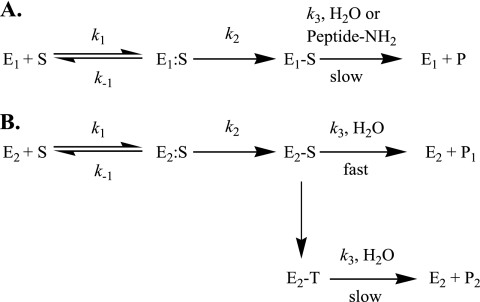
As a class of β-lactams, carbapenems are not easily diffusible through the bacterial cell wall (131). Generally speaking, carbapenems enter Gram-negative bacteria through outer membrane proteins (OMPs), also known as porins. After transversing the periplasmic space, carbapenems “permanently” acylate the PBPs (for the mechanism, see Fig. 3 A) (81, 228). PBPs are enzymes (i.e., transglycolases, transpeptidases, and carboxypeptidases) that catalyze the formation of peptidoglycan in the cell wall of bacteria. Current insights into this process suggest that the glycan backbone forms a right-handed helix with a periodicity of three per turn of the helix (137). Carbapenems act as mechanism-based inhibitors of the peptidase domain of PBPs and can inhibit peptide cross-linking as well as other peptidase reactions. A key factor of the efficacy of carbapenems is their ability to bind to multiple different PBPs (81). Since cell wall formation is a dynamic “three-dimensional process” with formation and autolysis occurring at the same time, when PBPs are inhibited, autolysis continues (237). Eventually the peptidoglycan weakens, and the cell bursts due to osmotic pressure.
Fig. 3.
(A) Enzymatic scheme for β-lactam inhibition of PBPs. In this reaction scheme, E1 corresponds to the PBP, S to the carbapenem, E1:S to the Michaelis complex, E1-S to the inactivated PBP, and P to the inactivated β-lactam product; k1, k−1, k2, and k3 represent the on, off, acylation, and deacylation rate constants, respectively. (B) Enzymatic scheme for carbapenem inhibition and hydrolysis by class A, C, and D β-lactamases. In this reaction scheme, E2 corresponds to the β-lactamase, S to the carbapenem, E2:S to the Michaelis complex, E2-S (Δ2) to the acyl-enzyme, E2-T (Δ1) to the tautomerized carbapenem, and P to the inactivated β-lactam product; k1, k−1, k2, and k3 represent the on, off, acylation, and deacylation rate constants, respectively. The conversion of E2-S to E2-T represents the biphasic nature of carbapenem hydrolysis, potentially due to tautomerization of the pyrroline double bond, which may or may not play a role in carbapenemase activity.
Microbiological activity.
Carbapenems demonstrate an overall broader antimicrobial spectrum in vitro than the available penicillins, cephalosporins, and β-lactam/β-lactamase inhibitor combinations (11). In general, imipenem, panipenem, and doripenem are potent antibiotics against Gram-positive bacteria (11, 72, 190, 194). Meropenem, biapenem, ertapenem, and doripenem are slightly more effective against Gram-negative organisms (11, 153, 181). Important considerations here are the following: (i) ertapenem has a more limited spectrum, because it is not as active as imipenem or meropenem against P. aeruginosa (164); (ii) meropenem is not as potent as imipenem or doripenem against Acinetobacter baumannii (164); (iii) doripenem has lower MICs than do imipenem and meropenem versus P. aeruginosa and A. baumannii (130). In addition, doripenem is the carbapenem least susceptible to hydrolysis by carbapenemases; hydrolysis of doripenem is 2- to 150-fold slower than that of imipenem (190); (iv) a unique application of meropenem is that when combined with clavulanic acid, it is potent at killing MDR Mycobacterium tuberculosis, a bacterium that typically is not susceptible to β-lactams due to a chromosomally expressed β-lactamase (90). This ability to inhibit or kill M. tuberculosis is likely to be a property of other carbapenems as research in this area grows.
Carbapenems can also be combined with other antimicrobials to treat serious infections. Combination therapy is a subject of intense interest, since the emergence of MDR pathogens often requires us to treat patients with more than one antibiotic (7, 32, 49, 53, 54, 108, 146, 169, 226). A list of the antibiotic combinations which have been tested in vitro against common MDR organisms and their effects is presented in Table 1. Some combinations demonstrate positive effects, such as extending the spectrum or working additively or synergistically. Adverse effects include increased resistance to one of the drugs used in the combination, as well as a lack of synergy or additivity and strain dependence. A full debate on the benefits and drawbacks of combination therapy with carbapenems is beyond the scope of this review.
Table 1.
In vitro-tested carbapenem combination therapiesa
| Drug 1 | Drug 2 | Bacterium (reference[s])b | Effect |
|---|---|---|---|
| Doripenem or imipenem | Vancomycin | MRSA (108, 139) | + |
| Doripenem | Teicoplanin | MRSA (108) | + |
| Imipenem | Linezolid | MRSA (94) | + |
| Imipenem | Teicoplanin | VRSA (76) | + |
| Meropenem | Levofloxacin | S. pneumoniae (41) | + |
| Meropenem | Rifampin | S. pneumoniae (60) | − |
| Imipenem or meropenem | Clavulanic acid | Nocardia brasiliensis (238) | − |
| Meropenem | Clavulanic acid | Mycobacterium tuberculosis (90) | + |
| Meropenem | Ciprofloxacin | A. baumannii (54, 169) | + |
| Imipenem or meropenem | Colistin (and sulbactam) | A. baumannii (54, 169, 188, 198) | + |
| Meropenem | Sulbactam | A. baumannii (104) | + |
| Imipenem | Azithromycin | A. baumannii (58) | + |
| Imipenem | Rifampin | A. baumannii (213) | + |
| Imipenem | Polymyxin B | A. baumannii (245) | − |
| Imipenem | Amikacin | A. baumannii (195) | − |
| Carbapenem | Fluoroquinolone | P. aeruginosa (41, 54, 104, 124, 169, 241) | + |
| Imipenem | Tachyplesin | P. aeruginosa (37) | + |
| Meropenem or imipenem | Colistin | P. aeruginosa (37, 54, 169) | +/− |
| Carbapenem | Aminoglycoside | P. aeruginosa (7, 49, 50, 53, 226) | − |
| Meropenem | Polymyxin B | P. aeruginosa (75) | − |
| Imipenem or meropenem | Tobramycin-rifampin | B. cepacia (19) | + |
| Imipenem or meropenem | Ciprofloxacin | B. cepacia (19) | + |
| Imipenem | Colistin | MBL K. pneumoniae (215) | + |
| Imipenem | Tigecycline | ESBL K. pneumoniae and E. coli (32) | − |
| Imipenem | Gentamicin | ESBL K. pneumoniae and E. coli (32) | − |
Some combinations demonstrate positive effects (+), such as extending spectrum or working additively or synergistically. Adverse effects (−) include increased resistance to one of the drugs used in the combination, as well as lack of synergy or additivity and strain dependence.
VRSA, vancomycin-resistant S. aureus; ESBL, extended-spectrum β-lactamase producing; MBL, metallo-β-lactamase producing.
Pharmacology and clinical use.
Several detailed reviews of the pharmacology of clinically available carbapenems exist (255). Briefly, all clinically available carbapenems have low oral bioavailability and thus do not cross gastrointestinal membranes readily and must be administered intravenously; imipenem-cilastatin and ertapenem can also be delivered intramuscularly (11, 72, 101, 153, 181, 194, 255). As with other β-lactams, all of these carbapenems are eliminated predominately by renal excretion. Carbapenems exhibit unique pharmacological properties and are typically used to treat complicated bacterial infections. A carbapenem is often combined with an antibiotic that targets Gram-positive bacteria when used for the empirical treatment of patients with serious nosocomial infections of unidentified origin.
Safety and tolerability.
Nephrotoxicity, neurotoxicity, and immunomodulation have been reported with the use of carbapenems, and thus predisposing factors should be considered when administering any carbapenem (45, 78, 96, 156, 212, 220, 234, 235). In addition, the use of carbapenems can alter the intestinal microflora and select for carbapenem-resistant isolates (119, 120, 136, 166, 232).
MECHANISMS OF RESISTANCE AGAINST CARBAPENEMS
Many nonfermenting Gram-negative bacteria (e.g., Pseudomonas spp., Acinetobacter spp., and Stenotrophomonas spp.), as well as the Enterobacteriaceae (e.g., Klebsiella spp., Escherichia coli, and Enterobacter spp.) and Gram-positive bacteria (e.g., Staphylococcus spp., Streptococcus spp., Enterococcus spp., and Nocardia spp.), are or are becoming resistant to most clinically available carbapenems. This distressing pattern poses a major public health threat.
Mechanisms of resistance to carbapenems include production of β-lactamases, efflux pumps, and mutations that alter the expression and/or function of porins and PBPs (Fig. 4). Combinations of these mechanisms can cause high levels of resistance to carbapenems in certain bacterial species, such as Klebsiella pneumoniae, P. aeruginosa, and A. baumannii (121, 136, 197).
Fig. 4.
Crystal structures of the carbapenemase, KPC-2 (Protein Data Bank [PDB] identifier 2OV5), present in the periplasm, P. aeruginosa porin, OprD (substrate binding loops highlighted in yellow) (PDB identifier 2ODJ) present in the outer membrane, P. aeruginosa efflux pump components, MexA, MexB, and OprM (PDB identifiers 1VF7, 2V50, and 3D5K), spanning the inner membrane and periplasmic and outer membranes, and P. aeruginosa PBP3 (PDB identifier 3PBN), anchored to the inner membrane.
A distinction exists between resistance to carbapenems in Gram-positive cocci and Gram-negative rods. In Gram-positive cocci, carbapenem resistance is typically the result of substitutions in amino acid sequences of PBPs or acquisition/production of a new carbapenem-resistant PBP (100, 109, 134). Expression of β-lactamases and efflux pumps, as well as porin loss and alterations in PBP, are all associated with carbapenem resistance in Gram-negative rods (155, 178, 245). The mechanism that has been investigated in the most detail is the production of β-lactamases, and thus it is discussed in greater detail here than the other mechanisms of resistance.
β-Lactamases.
β-Lactamases are a major antibiotic resistance mechanism employed by bacteria; these periplasmic enzymes hydrolyze β-lactam antibiotics, preventing the drug from reaching the PBP target. Presently, β-lactamases are classified into four distinct classes based on structural similarities (classes A, B, C, and D) or four groups based on hydrolytic and inhibitor profiles (1 to 4) (4, 26). Class B β-lactamases use Zn2+ to inactivate β-lactams, and all are carbapenemases. Class A, C, and D β-lactamases use a serine as a nucleophile to hydrolyze the β-lactam bond.
Carbapenemases are specific β-lactamases with the ability to hydrolyze carbapenems. Production of β-lactamases appears to be the most widespread cause of carbapenem resistance, since the documentation of their distribution in different bacterial species is extensive (189, 242, 243). An increasing number of class A carbapenemases (e.g., KPC and GES enzymes), class B metallo-β-lactamases (e.g., VIM, IMP, and NDM β-lactamases), and class D carbapenemases (e.g., OXA-23, −24/40, −48, −51, −55, −58, and −143) have recently emerged (187, 189, 242, 243, 253). In addition, overproduction of class C β-lactamases, such as CMY-10 and PDC β-lactamases, which are not robust carbapenemases, can lead to carbapenem resistance, especially when combined with other resistance mechanisms (e.g., porin loss) (65, 116, 126, 128, 197, 217).
Structure-function: considerations among carbapenemases.
Several class A carbapenemase β-lactamases (i.e., KPC-2, SME-1, and NmcA) have been crystallized (103, 182, 214, 222). These enzymes possess a distinctive set of active-site residues that are suspected to be involved in the hydrolysis of carbapenems. We will review here their important features.
A unique attribute of class A carbapenemases (i.e., KPC, SME, and NmcA) is the presence of a disulfide bond between Cys69 and Cys238 (Ambler numbering system [4]); this bond changes the overall shape of the active site by altering the distance between active-site residues. The distance between Ser70 and Thr237 is less, the length of the active site is decreased as indicated by the distance between Glu166 and Thr237, and the space between Asn132 and Asn170 is increased in comparison to SHV-1 and TEM-1. In addition, several active-site residues have different amino acids in comparison to SHV-1 and TEM-1 (e.g., Thr/Ser237, His/Trp105, Arg220, and Glu276). These significant structural changes decrease the steric hindrance caused by the C-6 hydroxyethyl side chain of carbapenems, which is a key determinant in inhibition of noncarbapenemase class A β-lactamases and allows class A carbapenemases to hydrolyze imipenem with kcat values (turnover rates) from 10 to 1,000 s−1. Mutagenesis studies of the SME-1 and KPC-2 β-lactamases have revealed several sites that may be necessary for carbapenem resistance (127, 170, 171). However, the finding of a single residue responsible for carbapenem resistance remains elusive.
GES-2 is unique to the class A carbapenemase family because a single amino acid substitution (Gly170Asn) changes GES-1, which is an extended-spectrum β-lactamase (ESBL), into a carbapenemase. GES-2 has a very low kcat for imipenem of ∼0.01 s−1. The Gly170Asn substitution is found in the Ω loop of GES-2. Molecular modeling studies with imipenem and the in silico-generated GES-2 suggest that the shape of the active site accommodates the hydroxyethyl moiety (211). In addition, Asn170 interacts with the predicted hydrolytic water molecule. Another mutation at Gly170 to Ser (mimicking GES-5) results in increased carbapenemase activity in comparison to that of GES-2; GES-5 exhibits a kcat for imipenem of 0.5 s−1 (61). Initial molecular modeling studies suggest that imipenem is bound in a similar manner in GES-5 and GES-1, not explaining the increase in kcat (211). Examining the microscopic rate constants for imipenem with GES-1, -2, and -5 reveals that the rate-limiting step for GES-1 and GES-2 is acylation (61). The rate of acylation for imipenem is enhanced by 5,000-fold for GES-5 and is no longer rate limiting. Deacylation is also enhanced in GES-5 but becomes the rate-limiting step in imipenem hydrolysis. Further molecular modeling studies with GES β-lactamases have disclosed the importance of the movement of Trp105 to the interior of the active site, which may alter the acylation rates (111).
Class B β-lactamases require one or two Zn2+ cations for activity and are subdivided into three groups, B1, B2, and B3, based on sequence alignments and structural analysis (14). All three groups hydrolyze carbapenems (kcat for imipenem of 2 to 1,000 s−1), but β-lactamases in group B2 are strictly carbapenemases. B1 and B3 enzymes typically exhibit maximum activity when bound by two Zn2+ ions. Conversely, B2 β-lactamases function as mono-Zn2+ enzymes, and binding of another Zn2+ decreases activity.
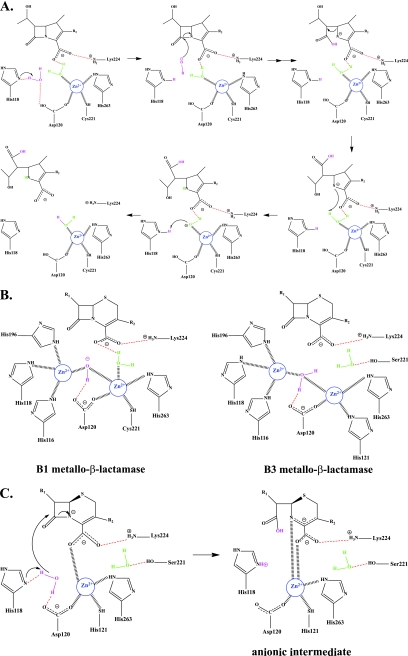
The CphA β-lactamase is a strict carbapenemase of subclass B2. CphA has been crystallized with one Zn2+ ion, two Zn2+ ions, and a biapenem intermediate trapped in the active site (15, 16, 40, 67, 208, 209, 250–252). Previously, the second inhibitory Zn2+ binding site was postulated to be remote from the active site (40, 42). However, the dizinc crystal structure of CphA reveals that this Zn2+ sits in the second Zn2+ binding site, similar to the case with subclass B1 and B3 metallo-β-lactamases (21, 22, 47, 77, 253, 254). Quantum mechanics and molecular mechanics have been used to dissect the mechanism of carbapenem turnover by CphA. A mechanism is presented in Fig. 5 A and is predicted to occur in a single step (67, 208, 250). In this mechanism, His118 is the general base that coordinates a water molecule with Asp120; this water serves as the nucleophile for β-lactam bond cleavage. Asp120, Cys221, and His263 coordinate Zn2+ along with a water molecule, which also hydrogen bonds to the carboxylate of carbapenems. This second water molecule donates a proton to β-lactam nitrogen to complete the hydrolysis event. Zn2+ anchors the critical deacylation water molecule and stabilizes the complex.
Fig. 5.
(A) Mechanism of carbapenem hydrolysis by CphA. (B) Subclass B1 and B3 metallo-β-lactamase active sites. (C) Stabilization of the anionic intermediate in GOB-18.
In contrast, with subclass B1 and B3 di-Zn2+ metallo-β-lactamases, one Zn2+ atom decreases the pKa of the water molecule to generate a hydroxyl nucleophile for the attack of the β-lactam, while the other Zn2+ stabilizes the tetrahedral intermediate (Fig. 5B) (14). A recent study reveals a common catalytic feature of mono-Zn2+ and di-Zn2+ metallo-β-lactamases using GOB-18, a member of the B3 metallo-β-lactamases (122). This enzyme is fully active with only one Zn2+ bound. Studies to date indicate that only one Zn2+ is essential for GOB-18 and its role is to anchor the substrate and stabilize the anionic intermediate and not for nucleophile activation (Fig. 5C) (59). A critical feature of the mono-Zn2+ mechanism in GOB-18 is the positioning of the Zn2+ atom in the active site.
Class C β-lactamases are not generally classified as carbapenemases. Most enzymes in this class have weak activity toward carbapenems (kcat < 4 s−1) if any activity at all (128). So when an AmpC enzyme is found in a strain with other resistance mechanisms, resistance toward carbapenems may be enhanced (129). Curiously, rare class C enzymes that can confer resistance to imipenem are described (106, 187, 196, 197). The preeminent candidate enzyme with this altered substrate profile is CMY-10 (106). Here, a three-amino-acid deletion in the R2 loop (near residue 303) significantly widens part of the active site, which accommodates the R2 side chains of carbapenems. The same deletion in P99 results in an enzyme with a similar phenotype. Consequently, the opening of the R2 loop of the active site by the deletion of some residues in the R2 loop can be considered an operative molecular strategy of class C β-lactamases to extend their substrate spectrum.
Hydrolysis of β-lactams by class D β-lactamases differs from that of class A and C enzymes. OXA enzymes are a very heterogeneous population of β-lactamases that have evolved through multiple mechanisms; their kcats for imipenem are <5 s−1. The structures of two OXA β-lactamases reveal two different enzyme architectures; in addition, the two enzymes have different substrate specificities for carbapenems.
The mechanism of carbapenem resistance mediated by OXA-24/40 was investigated first; a novel conformation of Tyr112 and Met223, which form a hydrophobic “tunnel” near the active site, was found and is believed to be the central mechanism by which these carbapenems are turned over by OXA-24/40 (203). The presence of the “tunnel” shrinks the active site of OXA-24/40. Consistent with the kinetic observations, the active site is hypothesized to accommodate smaller carbapenems as opposed to the larger oxacillin. On the other hand, OXA-48 is reminiscent of OXA-10, with subtle active-site differences (47). Changes in residues His109, Thr213, Arg214, and Arg244 appear to play a major role in the functional differences between OXA-48 and OXA-10. Interestingly, the orientations and sizes of the β5-β6 loop are similar in the OXA-24/40 and OXA-48 structures; this observation implies that the loop may be important for carbapenem turnover. However, the substrate specificity of the two enzymes differs; OXA-48 hydrolyzes imipenem, while OXA-24/40 displays a preference for meropenem.
The crystal structures of two deacylation-deficient variants (Lys84Asp and Val130Asp) of the carbapenemase OXA-24/40 in complex with doripenem were recently determined (205). The goal of this work was to investigate if the tautomeric state of the pyrroline ring contributes to the different carbapenem hydrolysis rates of OXA-1 and OXA-24/40. In these structures, doripenem's conformation in the active site differs significantly from that in the OXA-1/doripenem complex. In the doripenem structures of OXA-24/40, the hydroxyl side chain of the hydroxyethyl group is directed away from the general base carboxy-Lys84 (different numbering from that of OXA-1). The “tunnel” formed by the Tyr112/Met223 bridges in the apoenzyme form of OXA-24/40 is largely unchanged by the binding of doripenem. The presence of this bridge causes the pyrrolidine/sulfonamide group to bind in a conformation different from that of doripenem bound to OXA-1. This change in conformation is due to the different tautomeric state of doripenem in the active site and may correlate with why carbapenems are turned over by OXA-24/40 but not OXA-1.
Carbapenems and inhibition of β-lactamases.
Carbapenems can behave as “slow substrates” or inhibitors of noncarbapenemase (serine) β-lactamases (18, 29, 44, 145). This fortuitous observation serves as a major point of interest in these compounds and adds to their “dual function” (i.e., inhibits PBPs and β-lactamases). Many class A β-lactamases are susceptible to inhibition by clavulanic acid (26). In contrast, all class C and most class D β-lactamases are not inhibited by clavulanic acid. However, class A and C and certain class D enzymes are inhibited by carbapenems. This unique attribute serves as a starting point to consider for novel drug development.
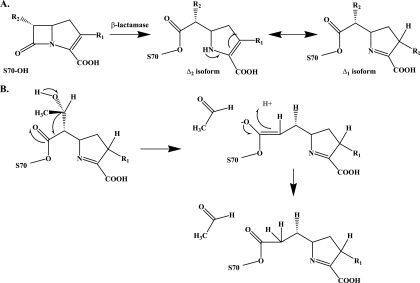
The initial characterization of the mechanism of carbapenem inhibition of β-lactamases was conducted with the olivanic acids (see above) and the class A β-lactamase, TEM-1, by Knowles's group in the early 1980s (34, 51). These landmark studies revealed that the kinetics of hydrolysis of olivanic acid were biphasic and that there is tautomerization of the pyrroline double bond from C-2—C-3 to C-3—C-4 (Δ2 → Δ1), resulting in two isoforms (Fig. 6 A). The insightful observation was made that deacylation of the carbapenem from TEM-1 proceeded more rapidly with the Δ2 form; the Δ1 isoform deacylated slowly (Fig. 3B). Knowles and colleagues (34, 51) concluded here that inhibition of β-lactamases depends on the rate of the formation of the Δ1 tautomer. Studies by Monks and Waley later examined the reaction of imipenem with the class A β-lactamase from Bacillus cereus and the chromosomal class C β-lactamase from P. aeruginosa. They found that imipenem behaved as a “slow substrate” against both β-lactamases and followed a “branched pathway” (145).
Fig. 6.
(A) The mechanism of carbapenem tautomerization. (B) The proposed mechanism for hydroxyethyl elimination in class A and C β-lactamases.
Zafaralla et al. explored the analysis of Knowles and studied the hydrolysis of imipenem by TEM-1 (254). This group found that the arginine residue at 244 in TEM-1 coordinates a water molecule that is the source of the proton for this Δ2 → Δ1 tautomerization (the crystal structure of TEM-1 inhibited by imipenem, Δ2 form, is discussed subsequently). In contrast to TEM-1, the Arg244Ser variant of TEM-1 displayed monophasic kinetics with imipenem, suggesting a loss of tautomerization (and coordination) with water. Molecular modeling of the Δ2 and Δ1 isoforms of imipenem in TEM-1 showed that for both tautomers the carbonyl is localized in the oxyanion hole without distortion of the deacylating water molecule (224). This observation led the authors to propose that conformational changes may occur in TEM-1 that may account for different orientations of the two tautomers (224).
At the same time, mass spectrometry also revealed that secondary changes to the carbapenem molecules may occur when they are reacted with a β-lactamase (48, 90, 230). Elimination of the C-6 hydroxyethyl group of carbapenems through a proposed retro-aldol reaction is suspected to occur with class A β-lactamase (BlaC) and class C β-lactamases (ADC-7 and CMY-2 and -32) (Fig. 7 B) (48, 52, 90). The loss of the hydroxyethyl group was assessed through molecular modeling studies, and a different acyl-enzyme conformation compared to that of the intact carbapenem is noted (48). The “fragmented” carbapenem may also be hydrolyzed. The loss of a hydroxyethyl group may be a very minor reaction, since crystallographic studies of β-lactamases with carbapenems do not seem to support this observation.
Fig. 7.
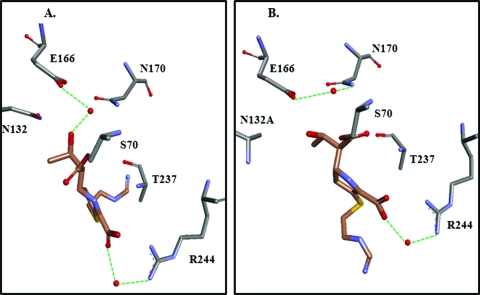
(A) TEM-1 and imipenem (PDB identifier 1BT5). (B) TEM-1 Asn132Ala and imipenem (PDB identifier 1JVJ).
Several crystal structures of β-lactamases complexed with different carbapenems are now known; these structures complement the above studies and significantly enhance our understanding of inhibition by carbapenems.
The first crystal structure of a carbapenem with a β-lactamases was TEM-1 with imipenem (Fig. 7A) (135). TEM-1 complexed with imipenem (1BT5) revealed that the electrostatic and steric hindrance caused by the bulky hydroxyethyl R2 side chain of imipenem at C-6 and Asn132 is one of the main driving forces behind the slow deacylation and inhibition of TEM-1 (135). This subsequent observation was confirmed in a another atomic structure of TEM-1, where the replacement of Asn132 with Ala (N132A) resulted in “space” for the hydroxylethyl (Fig. 7B) (244). In the TEM-1 imipenem complex analyzed by Maveyraud et al., the deacylation water molecule forms a hydrogen bond with the hydroxyethyl side chain at C-6, decreasing its nucleophilicity (135). Furthermore, the β-lactam carbonyl of imipenem is observed outside the electrophilic center (i.e., “oxyanion hole”), which is made up of the backbone amides of Ser70 and Ala237 in TEM-1. This was not predicted by earlier molecular modeling studies and was an unexpected observation since the Δ2 isoform of imipenem is predicted to be turned over rapidly (244). In the TEM-1 Asn132-imipenem complex, the carbonyl is located in the oxyanion hole (244). In both structures, a water molecule is positioned between Arg244 and the C-3 carboxylate; this water is postulated by Zafarella and Mobashery to play a key role in Δ2 → Δ1 tautomerization (254). Molecular dynamics further added to this understanding by suggesting that in the TEM-1 imipenem acyl enzyme complex, the β-lactam carbonyl can move from “outside” the electrophilic center to “inside” during the course of the simulation. The insights from the crystal structure of the E. coli class C β-lactamase AmpC and imipenem was very similar to those for TEM-1 with imipenem (carbonyl “outside” the oxyanion hole and Δ2 isoform). However, the tautomeric water molecule is missing, and the predicted deacylation water molecule is positioned between Ser64 and N4 of imipenem (13).
Subsequent studies were performed to investigate whether SHV-1, a β-lactamase with only 67% amino acid sequence similarity with TEM-1, was inactivated in the same manner as TEM-1. Surprisingly, in the SHV-1 and meropenem crystal structure, two conformers for meropenem exist: the β-lactam carbonyl rests both inside and outside the electrophilic center in the complex (159). The two conformers may coincide with the suspected presence of the two tautomers found during carbapenem hydrolysis; however, the double bond was not resolved in either structure (224, 254). In both SHV-1 meropenem complexes, the deacylation water interacts with the hydroxyethyl group of meropenem, and Glu166 is predicted to be protonated; these occurrences are anticipated to contribute to prolonged deacylation. At about the same time, Kalp and Carey observed the inactivation of SHV-1 with meropenem, ertapenem, and imipenem using Raman spectroscopy and identified ”long-lived“ Δ1 and”short-lived“ Δ2 isoforms (97).
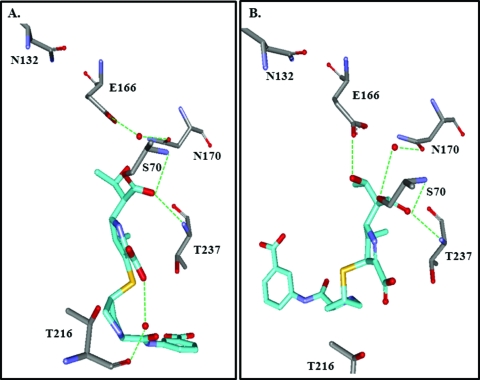
The M. tuberculosis BlaC class A β-lactamase was crystallized with meropenem, ertapenem, and doripenem (90, 230). Crystal structures with ertapenem revealed that ertapenem isomerizes from a Δ2-pyrroline to a Δ1-pyrroline (Fig. 8 A and B). In the other BlaC structures, meropenem and doripenem are in the Δ1-pyrroline isoform. Interestingly, the β-lactam carbonyl is oriented toward the oxyanion hole in all four structures; this orientation is speculated to occur because of a lack of an “Arg244 equivalent” in BlaC. Instead, a less-flexible Thr holds the tautomerization water molecule with the carboxylate in the preisomerization crystal, and this water molecule is absent in the postisomerization crystal. After isomerization, the hydroxylethyl of ertapenem reorients, preventing deacylation by disturbing the basicity of an active-site residue (i.e., Glu166) and the activation of the hydrolytic water molecule.
Fig. 8.
(A) BlaC with ertapenem preisomerization (PDB identifier 3M6B). (B) BlaC with ertapenem postisomerization (PDB identifier 3M6H).
Noncarbapenemase class D β-lactamases have been crystallized with carbapenems. These achievements are notable because class D β-lactamases are usually resistant to inhibitors. The two structures for these distinct enzymes (OXA-13 and OXA-1) reveal different findings. In both structures, the carbonyl of the carbapenem is inside the oxyanion hole. In the crystal structure of OXA-13 (a close variant of OXA-10) and meropenem, the water molecule necessary for deacylation is too far from the acyl-enzyme complex due to movement of the general base residue Lys70 (179). Examining the OXA-10 crystal structure, this hydrolytic water molecule is hydrogen bonded to Lys70 and Trp154 and is predicted to attack the acyl-enzyme intermediate upon activation by the general base Lys70 (167). In contrast to the OXA-13-meropenem complex, in the structure of OXA-1 with doripenem, the hydroxyethyl of doripenem forms a hydrogen bond with Lys70, thus preventing recruitment of a water molecule for deacylation (204). Additionally, doripenem appears to tautomerize to the Δ1-pyrroline isoform after acylation.
In summary, the key determinants in the inhibition of serine β-lactamases by carbapenems revealed from these studies are the hydroxyethyl side chain present on all carbapenems and the isomerization potential of the pyrroline ring. The hydroxyethyl side chain provides steric hindrance for the approach of the deacylating water molecule, while tautomerization can result in conformation changes of the enzyme-carbapenem complex. The factors that contribute to rapid deacylation of the Δ2 isoform remain to be determined. Interactions indicative of slow deacylation or inhibition revealed from these studies lead us to an important question: is carbonyl positioning and tautomerization a dynamic process in carbapenem turnover and does it contribute in a significant way to why these compounds are slow substrates?
By combining the knowledge obtained from studies conducted with carbapenemases and noncarbapenemase β-lactamase, we may identify rationale approaches for novel carbapenem compounds that will inhibit all β-lactamases. For example with class A, C, and D β-lactamases, tautomerization is a key factor for inhibition, and thus the carbapenem scaffold is ideal. The hydroxyethyl side chain is an important factor in the mechanism of stabilization of the acyl-enzyme complex. Further modifications of this side chain by keeping the R configuration at C-8 and trans configuration of the β-lactam ring at C-5 and C-6 may lead to novel carbapenems that are not hydrolyzed by carbapenemases.
Outer membrane proteins.
Outer membrane proteins (OMPs) are grouped into four large families: general/nonspecific porins, substrate-specific porins, gated porins, and efflux porins (77, 131). Porins allow the passage of molecules of ≤1,500 Da (77). General/nonspecific, substrate-specific, and efflux porins are the main families mediating resistance to carbapenems. Not all carbapenems interact with OMPs the same way; some OMPs are affected by certain carbapenems more than others (193).
(i) Porins. Substitutions in, or decreased expression of, nonefflux porins resulting in decreased entry of carbapenems into the periplasm exists in P. aeruginosa (89), K. pneumoniae (22, 247), Enterobacter aerogenes (36), E. coli (166), Serratia marcescens (132), Proteus mirabilis (240), Citrobacter freundii (126), A. baumannii (21, 210), Enterobacter cloacae (191), Proteus rettgeri (191), Shigella dysenteriae (69), and Salmonella enterica (5). Here we briefly describe resistance to clinically available carbapenems due to alterations in OprD of P. aeruginosa (77).
The physiological role of OprD is the transport of basic amino acids (i.e., amino acids with pKas in the range of 9) (227). OprD is the main porin used by carbapenems for diffusion into P. aeruginosa (63, 231). OprD is part of a larger family of 19 other porins in P. aeruginosa with 46 to 57% similarity. Eight of the 19 are even more closely related to OprD; however, only OprD participates in antibiotic uptake. OprD belongs to the substrate-specific family of porins and was first identified because loss of this porin resulted in imipenem resistance (89). This loss has been attributed to mutations and negative regulation of transcription of the oprD gene, which is present on the bacterial chromosome (161, 180). Since imipenem and all β-lactams are dipeptide mimics, cross-resistance to imipenem upon OprD loss occurs. Substitutions in loops 5, 7, and 8 of OprD, which bind dipeptides as well as carbapenems, also result in resistance to imipenem (87, 88).
(ii) Efflux pumps. Carbapenem resistance due to overexpression of efflux porins, which are a part of a tripartite protein complex, is reported mostly for P. aeruginosa (71, 110), E. aerogenes (20), and A. baumannii (70). These pumps can extrude some carbapenems but not others. Efflux pumps are grouped into several superfamilies: the small multidrug resistance (SMR) superfamily, the resistance-nodulation-division (RND) superfamily, the major facilitator superfamily (MFS), the ATP-binding cassette (ABC) superfamily, and the multidrug and toxic compound extrusion (MATE) superfamily (140).
Efflux pumps that eliminate carbapenems in P. aeruginosa belong to the RND superfamily and are a complex of proteins connecting the cytoplasm to the outside of the cell. These complex protein machines have three major components: a cytoplasmic membrane pump, a peripheral cytoplasmic membrane linker, and an outer membrane-periplasmic channel or efflux porin (2, 77, 140, 152, 223). Ligands can enter the efflux system either at the cytoplasm-membrane interface or the periplasm-membrane interface, and a proton motive force can actively extrude the ligand. OprM and OprJ are two efflux porins involved in carbapenem resistance in P. aeruginosa. These efflux porins assemble with MexA, MexC, or MexX, a peripheral cytoplasmic membrane linker, and either MexB, MexD, or MexY, a cytoplasmic membrane pump, to form a complete efflux complex. Resistance to carbapenems is mediated by overexpression of efflux pumps due to mutations in transcriptional regulatory proteins (168, 257). The true ligands of these efflux pumps are not known; however, they may be involved in the efflux of quorum sensing autoinducers or their metabolic precursors (178).
PBPs.
Mutations in the PBP protein and/or decreases in PBP transcription also result in carbapenem-resistant phenotypes. Expression of PBPs in P. aeruginosa, A. baumannii, P. mirabilis, and Rhodococcus equi is decreased, resulting in carbapenem resistance (57, 68, 71, 150, 154). In addition, amino acid substitutions in PBPs or acquisition of a novel PBP can cause carbapenem resistance in Haemophilus influenzae, B. fragilis, S. aureus, Enterococcus faecium, P. mirabilis, E. coli, Listeria monocytogenes, S. pneumoniae, and P. aeruginosa (6, 17, 31, 56, 100, 109, 134, 150, 158, 165, 184, 185, 218).
NOVEL CARBAPENEMS: ANYTHING IN THE PIPELINE?
Antipseudomonal and/or anti-MRSA carbapenems.
The discovery of “antipseudomonal” and/or “anti-MRSA” antibiotics is critical and is a major focus of many contemporary research groups. As reviewed herein, resistance in P. aeruginosa to β-lactams is more complex and involves the production of β-lactamases and poor membrane permeability (e.g., efflux pumps and porins). MRSA's resistance to β-lactams is mainly due to the low affinity of MRSA PBP2a for β-lactams (33). A significant research effort has been dedicated to using carbapenems to target these pathogens (2, 3, 8, 10–12, 71, 78, 79, 92, 93, 106–109, 119, 122, 125, 128, 179, 183, 197, 246). Of these compounds, only tomopenem (compound 12) made it to clinical trials (12), but development has since been discontinued (Fig. 9) (1).
Fig. 9.
Novel carbapenems.
Specific anti-MRSA carbapenems.
Several carbapenems were designed to target MRSA while maintaining activity against most Gram-negatives. The anti-MRSA activity is related to the high affinity of these compounds for PBP2a of MRSA. The R2 side chains present on these compounds are important affinity determinants for interactions with PBP2a of MRSA. Of these compounds, 15i (compound 13) and CP5484 (compound 14) may offer interesting possibilities (Fig. 9), while the rest are no longer being studied (33, 94, 120, 144, 145, 162, 206, 215, 236, 254).
Oral carbapenems.
Oral carbapenems are given as prodrugs to increase intestinal absorption (63, 101, 126–129, 209, 237, 248, 249). These prodrugs get activated by host enzymes in the intestinal wall or liver. An advantage for these compounds is the ability to treat patients in a nonhospital setting and to avoid the disadvantages of intravenous (i.v.) administration (i.e., inability of patients to self-administer, need for strict asepsis, and inconvenience).
Tebipenem-pivoxil (compound 15) is the world's first oral carbapenem in development in Japan (Fig. 9). Tebipenem-pivoxil is active against MDR S. pneumoniae (MIC = 0.06 mg/liter) and other Gram-positives, as well as the Enterobacteriaceae (82, 93, 143, 148). Tebipenem-pivoxil's spectrum does not include MRSA and P. aeruginosa. It is absorbed well in the intestine (93), with a half-life of 0.3 to 0.5 h in humans with otolaryngological infections (105). Tebipenem is also useful in treating pneumonia in children (115).
Trinem carbapenems.
A new class of β-lactams, known as the trinems or tribactams, includes tricyclic β-lactams that have a penicillin, cephalosporin, or carbapenem backbone. The most successful have been tricyclic β-lactams with a carbapenem backbone, and these compounds are described below.
The first carbapenem trinem to be explored was sanfetrinem (compound 16); it can enter phagocytes and thus has the potential to kill intracellular pathogens (Fig. 9) (43). But in 2009, sanfetrinem-cilexetil development ended after phase II clinical trials.
A rational drug design approach was used to combine the antimicrobial activity of the trinem sanfetrinem with the β-lactamase inhibitory activity of BRL 42715 (compound 17) (Fig. 9) (39). LK-157 (compound 18) differs from sanfetrinem in its BRL-42715-derived ethylidene R1 side chain (Fig. 9) (46, 239). It is speculated that the introduction of an ethylidene bond at C-6 was designed to stabilize the β-lactamase inhibitor-β-lactamase complex. In addition, the hydrophobic ring at the C-3-C-4 position was hypothesized to block the water molecule from accessing the acyl-enzyme complex, thus preventing deacylation (39). Additionally, LK-157 is a mechanism-based inactivator of class A and C β-lactamases (39, 186, 239) but lacks antibacterial activity on its own (177). LK-157 is poorly absorbed and thus must be used as an ester prodrug (91). LK-157 was in clinical trials but is no longer being pursued (206).
CONCLUSIONS
We believe that the discovery of a β-lactam (e.g., carbapenem) with PBP and β-lactamase inhibitory properties was a major breakthrough in infectious disease therapeutics. The carbapenems are often agents of “last resort” for many complicated bacterial infections. As MDR pathogens continue to emerge, the sustained study of the development of novel carbapenems is an essential undertaking.
What are the important lessons learned from the studies conducted with carbapenems? From the early years, the carbapenems isolated from Streptomyces were found to be chemically unstable and susceptible to hydrolysis by host enzymes (i.e., DHP-I). The region of the compound that results in this instability was identified, leading to modification of the carbapenems as a class (e.g., decreasing the basicity of R1 and adding 1-β-methyl). Additional work revealed the importance of the R2 side and stereochemistry of carbapenems; these factors aid in resistance to hydrolysis by β-lactamases, as well as increasing the spectrum of activity.
Work with β-lactamases and carbapenems revealed important features, which will directly aid in the future optimization of carbapenems. The different classes of β-lactamases are inhibited by carbapenems due to similar overall principles. Tautomerization of the pyrroline double bond of carbapenem is important for inhibition, since the Δ1 isoform deacylates at a much lower rate (224). The steric hindrance created by the R2 hydroxyethyl side chain plays a role in inhibition by preventing the deacylating water molecule from getting activated, as well as altering the reactivity of the general base (135, 159). Elimination of the hydroxyethyl group of carbapenems, seen with class A and C β-lactamases, is an intriguing preliminary observation, which may facilitate the hydrolysis of the carbapenem (48, 52, 90). The relative safety of these compounds is a real advantage; the primary concern is selection of carbapenem-resistant isolates, which is also the reason to continue development.
Future prospects include understanding the role of resistance determinants (e.g., carbapenemases, porins, PBPs, and efflux pumps), since overcoming resistance is essential in order to preserve longevity. Therefore, the modification of carbapenems so that they are not inactivated by β-lactamases is an important goal. The biggest challenges are the metallo-β-lactamases. However, some progress has been made in identifying potential inhibitors (cephalosporin-derived reverse hydroxamates and oximes, phthalic acid derivatives, mitoxantrone, 4-chloromercuribenzoic acid, sulfonyl-triazole analogs, and NH-1,2,3-triazole-based compounds) (66, 84, 138, 202, 248). Alternative inhibitors can include carbapenems with different stereochemistries. Quantum and molecular mechanics may hold the key to identifying a scaffold for competitive inhibitors of metallo-β-lactamases.
Generating carbapenems with increased permeability through the bacterial outer membrane is another avenue to explore, thus out-maneuvering the loss of porins. Several oral carbapenems with increased permeability through the host gastrointestinal membrane have been developed; notably, sanfetrinem-cilexetil also gets into phagocytes. Bypassing the continued evolution of PBPs with new carbapenems is attainable, as evidenced by the new anti-MRSA and antipseudomonal carbapenems. Yet structures of clinically relevant PBPs with carbapenems are needed if we are to understand the enhanced activity. Studies have shown that efflux pump inhibitors can restore the activity of antibiotics (85, 125, 256). Designing carbapenems that bypass efflux is another option, since efflux by bacteria needs to be studied in greater detail. The increasing number and type/diversity of carbapenems should compel us to revisit these compounds for new leads in the face of expanding resistance.
We conclude with a recollection of R. B. Woodward's essay in the Philosophical Transactions of the Royal Society. He noted, “Clearly, antibacterial activity is inherent in the bicyclic nuclear structures of the penems and the carbapenems. In constructing thienamycin, has Nature utilized the millions of years available to her, to endow the carbapenem nucleus with substituents which modulate the inherent activity of the nucleus in a manner upon which we cannot improve? We may doubt it. But we may not doubt that the chemist will accept the challenge provided by these fascinating new nuclei, and explore the opportunity to prepare new and perhaps superior antibiotics” (249).
ACKNOWLEDGMENTS
This work was supported by the Veterans Affairs Career Development Program (to K.M.P.-W.) and the Veterans Affairs Merit Review Program, the National Institutes of Health (RO1 AI063517-01), and the Veterans Integrated Service Network 10 Geriatric Research, Education, and Clinical Center (VISN 10 GRECC) (to R.A.B.).
We thank Sarah Drawz for her insightful comments and critical review of the manuscript.
Footnotes
Published ahead of print on 22 August 2011.
REFERENCES
- 1. Abbanat D., Morrow B., Bush K. 2008. New agents in development for the treatment of bacterial infections. Curr. Opin. Pharmacol. 8:582–592 [DOI] [PubMed] [Google Scholar]
- 2. Akama H., et al. 2004. Crystal structure of the membrane fusion protein, MexA, of the multidrug transporter in Pseudomonas aeruginosa. J. Biol. Chem. 279:25939–25942 [DOI] [PubMed] [Google Scholar]
- 3. Albers-Schonberg G., et al. 1976. Abstr. 16th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 229. American Society for Microbiology, Washington, DC [Google Scholar]
- 4. Ambler R. P., et al. 1991. A standard numbering scheme for the class A β-lactamases. Biochem. J. 276(Pt. 1):269–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Armand-Lefevre L., et al. 2003. Imipenem resistance in Salmonella enterica serovar Wien related to porin loss and CMY-4 β-lactamase production. Antimicrob. Agents Chemother. 47:1165–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ayala J., Quesada A., Vadillo S., Criado J., Piriz S. 2005. Penicillin-binding proteins of Bacteroides fragilis and their role in the resistance to imipenem of clinical isolates. J. Med. Microbiol. 54:1055–1064 [DOI] [PubMed] [Google Scholar]
- 7. Balke B., et al. 2006. Evaluation of the E test for the assessment of synergy of antibiotic combinations against multiresistant Pseudomonas aeruginosa isolates from cystic fibrosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 25:25–30 [DOI] [PubMed] [Google Scholar]
- 8. Basker M. J. 1982. The carbapenem family. J. Antimicrob. Chemother. 10:4–7 [DOI] [PubMed] [Google Scholar]
- 9. Basker M. J., et al. 1981. Synthesis of 6-unsubstituted olivanic acid analogues and their antibacterial activities. J. Antibiot. (Tokyo) 34:1224–1226 [DOI] [PubMed] [Google Scholar]
- 10. Basker M. J., Boon R. J., Hunter P. A. 1980. Comparative antibacterial properties in vitro of seven olivanic acid derivatives: MM 4550, MM 13902, MM 17880, MM 22380, MM 22381, MM 22382 and MM 22383. J. Antibiot. (Tokyo) 33:878–884 [DOI] [PubMed] [Google Scholar]
- 11. Bassetti M., Nicolini L., Esposito S., Righi E., Viscoli C. 2009. Current status of newer carbapenems. Curr. Med. Chem. 16:564–575 [DOI] [PubMed] [Google Scholar]
- 12. Bayes M., Rabasseda X., Prous J. R. 2007. Gateways to clinical trials. Methods Find. Exp. Clin. Pharmacol. 29:697–735 [PubMed] [Google Scholar]
- 13. Beadle B. M., Shoichet B. K. 2002. Structural basis for imipenem inhibition of class C β-lactamases. Antimicrob. Agents Chemother. 46:3978–3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bebrone C. 2007. Metallo-β-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily. Biochem. Pharmacol. 74:1686–1701 [DOI] [PubMed] [Google Scholar]
- 15. Bebrone C., et al. 2008. Mutational analysis of the zinc- and substrate-binding sites in the CphA metallo-β-lactamase from Aeromonas hydrophila. Biochem. J. 414:151–159 [DOI] [PubMed] [Google Scholar]
- 16. Bebrone C., et al. 2009. The structure of the dizinc subclass B2 metallo-β-lactamase CphA reveals that the second inhibitory zinc ion binds in the histidine site. Antimicrob. Agents Chemother. 53:4464–4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bellido F., Veuthey C., Blaser J., Bauernfeind A., Pechere J. C. 1990. Novel resistance to imipenem associated with an altered PBP-4 in a Pseudomonas aeruginosa clinical isolate. J. Antimicrob. Chemother. 25:57–68 [DOI] [PubMed] [Google Scholar]
- 18. Bethel C. R., et al. 2011. Exploring the inhibition of CTX-M-9 by β-lactamase inhibitors and carbapenems. Antimicrob. Agents Chemother. 55:3465–3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bonacorsi S., Fitoussi F., Lhopital S., Bingen E. 1999. Comparative in vitro activities of meropenem, imipenem, temocillin, piperacillin, and ceftazidime in combination with tobramycin, rifampin, or ciprofloxacin against Burkholderia cepacia isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 43:213–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bornet C., et al. 2003. Imipenem and expression of multidrug efflux pump in Enterobacter aerogenes. Biochem. Biophys. Res. Commun. 301:985–990 [DOI] [PubMed] [Google Scholar]
- 21. Bou G., Cervero G., Dominguez M. A., Quereda C., Martinez-Beltran J. 2000. Characterization of a nosocomial outbreak caused by a multiresistant Acinetobacter baumannii strain with a carbapenem-hydrolyzing enzyme: high-level carbapenem resistance in A. baumannii is not due solely to the presence of β-lactamases. J. Clin. Microbiol. 38:3299–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bradford P. A., et al. 1997. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob. Agents Chemother. 41:563–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bradley J. S., et al. 1999. Carbapenems in clinical practice: a guide to their use in serious infection. Int. J. Antimicrob. Agents 11:93–100 [DOI] [PubMed] [Google Scholar]
- 24. Branch C. L., et al. 1998. Novel C-2 substituted carbapenem derivatives. Part IV. Synthesis and biological activity of five membered heteroaromatic derivatives. J. Antibiot. (Tokyo) 51:210–220 [DOI] [PubMed] [Google Scholar]
- 25. Brown A. G., et al. 1976. Naturally-occurring β-lactamase inhibitors with antibacterial activity. J. Antibiot. (Tokyo) 29:668–669 [DOI] [PubMed] [Google Scholar]
- 26. Bush K., Jacoby G. A. 2010. Updated functional classification of β-lactamases. Antimicrob. Agents Chemother. 54:969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Butterworth D., Cole M., Hanscomb G., Rolinson G. N. 1979. Olivanic acids, a family of β-lactam antibiotics with β-lactamase inhibitory properties produced by Streptomyces species. I. Detection, properties and fermentation studies. J. Antibiot. (Tokyo) 32:287–294 [DOI] [PubMed] [Google Scholar]
- 28. Cama L. D., Christensen B. G. 1978. Total synthesis of thienamycin analogs. 1. Synthesis of the thienamycin nucleus and dl-decysteaminylthienamycin. J. Am. Chem. Soc. 100:8006–8007 [Google Scholar]
- 29. Cartwright S. J., Waley S. G. 1983. β-Lactamase inhibitors. Med. Res. Rev. 3:341–382 [DOI] [PubMed] [Google Scholar]
- 30. Cassidy P. J., et al. 1981. Epithienamycins. II. Isolation and structure assignment. J. Antibiot. (Tokyo) 34:637–648 [DOI] [PubMed] [Google Scholar]
- 31. Cerquetti M., Giufre M., Cardines R., Mastrantonio P. 2007. First characterization of heterogeneous resistance to imipenem in invasive nontypeable Haemophilus influenzae isolates. Antimicrob. Agents Chemother. 51:3155–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cha R. 2008. In vitro activity of cefepime, imipenem, tigecycline, and gentamicin, alone and in combination, against extended-spectrum β-lactamase-producing Klebsiella pneumoniae and Escherichia coli. Pharmacotherapy 28:295–300 [DOI] [PubMed] [Google Scholar]
- 33. Chambers H. F., Sachdeva M., Kennedy S. 1990. Binding affinity for penicillin-binding protein 2a correlates with in vivo activity of β-lactam antibiotics against methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 162:705–710 [DOI] [PubMed] [Google Scholar]
- 34. Charnas R. L., Knowles J. R. 1981. Inhibition of the RTEM β-lactamase from Escherichia coli. Interaction of enzyme with derivatives of olivanic acid. Biochemistry 20:2732–2737 [DOI] [PubMed] [Google Scholar]
- 35. Chouchani C., Marrakchi R., El Salabi A. 2011. Evolution of β-lactams resistance in Gram-negative bacteria in Tunisia. Crit. Rev. Microbiol. 37:167–177 [DOI] [PubMed] [Google Scholar]
- 36. Chow J. W., Shlaes D. M. 1991. Imipenem resistance associated with the loss of a 40 kDa outer membrane protein in Enterobacter aerogenes. J. Antimicrob. Chemother. 28:499–504 [DOI] [PubMed] [Google Scholar]
- 37. Cirioni O., et al. 2007. Efficacy of tachyplesin III, colistin, and imipenem against a multiresistant Pseudomonas aeruginosa strain. Antimicrob. Agents Chemother. 51:2005–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cole M. 1980. ‘β-Lactams’ as β-lactamase inhibitors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 289:207–223 [DOI] [PubMed] [Google Scholar]
- 39. Copar A., et al. 2002. Design, synthesis and bioactivity evaluation of tribactam beta lactamase inhibitors. Bioorg. Med. Chem. Lett. 12:971–975 [DOI] [PubMed] [Google Scholar]
- 40. Costello A. L., Sharma N. P., Yang K. W., Crowder M. W., Tierney D. L. 2006. X-ray absorption spectroscopy of the zinc-binding sites in the class B2 metallo-β-lactamase ImiS from Aeromonas veronii bv. sobria. Biochemistry 45:13650–13658 [DOI] [PubMed] [Google Scholar]
- 41. Cottagnoud P., et al. 2003. Meropenem prevents levofloxacin-induced resistance in penicillin-resistant pneumococci and acts synergistically with levofloxacin in experimental meningitis. Eur. J. Clin. Microbiol. Infect. Dis. 22:656–662 [DOI] [PubMed] [Google Scholar]
- 42. Crawford P. A., Yang K. W., Sharma N., Bennett B., Crowder M. W. 2005. Spectroscopic studies on cobalt(II)-substituted metallo-β-lactamase ImiS from Aeromonas veronii bv. sobria. Biochemistry 44:5168–5176 [DOI] [PubMed] [Google Scholar]
- 43. Cuffini A. M., et al. 1998. Entry of sanfetrinem into human polymorphonuclear granulocytes and its cell-associated activity against intracellular, penicillin-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:1745–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cullmann W., Dick W. 1983. Investigations on β-lactamase stability of recently developed β-lactam compounds: study of enzyme kinetics. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 254:413–422 [PubMed] [Google Scholar]
- 45. Cunha B. A., Hamid N. S., Krol V., Eisenstein L. 2008. Safety of meropenem in patients reporting penicillin allergy: lack of allergic cross reactions. J. Chemother. 20:233–237 [DOI] [PubMed] [Google Scholar]
- 46. Di Modugno E., et al. 1994. In vitro activity of the tribactam GV104326 against gram-positive, gram-negative, and anaerobic bacteria. Antimicrob. Agents Chemother. 38:2362–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Docquier J. D., et al. 2009. Crystal structure of the OXA-48 β-lactamase reveals mechanistic diversity among class D carbapenemases. Chem. Biol. 16:540–547 [DOI] [PubMed] [Google Scholar]
- 48. Drawz S. M., et al. 2010. Inhibition of the class C β-lactamase from Acinetobacter spp.: insights into effective inhibitor design. Biochemistry 49:329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Drusano G. L., Liu W., Fregeau C., Kulawy R., Louie A. 2009. Differing effects of combination chemotherapy with meropenem and tobramycin on cell kill and suppression of resistance of wild-type Pseudomonas aeruginosa PAO1 and its isogenic MexAB efflux pump-overexpressed mutant. Antimicrob. Agents Chemother. 53:2266–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dundar D., Otkun M. 2010. In-vitro efficacy of synergistic antibiotic combinations in multidrug resistant Pseudomonas aeruginosa strains. Yonsei Med. J. 51:111–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Easton C. J., Knowles J. R. 1982. Inhibition of the RTEM β-lactamase from Escherichia coli. Interaction of the enzyme with derivatives of olivanic acid. Biochemistry 21:2857–2862 [DOI] [PubMed] [Google Scholar]
- 52. Endimiani A., et al. 2010. Enhancing resistance to cephalosporins in class C β-lactamases: impact of Gly214Glu in CMY-2. Biochemistry 49:1014–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Endimiani A., et al. 2006. Pseudomonas aeruginosa bloodstream infections: risk factors and treatment outcome related to expression of the PER-1 extended-spectrum β-lactamase. BMC Infect. Dis. 6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ermertcan S., Hosgor M., Tunger O., Cosar G. 2001. Investigation of synergism of meropenem and ciprofloxacin against Pseudomonas aeruginosa and Acinetobacter strains isolated from intensive care unit infections. Scand. J. Infect. Dis. 33:818–821 [DOI] [PubMed] [Google Scholar]
- 55. Fainstein V., LeBlanc B., Weaver S., Bodey G. P. 1982. A comparative in vitro study of thienamycin. Infection 10:50–52 [DOI] [PubMed] [Google Scholar]
- 56. Farra A., Islam S., Stralfors A., Sorberg M., Wretlind B. 2008. Role of outer membrane protein OprD and penicillin-binding proteins in resistance of Pseudomonas aeruginosa to imipenem and meropenem. Int. J. Antimicrob. Agents 31:427–433 [DOI] [PubMed] [Google Scholar]
- 57. Fernandez-Cuenca F., et al. 2003. Relationship between β-lactamase production, outer membrane protein and penicillin-binding protein profiles on the activity of carbapenems against clinical isolates of Acinetobacter baumannii. J. Antimicrob. Chemother. 51:565–574 [DOI] [PubMed] [Google Scholar]
- 58. Fernandez-Cuenca F., Martinez-Martinez L., Pascual A., Perea E. J. 2003. In vitro activity of azithromycin in combination with amikacin, ceftazidime, ciprofloxacin or imipenem against clinical isolates of Acinetobacter baumannii. Chemotherapy 49:24–26 [DOI] [PubMed] [Google Scholar]
- 59. Fonseca F., Bromley E. H., Saavedra M. J., Correia A., Spencer J. 2011. Crystal structure of Serratia fonticola Sfh-I: activation of the nucleophile in mono-zinc metallo-β-lactamases. J. Mol. Biol. 411:951–959 [DOI] [PubMed] [Google Scholar]
- 60. Force E., et al. 2009. Evaluation of meropenem alone and combined with rifampin in the guinea pig model of pneumococcal meningitis. Eur. J. Clin. Microbiol. Infect. Dis. 28:807–811 [DOI] [PubMed] [Google Scholar]
- 61. Frase H., Shi Q., Testero S. A., Mobashery S., Vakulenko S. B. 2009. Mechanistic basis for the emergence of catalytic competence against carbapenem antibiotics by the GES family of β-lactamases. J. Biol. Chem. 284:29509–29513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fukasawa M., et al. 1992. Stability of meropenem and effect of 1 β-methyl substitution on its stability in the presence of renal dehydropeptidase I. Antimicrob. Agents Chemother. 36:1577–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fukuoka T., et al. 1993. Activity of the carbapenem panipenem and role of the OprD (D2) protein in its diffusion through the Pseudomonas aeruginosa outer membrane. Antimicrob. Agents Chemother. 37:322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gaibani P., et al. 2011. Rapid increase of carbapenemase-producing Klebsiella pneumoniae strains in a large Italian hospital: surveillance period 1 March-30 September 2010. Euro Surveill. 16:pii=19800. [PubMed] [Google Scholar]
- 65. Galleni M., Amicosante G., Frere J. M. 1988. A survey of the kinetic parameters of class C β-lactamases. Cephalosporins and other β-lactam compounds. Biochem. J. 255:123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ganta S. R., et al. 2009. Approaches to the simultaneous inactivation of metallo- and serine-β-lactamases. Bioorg. Med. Chem. Lett. 19:1618–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Garau G., et al. 2005. A metallo-β-lactamase enzyme in action: crystal structures of the monozinc carbapenemase CphA and its complex with biapenem. J. Mol. Biol. 345:785–795 [DOI] [PubMed] [Google Scholar]
- 68. Gehrlein M., Leying H., Cullmann W., Wendt S., Opferkuch W. 1991. Imipenem resistance in Acinetobacter baumannii is due to altered penicillin-binding proteins. Chemotherapy 37:405–412 [DOI] [PubMed] [Google Scholar]
- 69. Ghosh A. S., Kar A. K., Kundu M. 1999. Impaired imipenem uptake associated with alterations in outer membrane proteins and lipopolysaccharides in imipenem-resistant Shigella dysenteriae. J. Antimicrob. Chemother. 43:195–201 [DOI] [PubMed] [Google Scholar]
- 70. Giamarellou H., Antoniadou A., Kanellakopoulou K. 2008. Acinetobacter baumannii: a universal threat to public health? Int. J. Antimicrob. Agents 32:106–119 [DOI] [PubMed] [Google Scholar]
- 71. Giske C. G., Buaro L., Sundsfjord A., Wretlind B. 2008. Alterations of porin, pumps, and penicillin-binding proteins in carbapenem resistant clinical isolates of Pseudomonas aeruginosa. Microb. Drug Resist. 14:23–30 [DOI] [PubMed] [Google Scholar]
- 72. Goa K. L., Noble S. 2003. Panipenem/betamipron. Drugs 63:913–926 [DOI] [PubMed] [Google Scholar]
- 73. Gopalakrishnan R., Sureshkumar D. 2010. Changing trends in antimicrobial susceptibility and hospital acquired infections over an 8 year period in a tertiary care hospital in relation to introduction of an infection control programme. J. Assoc. Physicians India 58(Suppl.):25–31 [PubMed] [Google Scholar]
- 74. Graham D. W., et al. 1987. Inhibition of the mammalian β-lactamase renal dipeptidase (dehydropeptidase-I) by (Z)-2-(acylamino)-3-substituted-propenoic acids. J. Med. Chem. 30:1074–1090 [DOI] [PubMed] [Google Scholar]
- 75. Guelfi K. C., et al. 2008. In vitro evaluation of the antimicrobial activity of meropenem in combination with polymyxin B and gatifloxacin against Pseudomonas aeruginosa and Acinetobacter baumannii. J. Chemother. 20:180–185 [DOI] [PubMed] [Google Scholar]
- 76. Hanaki H., Hiramatsu K. 1999. Combination effect of teicoplanin and various antibiotics against hetero-VRSA and VRSA. Kansenshogaku Zasshi 73:1048–1053 (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 77. Hancock R. E., Brinkman F. S. 2002. Function of Pseudomonas porins in uptake and efflux. Annu. Rev. Microbiol. 56:17–38 [DOI] [PubMed] [Google Scholar]
- 78. Hantson P., Leonard F., Maloteaux J. M., Mahieu P. 1999. How epileptogenic are the recent antibiotics? Acta Clin. Belg. 54:80–87 [DOI] [PubMed] [Google Scholar]
- 79. Hashihayata T., et al. 2001. Diastereoselective synthesis of (2R,4R)-2-aryl-4-hydroxypyrrolidine: preparation of the side chain of novel carbapenem. Chem. Pharm. Bull. (Tokyo) 49:1500–1502 [DOI] [PubMed] [Google Scholar]
- 80. Hashihayata T., Sakoh H., Goto Y., Yamada K., Morishima H. 2002. Synthesis of the side chain of a novel carbapenem via iodine-mediated oxidative cyclization of (1R)-N-(1-aryl-3-butenyl)acetamide. Chem. Pharm. Bull. (Tokyo) 50:423–425 [DOI] [PubMed] [Google Scholar]
- 81. Hashizume T., Ishino F., Nakagawa J., Tamaki S., Matsuhashi M. 1984. Studies on the mechanism of action of imipenem (N-formimidoylthienamycin) in vitro: binding to the penicillin-binding proteins (PBPs) in Escherichia coli and Pseudomonas aeruginosa, and inhibition of enzyme activities due to the PBPs in E. coli. J. Antibiot. (Tokyo) 37:394–400 [DOI] [PubMed] [Google Scholar]
- 82. Hikida M., Itahashi K., Igarashi A., Shiba T., Kitamura M. 1999. In vitro antibacterial activity of LJC 11,036, an active metabolite of L-084, a new oral carbapenem antibiotic with potent antipneumococcal activity. Antimicrob. Agents Chemother. 43:2010–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hikida M., Kawashima K., Yoshida M., Mitsuhashi S. 1992. Inactivation of new carbapenem antibiotics by dehydropeptidase-I from porcine and human renal cortex. J. Antimicrob. Chemother. 30:129–134 [DOI] [PubMed] [Google Scholar]
- 84. Hiraiwa Y., Morinaka A., Fukushima T., Kudo T. 2009. Metallo-β-lactamase inhibitory activity of phthalic acid derivatives. Bioorg. Med. Chem. Lett. 19:5162–5165 [DOI] [PubMed] [Google Scholar]
- 85. Hirakata Y., et al. 2009. Efflux pump inhibitors reduce the invasiveness of Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 34:343–346 [DOI] [PubMed] [Google Scholar]
- 86. Hodgson S. T., Hollinshead D. M., Ley S. V. 1984. π-Allyltricarbonyliron lactone complexes in synthesis: application to the synthesis of the β-lactam antibiotic (+)-thienamycin J. Chem. Soc. Chem. Commun. (Camb.) 1984:494–496 [Google Scholar]
- 87. Huang H., Hancock R. E. 1996. The role of specific surface loop regions in determining the function of the imipenem-specific pore protein OprD of Pseudomonas aeruginosa. J. Bacteriol. 178:3085–3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Huang H., Jeanteur D., Pattus F., Hancock R. E. 1995. Membrane topology and site-specific mutagenesis of Pseudomonas aeruginosa porin OprD. Mol. Microbiol. 16:931–941 [DOI] [PubMed] [Google Scholar]
- 89. Huang H., Siehnel R. J., Bellido F., Rawling E., Hancock R. E. 1992. Analysis of two gene regions involved in the expression of the imipenem-specific, outer membrane porin protein OprD of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 76:267–273 [DOI] [PubMed] [Google Scholar]
- 90. Hugonnet J. E., Tremblay L. W., Boshoff H. I., Barry C. E., III, Blanchard J. S. 2009. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 323:1215–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Iglicar P., Legen I., Vilfan G., Selic L., Prezelj A. 2009. Permeability of a novel β-lactamase inhibitor LK-157 and its ester prodrugs across rat jejunum in vitro. J. Pharm. Pharmacol. 61:1211–1218 [DOI] [PubMed] [Google Scholar]
- 92. Imada A., et al. 1980. C-19393 S2 and H2, new carbapenem antibiotics. I. Taxonomy of the producing strain, fermentation and antibacterial properties. J. Antibiot. (Tokyo) 33:1417–1424 [DOI] [PubMed] [Google Scholar]
- 93. Isoda T., et al. 2006. Syntheses and pharmacokinetic studies of prodrug esters for the development of oral carbapenem, L-084. J. Antibiot. (Tokyo). 59:241–247 [DOI] [PubMed] [Google Scholar]
- 94. Jacqueline C., et al. 2005. In vitro and in vivo synergistic activities of linezolid combined with subinhibitory concentrations of imipenem against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kahan J. S., et al. 1979. Thienamycin, a new β-lactam antibiotic. I. Discovery, taxonomy, isolation and physical properties. J. Antibiot. (Tokyo) 32:1–12 [DOI] [PubMed] [Google Scholar]
- 96. Kaloyanides G. J. 1994. Antibiotic-related nephrotoxicity. Nephrol. Dial. Transplant. 9(Suppl. 4):130–134 [PubMed] [Google Scholar]
- 97. Kalp M., Carey P. R. 2008. Carbapenems and SHV-1 β-lactamase form different acyl-enzyme populations in crystals and solution. Biochemistry 47:11830–11837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kang Y. K., et al. 2003. Synthesis and biological evaluation of novel 1 β-methylcarbapenems with isothiazoloethenyl side chains. Bioorg. Med. Chem. Lett. 13:463–466 [DOI] [PubMed] [Google Scholar]
- 99. Kang Y. K., et al. 1999. Synthesis and biological evaluation of novel 1β-methylcarbapenems having a new moiety at C-2. Bioorg. Med. Chem. Lett. 9:2385–2390 [DOI] [PubMed] [Google Scholar]
- 100. Katayama Y., Zhang H. Z., Chambers H. F. 2004. PBP 2a mutations producing very-high-level resistance to β-lactams. Antimicrob. Agents Chemother. 48:453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kattan J. N., Villegas M. V., Quinn J. P. 2008. New developments in carbapenems. Clin. Microbiol. Infect. 14:1102–1111 [DOI] [PubMed] [Google Scholar]
- 102. Kawamoto I., et al. 2001. Synthesis and antibacterial activity of novel 1β-methyl carbapenems with cycloalkylamine moiety at the C-2 position. J. Antibiot. (Tokyo) 54:1080–1092 [DOI] [PubMed] [Google Scholar]
- 103. Ke W., Bethel C. R., Thomson J. M., Bonomo R. A., van den Akker F. 2007. Crystal structure of KPC-2: insights into carbapenemase activity in class A β-lactamases. Biochemistry 46:5732–5740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kiffer C. R., et al. 2005. In vitro synergy test of meropenem and sulbactam against clinical isolates of Acinetobacter baumannii. Diagn. Microbiol. Infect. Dis. 52:317–322 [DOI] [PubMed] [Google Scholar]
- 105. Kijima K., et al. 2009. Pharmacokinetics analysis of tebipenem pivoxil in a phase II clinical trial in otolaryngological infections. Jpn. J. Antibiot. 62:143–154 (In Japanese.) [PubMed] [Google Scholar]
- 106. Kim J. Y., et al. 2006. Structural basis for the extended substrate spectrum of CMY-10, a plasmid-encoded class C β-lactamase. Mol. Microbiol. 60:907–916 [DOI] [PubMed] [Google Scholar]
- 107. Kobayashi F., et al. 1982. Antimicrobial and β-lactamase inhibitory activities of carpetimycins A and B, new carbapenem antibiotics. Antimicrob. Agents Chemother. 21:536–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kobayashi Y. 2005. Study of the synergism between carbapenems and vancomycin or teicoplanin against MRSA, focusing on S-4661, a carbapenem newly developed in Japan. J. Infect. Chemother. 11:259–261 [DOI] [PubMed] [Google Scholar]
- 109. Koga T., et al. 2009. Affinity of tomopenem (CS-023) for penicillin-binding proteins in Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:1238–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kohler T., Michea-Hamzehpour M., Epp S. F., Pechere J. C. 1999. Carbapenem activities against Pseudomonas aeruginosa: respective contributions of OprD and efflux systems. Antimicrob. Agents Chemother. 43:424–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kotsakis S. D., Miriagou V., Tzelepi E., Tzouvelekis L. S. 2010. Comparative biochemical and computational study of the role of naturally occurring mutations at Ambler positions 104 and 170 in GES β-lactamases. Antimicrob. Agents Chemother. 54:4864–4871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kropp H., et al. 1976. Abstr. 16th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 228. American Society for Microbiology, Washington, DC [Google Scholar]
- 113. Kropp H., Sundelof J. G., Kahan J. S., Kahan F. M., Birnbaum J. 1979. Abstr. 19th Intersci. Conf Antimicrob. Agents Chemother., abstr. 231. American Society for Microbiology, Washington, DC [Google Scholar]
- 114. Kropp H., Sundelof J. G., Hajdu R., Kahan F. M. 1982. Metabolism of thienamycin and related carbapenem antibiotics by the renal dipeptidase, dehydropeptidase. Antimicrob. Agents Chemother. 22:62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kuroki H., Tateno N., Ikeda H., Saito N. 2010. Investigation of pneumonia-causing pathogenic organisms in children and the usefulness of tebipenem pivoxil for their treatment. J. Infect. Chemother. 16:280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lakaye B., Dubus A., Lepage S., Groslambert S., Frere J. M. 1999. When drug inactivation renders the target irrelevant to antibiotic resistance: a case story with β-lactams. Mol. Microbiol. 31:89–101 [DOI] [PubMed] [Google Scholar]
- 117. Lee J. H., et al. 2003. Synthesis and biological activity of novel 1β-methylcarbapenems with oxyiminopyrrolidinylamide moiety. Bioorg. Med. Chem. Lett. 13:4399–4403 [DOI] [PubMed] [Google Scholar]
- 118. Lee K. S., et al. 2005. Novel 1β-methylcarbapenems with isoxazoloethenyl moieties containing carboxylic acid sodium salt. Bioorg. Med. Chem. Lett. 15:231–234 [DOI] [PubMed] [Google Scholar]
- 119. Lee S. O., et al. 2004. Risk factors for acquisition of imipenem-resistant Acinetobacter baumannii: a case-control study. Antimicrob. Agents Chemother. 48:224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lepelletier D., et al. 2010. Imipenem-resistant Pseudomonas aeruginosa gastrointestinal carriage among hospitalized patients: risk factors and resistance mechanisms. Diagn. Microbiol. Infect. Dis. 66:1–6 [DOI] [PubMed] [Google Scholar]
- 121. Limansky A. S., Mussi M. A., Viale A. M. 2002. Loss of a 29-kilodalton outer membrane protein in Acinetobacter baumannii is associated with imipenem resistance. J. Clin. Microbiol. 40:4776–4778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lisa M. N., Hemmingsen L., Vila A. J. 2010. Catalytic role of the metal ion in the metallo-β-lactamase GOB. J. Biol. Chem. 285:4570–4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Livermore D. M., et al. 2011. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int. J. Antimicrob. Agents 37:415–419 [DOI] [PubMed] [Google Scholar]
- 124. Louie A., et al. 2010. The combination of meropenem and levofloxacin is synergistic with respect to both Pseudomonas aeruginosa kill rate and resistance suppression. Antimicrob. Agents Chemother. 54:2646–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mahamoud A., Chevalier J., Baitiche M., Adam E., Pages J. M. 2010. An alkylaminoquinazoline restores antibiotic activity in Gram-negative resistant isolates. Microbiology 157:566–571 [DOI] [PubMed] [Google Scholar]
- 126. Mainardi J. L., et al. 1997. Carbapenem resistance in a clinical isolate of Citrobacter freundii. Antimicrob. Agents Chemother. 41:2352–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Majiduddin F. K., Palzkill T. 2005. Amino acid residues that contribute to substrate specificity of class A β-lactamase SME-1. Antimicrob. Agents Chemother. 49:3421–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Mammeri H., Guillon H., Eb F., Nordmann P. 2010. Phenotypic and biochemical comparison of the carbapenem hydrolyzing activity of five plasmid-borne AmpC β-lactamases. Antimicrob. Agents Chemother. 54:4556–4560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Mammeri H., Nordmann P., Berkani A., Eb F. 2008. Contribution of extended-spectrum AmpC (ESAC) β-lactamases to carbapenem resistance in Escherichia coli. FEMS Microbiol. Lett. 282:238–240 [DOI] [PubMed] [Google Scholar]
- 130. Mandell L. 2009. Doripenem: a new carbapenem in the treatment of nosocomial infection. Clin. Infect. Dis. 49(Suppl. 1):S1–S3 [DOI] [PubMed] [Google Scholar]
- 131. Martinez-Martinez L. 2008. Extended-spectrum β-lactamases and the permeability barrier. Clin. Microbiol. Infect. 14(Suppl. 1):82–89 [DOI] [PubMed] [Google Scholar]
- 132. Marumo K., Nagaki T., Nakamura Y. 1996. Evaluation of high-level carbapenem resistance in atypical Serratia marcescens by a comparison with its revertants. J. Antimicrob. Chemother. 38:47–58 [DOI] [PubMed] [Google Scholar]
- 133. Mastalerz H., Menard M., Ruediger E., Fung-Tomc J. 1992. Synthesis and antibacterial activity of some novel 6-methyl- and 6-propenyl-substituted carbapenems. J. Med. Chem. 35:953–958 [DOI] [PubMed] [Google Scholar]
- 134. Matsumoto A., et al. 2007. The emergence of drug-resistant Streptococcus pneumoniae and host risk factors for carriage of drug-resistant genes in northeastern Japan. Jpn. J. Infect. Dis. 60:10–13 [PubMed] [Google Scholar]
- 135. Maveyraud L., et al. 1998. Structural basis for clinical longevity of carbapenem antibiotics in the face of challenge by the common class A β-lactamases from the antibiotic-resistant bacteria. J. Am. Chem. Soc. 120:9748–9752 [Google Scholar]
- 136. Mena A., et al. 2006. Characterization of a large outbreak by CTX-M-1-producing Klebsiella pneumoniae and mechanisms leading to in vivo carbapenem resistance development. J. Clin. Microbiol. 44:2831–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Meroueh S. O., et al. 2006. Three-dimensional structure of the bacterial cell wall peptidoglycan. Proc. Natl. Acad. Sci. U. S. A. 103:4404–4409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Minond D., et al. 2009. Inhibitors of VIM-2 by screening pharmacologically active and click-chemistry compound libraries. Bioorg. Med. Chem. 17:5027–5037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Miranda-Novales G., Leanos-Miranda B. E., Vilchis-Perez M., Solorzano-Santos F. 2006. In vitro activity effects of combinations of cephalothin, dicloxacillin, imipenem, vancomycin and amikacin against methicillin-resistant Staphylococcus spp. strains. Ann. Clin. Microbiol. Antimicrob. 5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Misra R., Bavro V. N. 2009. Assembly and transport mechanism of tripartite drug efflux systems. Biochim. Biophys. Acta 1794:817–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Miyadera T., et al. 1983. Synthesis and in vitro activity of a new carbapenem, RS-533. J. Antibiot. (Tokyo) 36:1034–1039 [DOI] [PubMed] [Google Scholar]
- 142. Miyashita M., Chida M. N., Yoshikoshi A. 1982. Synthesis of the precursor of (+)-thienamycin utilizing D-glucosamine. J. Chem. Soc Chem. Commun. (Camb.) 1982:1354–1356 [Google Scholar]
- 143. Miyazaki S., et al. 2001. In vitro and in vivo antibacterial activities of L-084, a novel oral carbapenem, against causative organisms of respiratory tract infections. Antimicrob. Agents Chemother. 45:203–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Moellering R. C., Jr., Eliopoulos G. M., Sentochnik D. E. 1989. The carbapenems: new broad spectrum β-lactam antibiotics. J. Antimicrob. Chemother. 24(Suppl. A):1–7 [DOI] [PubMed] [Google Scholar]
- 145. Monks J., Waley S. G. 1988. Imipenem as substrate and inhibitor of β-lactamases. Biochem. J. 253:323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Montravers P., Andremont A., Massias L., Carbon C. 1994. Investigation of the potential role of Enterococcus faecalis in the pathophysiology of experimental peritonitis. J. Infect. Dis. 169:821–830 [DOI] [PubMed] [Google Scholar]
- 147. Murakami K., Doi M., Yoshida T. 1982. Asparenomycins A, B and C, new carbapenem antibiotics. V. Inhibition of β-lactamases. J. Antibiot. (Tokyo) 35:39–45 [DOI] [PubMed] [Google Scholar]
- 148. Muratani T., Doi K., Kobayashi T., Nakamura T., Matsumoto T. 2009. Antimicrobial activity of tebipenem against various clinical isolates from various specimen, mainly urinary tract. Jpn. J. Antibiot. 62:116–126 (In Japanese.) [PubMed] [Google Scholar]
- 149. Nakayama M., et al. 1980. Carpetimycins A and B, new β-lactam antibiotics. J. Antibiot. (Tokyo) 33:1388–1390 [DOI] [PubMed] [Google Scholar]
- 150. Neuwirth C., Siebor E., Duez J. M., Pechinot A., Kazmierczak A. 1995. Imipenem resistance in clinical isolates of Proteus mirabilis associated with alterations in penicillin-binding proteins. J. Antimicrob. Chemother. 36:335–342 [DOI] [PubMed] [Google Scholar]
- 151. Nicasio A. M., Kuti J. L., Nicolau D. P. 2008. The current state of multidrug-resistant gram-negative bacilli in North America. Pharmacotherapy 28:235–249 [DOI] [PubMed] [Google Scholar]
- 152. Nikaido H. 1996. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 178:5853–5859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Nix D. E., Majumdar A. K., DiNubile M. J. 2004. Pharmacokinetics and pharmacodynamics of ertapenem: an overview for clinicians. J. Antimicrob. Chemother. 53(Suppl. 2):ii23–ii28 [DOI] [PubMed] [Google Scholar]
- 154. Nordmann P., Nicolas M. H., Gutmann L. 1993. Penicillin-binding proteins of Rhodococcus equi: potential role in resistance to imipenem. Antimicrob. Agents Chemother. 37:1406–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Nordmann P., et al. 2011. Comparative activity of carbapenem testing: the COMPACT study. J. Antimicrob. Chemother. 66:1070–1078 [DOI] [PubMed] [Google Scholar]
- 156. Norrby S. R. 1996. Neurotoxicity of carbapenem antibacterials. Drug Saf. 15:87–90 [DOI] [PubMed] [Google Scholar]
- 157. Norrby S. R., et al. 1983. Urinary recovery of N-formimidoyl thienamycin (MK0787) as affected by coadministration of N-formimidoyl thienamycin dehydropeptidase inhibitors. Antimicrob. Agents Chemother. 23:300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Nozaki Y., Harada S., Kitano K., Imada A. 1984. Structure-activity relations of 5,6-cis carbapenem antibiotics and role of factors determining susceptibility of Escherichia coli to β-lactam antibiotics. J. Antibiot. (Tokyo) 37:218–226 [DOI] [PubMed] [Google Scholar]
- 159. Nukaga M., et al. 2008. Inhibition of class A β-lactamases by carbapenems: crystallographic observation of two conformations of meropenem in SHV-1. J. Am. Chem. Soc. 130:12656–12662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Nunez L. E., Mendez C., Brana A. F., Blanco G., Salas J. A. 2003. The biosynthetic gene cluster for the β-lactam carbapenem thienamycin in Streptomyces cattleya. Chem. Biol. 10:301–311 [DOI] [PubMed] [Google Scholar]
- 161. Ochs M. M., McCusker M. P., Bains M., Hancock R. E. 1999. Negative regulation of the Pseudomonas aeruginosa outer membrane porin OprD selective for imipenem and basic amino acids. Antimicrob. Agents Chemother. 43:1085–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Okabe M., et al. 1982. Studies on the OA-6129 group of antibiotics, new carbapenem compounds. I. Taxonomy, isolation and physical properties. J. Antibiot. (Tokyo) 35:1255–1263 [DOI] [PubMed] [Google Scholar]
- 163. Okamura K., et al. 1978. PS-5, a new β-lactam antibiotic from Streptomyces. J. Antibiot. (Tokyo) 31:480–482 [DOI] [PubMed] [Google Scholar]
- 164. Oliver A., Levin B. R., Juan C., Baquero F., Blazquez J. 2004. Hypermutation and the preexistence of antibiotic-resistant Pseudomonas aeruginosa mutants: implications for susceptibility testing and treatment of chronic infections. Antimicrob. Agents Chemother. 48:4226–4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Osaki Y., et al. 2005. Genetic approach to study the relationship between penicillin-binding protein 3 mutations and Haemophilus influenzae β-lactam resistance by using site-directed mutagenesis and gene recombinants. Antimicrob. Agents Chemother. 49:2834–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Oteo J., et al. 2008. Emergence of imipenem resistance in clinical Escherichia coli during therapy. Int. J. Antimicrob. Agents 32:534–537 [DOI] [PubMed] [Google Scholar]
- 167. Paetzel M., et al. 2000. Crystal structure of the class D β-lactamase OXA-10. Nat. Struct. Biol. 7:918–925 [DOI] [PubMed] [Google Scholar]
- 168. Pai H., et al. 2001. Carbapenem resistance mechanisms in Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 45:480–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Pankuch G. A., Lin G., Seifert H., Appelbaum P. C. 2008. Activity of meropenem with and without ciprofloxacin and colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob. Agents Chemother. 52:333–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Papp-Wallace K. M., et al. 2010. Substrate selectivity and a novel role in inhibitor discrimination by position 237 in the KPC-2 β-lactamase. Antimicrob. Agents Chemother. 54:2867–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Papp-Wallace K. M., et al. 2010. Elucidating the role of Trp105 in the KPC-2 β-lactamase. Protein Sci. 19:1714–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Parker W. L., et al. 1982. SQ 27,860, a simple carbapenem produced by species of Serratia and Erwinia. J. Antibiot. (Tokyo) 35:653–660 [DOI] [PubMed] [Google Scholar]
- 173. Patel G., Bonomo R. A. 2011. Status report on carbapenemases: challenges and prospects. Expert Rev. Anti Infect. Ther. 9:555–570 [DOI] [PubMed] [Google Scholar]
- 174. Paterson D. L. 2000. Recommendation for treatment of severe infections caused by Enterobacteriaceae producing extended-spectrum β-lactamases (ESBLs). Clin. Microbiol. Infect. 6:460–463 [DOI] [PubMed] [Google Scholar]
- 175. Paterson D. L. 2002. Serious infections caused by enteric gram-negative bacilli—mechanisms of antibiotic resistance and implications for therapy of gram-negative sepsis in the transplanted patient. Semin. Respir. Infect. 17:260–264 [DOI] [PubMed] [Google Scholar]
- 176. Paterson D. L., Bonomo R. A. 2005. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Paukner S., Hesse L., Prezelj A., Solmajer T., Urleb U. 2009. In vitro activity of LK-157, a novel tricyclic carbapenem as broad-spectrum β-lactamase inhibitor. Antimicrob. Agents Chemother. 53:505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Pearson J. P., Van Delden C., Iglewski B. H. 1999. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 181:1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Pernot L., et al. 2001. Crystal structures of the class D β-lactamase OXA-13 in the native form and in complex with meropenem. J. Mol. Biol. 310:859–874 [DOI] [PubMed] [Google Scholar]
- 180. Perron K., et al. 2004. CzcR-CzcS, a two-component system involved in heavy metal and carbapenem resistance in Pseudomonas aeruginosa. J. Biol. Chem. 279:8761–8768 [DOI] [PubMed] [Google Scholar]
- 181. Perry C. M., Ibbotson T. 2002. Biapenem. Drugs 62:2221–2235 [DOI] [PubMed] [Google Scholar]
- 182. Petrella S., et al. 2008. Genetic and structural insights into the dissemination potential of the extremely broad-spectrum class A β-lactamase KPC-2 identified in an Escherichia coli strain and an Enterobacter cloacae strain isolated from the same patient in France. Antimicrob. Agents Chemother. 52:3725–3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Pfaendler H. R., Gostel J. R., Woodward R. B., Rihs G. 1981. Structure, reactivity, and biological activity of strained bicyclic β-lactams. J. Am. Chem. Soc. 103:4526–4531 [Google Scholar]
- 184. Piddock L. J., Jin Y. F. 1995. Activity of biapenem (LJC 10627) against 51 imipenem-resistant bacteria and selection and characterisation of biapenem-resistant mutants. J. Antimicrob. Chemother. 36:845–850 [DOI] [PubMed] [Google Scholar]
- 185. Pierre J., Boisivon A., Gutmann L. 1990. Alteration of PBP 3 entails resistance to imipenem in Listeria monocytogenes. Antimicrob. Agents Chemother. 34:1695–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Plantan I., et al. 2007. 4-Substituted trinems as broad spectrum β-lactamase inhibitors: structure-based design, synthesis, and biological activity. J. Med. Chem. 50:4113–4121 [DOI] [PubMed] [Google Scholar]
- 187. Poirel L., Pitout J. D., Nordmann P. 2007. Carbapenemases: molecular diversity and clinical consequences. Future Microbiol. 2:501–512 [DOI] [PubMed] [Google Scholar]
- 188. Pongpech P., et al. 2010. Antibacterial activity of carbapenem-based combinations against multidrug-resistant Acinetobacter baumannii. J. Med. Assoc. Thai. 93:161–171 [PubMed] [Google Scholar]
- 189. Queenan A. M., Bush K. 2007. Carbapenemases: the versatile β-lactamases. Clin. Microbiol. Rev. 20:440–458, table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Queenan A. M., Shang W., Flamm R., Bush K. 2010. Hydrolysis and inhibition profiles of β-lactamases from molecular classes A to D with doripenem, imipenem, and meropenem. Antimicrob. Agents Chemother. 54:565–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Raimondi A., Traverso A., Nikaido H. 1991. Imipenem- and meropenem-resistant mutants of Enterobacter cloacae and Proteus rettgeri lack porins. Antimicrob. Agents Chemother. 35:1174–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Reading C., Farmer T. 1984. The inhibition of periplasmic β-lactamase in Escherichia coli by clavulanic acid and other β-lactamase inhibitors. McGraw-Hill, New York, NY [Google Scholar]
- 193. Riera E., et al. 8 June 2011. Pseudomonas aeruginosa carbapenem resistance mechanisms in Spain: impact on the activity of imipenem, meropenem and doripenem. J. Antimicrob. Chemother. doi: 10.1093/jac/dkr232 [DOI] [PubMed] [Google Scholar]
- 194. Rodloff A. C., Goldstein E. J., Torres A. 2006. Two decades of imipenem therapy. J. Antimicrob. Chemother. 58:916–929 [DOI] [PubMed] [Google Scholar]
- 195. Rodriguez-Hernandez M. J., et al. 2000. Imipenem, doxycycline and amikacin in monotherapy and in combination in Acinetobacter baumannii experimental pneumonia. J. Antimicrob. Chemother. 45:493–501 [DOI] [PubMed] [Google Scholar]
- 196. Rodriguez-Martinez J. M., Poirel L., Nordmann P. 2009. Extended-spectrum cephalosporinases in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:1766–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Rodriguez-Martinez J. M., Poirel L., Nordmann P. 2009. Molecular epidemiology and mechanisms of carbapenem resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:4783–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Rodriguez C. H., et al. 2010. In vitro antimicrobials activity against endemic Acinetobacter baumannii multiresistant clones. J. Infect. Dev. Ctries. 4:164–167 [DOI] [PubMed] [Google Scholar]
- 199. Rolinson G. N. 1991. Evolution of β-lactamase inhibitors. Rev. Infect. Dis. 13(Suppl. 9):S727–S732 [DOI] [PubMed] [Google Scholar]
- 200. Rossi F. 2011. The challenges of antimicrobial resistance in Brazil. Clin. Infect. Dis. 52:1138–1143 [DOI] [PubMed] [Google Scholar]
- 201. Salzmann T. N., Ratcliffe R. W., Christensen B. G., Boufford F. A. 1980. A stereocontrolled synthesis of (+)-thienamycin. J. Am. Chem. Soc. 102:6161–6163 [DOI] [PubMed] [Google Scholar]
- 202. Sanchez P. A., Toney J. H., Thomas J. D., Berger J. M. 2009. A sensitive coupled HPLC/electrospray mass spectrometry assay for SPM-1 metallo-β-lactamase inhibitors. Assay Drug Dev. Technol. 7:170–179 [DOI] [PubMed] [Google Scholar]
- 203. Santillana E., Beceiro A., Bou G., Romero A. 2007. Crystal structure of the carbapenemase OXA-24 reveals insights into the mechanism of carbapenem hydrolysis. Proc. Natl. Acad. Sci. U. S. A. 104:5354–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Schneider K. D., Karpen M. E., Bonomo R. A., Leonard D. A., Powers R. A. 2009. The 1.4 A crystal structure of the class D β-lactamase OXA-1 complexed with doripenem. Biochemistry 48:11840–11847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Schneider K. D., et al. 2011. Structures of the class D carbapenemase OXA-24 from Acinetobacter baumannii in complex with doripenem. J. Mol. Biol. 406:583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Shahid M., et al. 2009. β-lactams and β-lactamase-inhibitors in current- or potential-clinical practice: a comprehensive update. Crit. Rev. Microbiol. 35:81–108 [DOI] [PubMed] [Google Scholar]
- 207. Shin K. J., et al. 1998. Synthesis and biological properties of new 1 β-methylcarbapenems. Bioorg. Med. Chem. Lett. 8:1607–1612 [DOI] [PubMed] [Google Scholar]
- 208. Simona F., et al. 2009. Common mechanistic features among metallo-β-lactamases: a computational study of Aeromonas hydrophila CphA enzyme. J. Biol. Chem. 284:28164–28171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Simona F., et al. 2007. Protonation state and substrate binding to B2 metallo-β-lactamase CphA from Aeromonas hydrofila. Proteins 69:595–605 [DOI] [PubMed] [Google Scholar]
- 210. Siroy A., et al. 2005. Channel formation by CarO, the carbapenem resistance-associated outer membrane protein of Acinetobacter baumannii. Antimicrob. Agents Chemother. 49:4876–4883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Smith C. A., Caccamo M., Kantardjieff K. A., Vakulenko S. 2007. Structure of GES-1 at atomic resolution: insights into the evolution of carbapenamase activity in the class A extended-spectrum β-lactamases. Acta Crystallogr. D Biol. Crystallogr. 63:982–992 [DOI] [PubMed] [Google Scholar]
- 212. Sodhi M., Axtell S. S., Callahan J., Shekar R. 2004. Is it safe to use carbapenems in patients with a history of allergy to penicillin? J. Antimicrob. Chemother. 54:1155–1157 [DOI] [PubMed] [Google Scholar]
- 213. Song J. Y., Cheong H. J., Lee J., Sung A. K., Kim W. J. 2009. Efficacy of monotherapy and combined antibiotic therapy for carbapenem-resistant Acinetobacter baumannii pneumonia in an immunosuppressed mouse model. Int. J. Antimicrob. Agents 33:33–39 [DOI] [PubMed] [Google Scholar]
- 214. Sougakoff W., et al. 2002. Structure of the imipenem-hydrolyzing class A β-lactamase SME-1 from Serratia marcescens. Acta Crystallogr. D Biol. Crystallogr. 58:267–274 [DOI] [PubMed] [Google Scholar]
- 215. Souli M., et al. 2009. Does the activity of the combination of imipenem and colistin in vitro exceed the problem of resistance in metallo-β-lactamase-producing Klebsiella pneumoniae isolates? Antimicrob. Agents Chemother. 53:2133–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Spratt B. G., Jobanputra V., Zimmermann W. 1977. Binding of thienamycin and clavulanic acid to the penicillin-binding proteins of Escherichia coli K-12. Antimicrob. Agents Chemother. 12:406–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Stapleton P. D., Shannon K. P., French G. L. 1999. Carbapenem resistance in Escherichia coli associated with plasmid-determined CMY-4 β-lactamase production and loss of an outer membrane protein. Antimicrob. Agents Chemother. 43:1206–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Sumita Y., Fukasawa M. 1995. Potent activity of meropenem against Escherichia coli arising from its simultaneous binding to penicillin-binding proteins 2 and 3. J. Antimicrob. Chemother. 36:53–64 [DOI] [PubMed] [Google Scholar]
- 219. Sunagawa M., Matsumura H., Inoue T., Fukasawa M., Kato M. 1990. A novel carbapenem antibiotic, SM-7338 structure-activity relationships. J. Antibiot. (Tokyo) 43:519–532 [DOI] [PubMed] [Google Scholar]
- 220. Sunagawa M., Matsumura H., Sumita Y., Nouda H. 1995. Structural features resulting in convulsive activity of carbapenem compounds: effect of C-2 side chain. J. Antibiot. (Tokyo) 48:408–416 [DOI] [PubMed] [Google Scholar]
- 221. Sunagawa M., et al. 1994. Novel quaternary ammonium carbapenems: 1 β-methyl-2-(5′-substituted pyrrolidinylthio) carbapenems. J. Antibiot. (Tokyo) 47:1337–1340 [DOI] [PubMed] [Google Scholar]
- 222. Swaren P., et al. 1998. X-ray analysis of the NMC-A β-lactamase at 1.64-A resolution, a class A carbapenemase with broad substrate specificity. J. Biol. Chem. 273:26714–26721 [DOI] [PubMed] [Google Scholar]
- 223. Symmons M. F., Bokma E., Koronakis E., Hughes C., Koronakis V. 2009. The assembled structure of a complete tripartite bacterial multidrug efflux pump. Proc. Natl. Acad. Sci. U. S. A. 106:7173–7178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Taibi P., Mobashery S. 1995. Mechanism of turnover of imipenem by the TEM β-lactamase revisited. J. Am. Chem. Soc. 117:7600–7605 [Google Scholar]
- 225. Tally F. P., Jacobus N. V., Gorbach S. L. 1978. In vitro activity of thienamycin. Antimicrob. Agents Chemother. 14:436–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Tam V. H., Schilling A. N., Lewis R. E., Melnick D. A., Boucher A. N. 2004. Novel approach to characterization of combined pharmacodynamic effects of antimicrobial agents. Antimicrob. Agents Chemother. 48:4315–4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Tamber S., Hancock R. E. 2006. Involvement of two related porins, OprD and OpdP, in the uptake of arginine by Pseudomonas aeruginosa. FEMS Microbiol. Lett. 260:23–29 [DOI] [PubMed] [Google Scholar]
- 228. Tipper D. J., Strominger J. L. 1965. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc. Natl. Acad. Sci. U. S. A. 54:1133–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Torres J. A., Villegas M. V., Quinn J. P. 2007. Current concepts in antibiotic-resistant gram-negative bacteria. Expert Rev. Anti Infect. Ther. 5:833–843 [DOI] [PubMed] [Google Scholar]
- 230. Tremblay L. W., Fan F., Blanchard J. S. 2010. Biochemical and structural characterization of Mycobacterium tuberculosis β-lactamase with the carbapenems ertapenem and doripenem. Biochemistry 49:3766–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Trias J., Nikaido H. 1990. Outer membrane protein D2 catalyzes facilitated diffusion of carbapenems and penems through the outer membrane of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 34:52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Tsai H. T., Wang J. T., Chen C. J., Chang S. C. 2008. Association between antibiotic usage and subsequent colonization or infection of extensive drug-resistant Acinetobacter baumannii: a matched case-control study in intensive care units. Diagn. Microbiol. Infect. Dis. 62:298–305 [DOI] [PubMed] [Google Scholar]
- 233. Tsuji N., et al. 1982. The structures of pluracidomycins, new carbapenem antibiotics. J. Antibiot. (Tokyo) 35:536–540 [DOI] [PubMed] [Google Scholar]
- 234. Tune B. M. 1994. Renal tubular transport and nephrotoxicity of beta lactam antibiotics: structure-activity relationships. Miner Electrolyte Metab. 20:221–231 [PubMed] [Google Scholar]
- 235. Tune B. M., Fravert D., Hsu C. Y. 1989. Thienamycin nephrotoxicity. Mitochondrial injury and oxidative effects of imipenem in the rabbit kidney. Biochem. Pharmacol. 38:3779–3783 [DOI] [PubMed] [Google Scholar]
- 236. Ueda Y., Vinet V. 1992. Synthesis and in vitro activity of novel quaternary ammonium carbapenems: 2-pyridiniopropyl and 1-pyridinioethyl carbapenems. J. Antibiot. (Tokyo) 45:940–953 [DOI] [PubMed] [Google Scholar]
- 237. van Dam V., Olrichs N., Breukink E. 2009. Specific labeling of peptidoglycan precursors as a tool for bacterial cell wall studies. Chembiochem 10:617–624 [DOI] [PubMed] [Google Scholar]
- 238. Vera-Cabrera L., et al. 2010. In vitro activity of ACH-702, a new isothiazoloquinolone, against Nocardia brasiliensis compared with econazole and the carbapenems imipenem and meropenem alone or in combination with clavulanic acid. Antimicrob. Agents Chemother. 54:2191–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Vilar M., et al. 2001. Kinetic study of two novel enantiomeric tricyclic β-lactams which efficiently inactivate class C β-lactamases. Antimicrob. Agents Chemother. 45:2215–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Villar H. E., Danel F., Livermore D. M. 1997. Permeability to carbapenems of Proteus mirabilis mutants selected for resistance to imipenem or other β-lactams. J. Antimicrob. Chemother. 40:365–370 [DOI] [PubMed] [Google Scholar]
- 241. Visalli M. A., Jacobs M. R., Appelbaum P. C. 1998. Determination of activities of levofloxacin, alone and combined with gentamicin, ceftazidime, cefpirome, and meropenem, against 124 strains of Pseudomonas aeruginosa by checkerboard and time-kill methodology. Antimicrob. Agents Chemother. 42:953–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Walsh T. R. 2008. Clinically significant carbapenemases: an update. Curr. Opin. Infect. Dis. 21:367–371 [DOI] [PubMed] [Google Scholar]
- 243. Walsh T. R. 2010. Emerging carbapenemases: a global perspective. Int. J. Antimicrob. Agents 36(Suppl. 3):S8–S14 [DOI] [PubMed] [Google Scholar]
- 244. Wang X., Minasov G., Shoichet B. K. 2002. Noncovalent interaction energies in covalent complexes: TEM-1 β-lactamase and β-lactams. Proteins 47:86–96 [PubMed] [Google Scholar]
- 245. Wareham D. W., Bean D. C. 2006. In-vitro activity of polymyxin B in combination with imipenem, rifampicin and azithromycin versus multidrug resistant strains of Acinetobacter baumannii producing OXA-23 carbapenemases. Ann. Clin. Microbiol. Antimicrob. 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Weaver S. S., Bodey G. P., LeBlanc B. M. 1979. Thienamycin: new β-lactam antibiotic with potent broad-spectrum activity. Antimicrob. Agents Chemother. 15:518–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Webster D. P., et al. 2010. Emergence of carbapenem resistance due to porin loss in an extended-spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae strain during meropenem therapy. Int. J. Antimicrob. Agents 36:575–576 [DOI] [PubMed] [Google Scholar]
- 248. Weide T., et al. 2010. NH-1,2,3-triazole-based inhibitors of the VIM-2 metallo-β-lactamase: synthesis and structure-activity studies. ACS Med. Chem. Lett. 1:150–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Woodward R. B. 1980. Penems and related substances. Philos. Trans. R. Soc. Lond. B Biol. Sci. 289:239–250 [DOI] [PubMed] [Google Scholar]
- 250. Wu S., Xu D., Guo H. 2010. QM/MM studies of monozinc β-lactamase CphA suggest that the crystal structure of an enzyme-intermediate complex represents a minor pathway. J. Am. Chem. Soc. 132:17986–17988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Xu D., Xie D., Guo H. 2006. Catalytic mechanism of class B2 metallo-β-lactamase. J. Biol. Chem. 281:8740–8747 [DOI] [PubMed] [Google Scholar]
- 252. Xu D., Zhou Y., Xie D., Guo H. 2005. Antibiotic binding to monozinc CphA β-lactamase from Aeromonas hydropila: quantum mechanical/molecular mechanical and density functional theory studies. J. Med. Chem. 48:6679–6689 [DOI] [PubMed] [Google Scholar]
- 253. Yong D., et al. 2009. Characterization of a new metallo-β-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 53:5046–5054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Zafaralla G., Mobashery S. 1992. Facilitation of the delta2 to delta1 pyrroline tautomerization of carbapenem antibiotics by the highly conserved arginine-244 of class A beta-lactamases during the course of turnover. J. Am. Chem. Soc. 114:1506–1507 [Google Scholar]
- 255. Zhanel G. G., et al. 2007. Comparative review of the carbapenems. Drugs 67:1027–1052 [DOI] [PubMed] [Google Scholar]
- 256. Zhang L., Ma S. 2010. Efflux pump inhibitors: a strategy to combat P-glycoprotein and the NorA multidrug resistance pump. ChemMedChem 5:811–822 [DOI] [PubMed] [Google Scholar]
- 257. Ziha-Zarifi I., Llanes C., Kohler T., Pechere J. C., Plesiat P. 1999. In vivo emergence of multidrug-resistant mutants of Pseudomonas aeruginosa overexpressing the active efflux system MexA-MexB-OprM. Antimicrob. Agents Chemother. 43:287–291 [DOI] [PMC free article] [PubMed] [Google Scholar]