Abstract
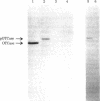
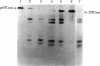
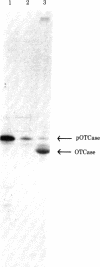
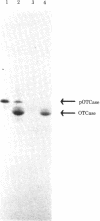
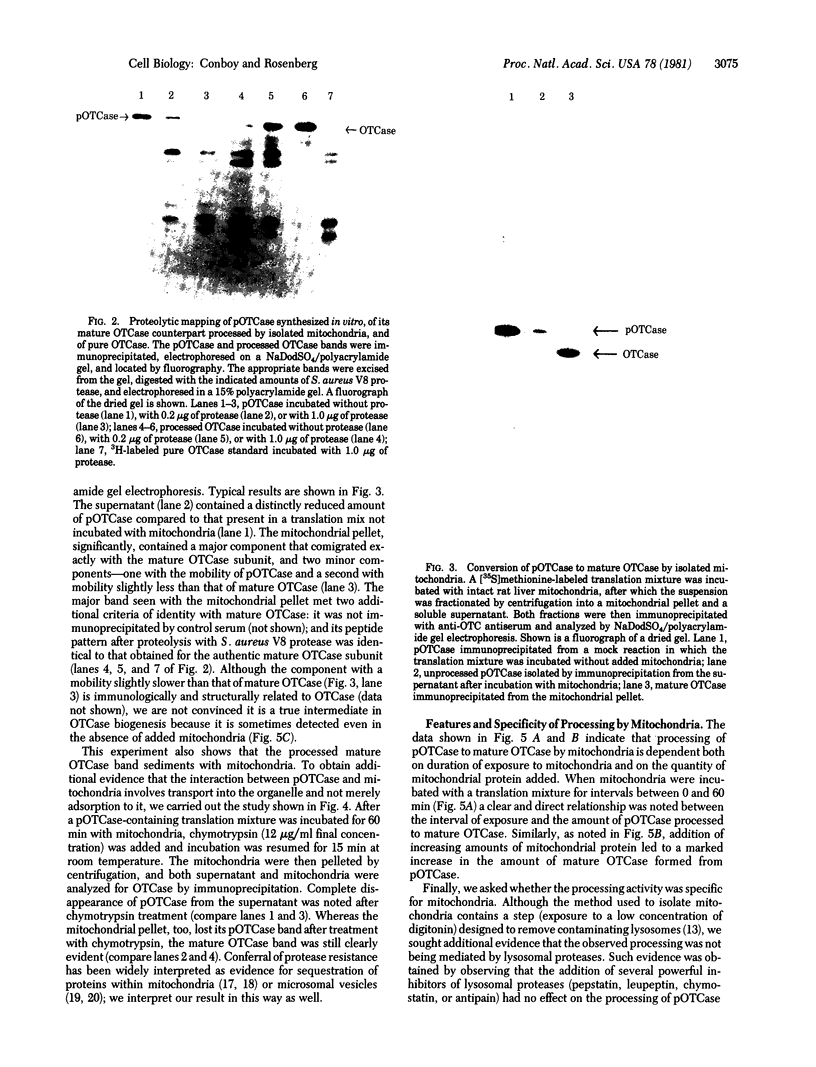
The mitochondrial matrix enzyme ornithine transcarbamoylase (OTCase; ornithine carbamoyltransferase; carbamoylphosphate:L-ornithine carbamoyltransferase, EC 2.1.3.3) is encoded by a nuclear gene on the X chromosome, synthesized on cytoplasmic ribosomes, and translocated across both mitochondrial membranes. Using specific immunoprecipitation, we presented evidence previously that the primary in vitro translation product of OTCase in rat liver is a polypeptide about 4000 daltons larger than the "mature" OTCase augment subunit purified from homologous mitochondria. In this report we augment the immunological identification of this cell-free translation product (pOTCase) with structural information and show, by electrophoresis of proteolysis products, that pOTCase is structurally similar to mitochondrial OTCase. Moreover, we now demonstrate that, when pOTCase is incubated posttranslationally with isolated rat liver mitochondria, it is converted to the size of mature OTCase and is sequestered within the mitochondria in such a way that it becomes resistant to externally added proteases. Such posttranslational processing is catalyzed specifically by the mitochondrial fraction of rat liver cells and is dependent both on the duration of incubation with mitochondria and on the amount of mitochondrial protein added. We conclude that pOTCase is indeed the bona fide precursor of mitochondrial OTCase and that use of this simplified cell-free system will facilitate analysis of OTCase biogenesis at both the cellular and the molecular level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ades I. Z., Butow R. A. The products of mitochondria-bound cytoplasmic polysomes in yeast. J Biol Chem. 1980 Oct 25;255(20):9918–9924. [PubMed] [Google Scholar]
- Ades I. Z., Butow R. A. The transport of proteins into yeast mitochondria. Kinetics and pools. J Biol Chem. 1980 Oct 25;255(20):9925–9935. [PubMed] [Google Scholar]
- Clarke S. The polypeptides of rat liver mitochondria: identification of a 36,000 dalton polypeptide as the subunit of ornithine transcarbamylase. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1118–1124. doi: 10.1016/0006-291x(76)90769-5. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Conboy J. G., Kalousek F., Rosenberg L. E. In vitro synthesis of a putative precursor of mitochondrial ornithine transcarbamoylase. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5724–5727. doi: 10.1073/pnas.76.11.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté C., Solioz M., Schatz G. Biogenesis of the cytochrome bc1 complex of yeast mitochondria. A precursor form of the cytoplasmically made subunit V. J Biol Chem. 1979 Mar 10;254(5):1437–1439. [PubMed] [Google Scholar]
- DeMars R., LeVan S. L., Trend B. L., Russell L. B. Abnormal ornithine carbamoyltransferase in mice having the sparse-fur mutation. Proc Natl Acad Sci U S A. 1976 May;73(5):1693–1697. doi: 10.1073/pnas.73.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton W. A., Ambani L. M., Rosenberg L. E. Uptake of hydroxocobalamin by rat liver mitochondria. Binding to a mitochondrial protein. J Biol Chem. 1976 Nov 10;251(21):6616–6623. [PubMed] [Google Scholar]
- Gamble J. G., Lehninger A. L. Transport of ornithine and citrulline across the mitochondrial membrane. J Biol Chem. 1973 Jan 25;248(2):610–618. [PubMed] [Google Scholar]
- Harmey M. A., Hallermayer G., Korb H., Neupert W. Transport of cytoplasmically synthesized proteins into the mitochondria in a cell free system from Neurospora crassa. Eur J Biochem. 1977 Dec;81(3):533–544. doi: 10.1111/j.1432-1033.1977.tb11979.x. [DOI] [PubMed] [Google Scholar]
- Harmey M. A., Neupert W. Biosynthesis of mitochondrial citrate synthase in Neurospora crassa. FEBS Lett. 1979 Dec 15;108(2):385–389. doi: 10.1016/0014-5793(79)80569-4. [DOI] [PubMed] [Google Scholar]
- Hoogenraad N. J., Sutherland T. M., Howlett G. J. Purification of ornithine transcarbamylase from rat liver by affinity chromatography with immobilized transition-state analog. Anal Biochem. 1980 Jan 1;101(1):97–102. doi: 10.1016/0003-2697(80)90045-7. [DOI] [PubMed] [Google Scholar]
- Kalousek F., François B., Rosenberg L. E. Isolation and characterization of ornithine transcarbamylase from normal human liver. J Biol Chem. 1978 Jun 10;253(11):3939–3944. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewin A. S., Gregor I., Mason T. L., Nelson N., Schatz G. Cytoplasmically made subunits of yeast mitochondrial F1-ATPase and cytochrome c oxidase are synthesized as individual precursors, not as polyproteins. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3998–4002. doi: 10.1073/pnas.77.7.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Cunningham B. A., Jazwinski S. M., Hopp T. P., Blobel G., Edelman G. M. Cell-free synthesis and segregation of beta 2-microglobulin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3651–3655. doi: 10.1073/pnas.76.8.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein J., Scholte H. R., Wit-Peeters E. M. A rapid and simple procedure to deplete rat-liver mitochondria of lysosomal activity. Biochim Biophys Acta. 1970 Dec 8;223(2):432–436. doi: 10.1016/0005-2728(70)90201-x. [DOI] [PubMed] [Google Scholar]
- Lusty C. J., Jilka R. L., Nietsch E. H. Ornithine transcarbamylase of rat liver. Kinetic, physical, and chemical properties. J Biol Chem. 1979 Oct 25;254(20):10030–10036. [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Schatz G. Transport of proteins across the mitochondrial outer membrane. A precursor form of the cytoplasmically made intermembrane enzyme cytochrome c peroxidase. J Biol Chem. 1979 Aug 25;254(16):7468–7471. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. I. Isolation and subunit structure. J Biol Chem. 1972 Mar 25;247(6):1641–1653. [PubMed] [Google Scholar]
- Michel R., Wachter E., Sebald W. Synthesis of a larger precursor for the proteolipid subunit of the mitochondrial ATPase complex of Neurospora crassa in a cell-free wheat germ system. FEBS Lett. 1979 May 15;101(2):373–376. doi: 10.1016/0014-5793(79)81047-9. [DOI] [PubMed] [Google Scholar]
- Mihara K., Blobel G. The four cytoplasmically made subunits of yeast mitochondrial cytochrome c oxidase are synthesized individually and not as a polyprotein. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4160–4164. doi: 10.1073/pnas.77.7.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Cell-free synthesis and processing of a putative precursor for mitochondrial carbamyl phosphate synthetase I of rat liver. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5071–5075. doi: 10.1073/pnas.76.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Characterization of a protease apparently involved in processing of pre-ornithine transcarbamylase of rat liver. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7044–7048. doi: 10.1073/pnas.77.12.7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Processing of a putative precursor of rat liver ornithine transcarbamylase, a mitochondrial matrix enzyme. J Biochem. 1980 Dec;88(6):1829–1836. doi: 10.1093/oxfordjournals.jbchem.a133158. [DOI] [PubMed] [Google Scholar]
- Nelson N., Schatz G. Energy-dependent processing of cytoplasmically made precursors to mitochondrial proteins. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4365–4369. doi: 10.1073/pnas.76.9.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raijman L. Citrulline synthesis in rat tissues and liver content of carbamoyl phosphate and ornithine. Biochem J. 1974 Feb;138(2):225–232. doi: 10.1042/bj1380225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond Y., Shore G. C. The precursor for carbamyl phosphate synthetase is transported to mitochondria via a cytosolic route. J Biol Chem. 1979 Oct 10;254(19):9335–9338. [PubMed] [Google Scholar]
- Ricciuti F. C., Gelehrter T. D., Rosenberg L. E. X-chromosome inactivation in human liver: confirmation of X-linkage of ornithine transcarbamylase. Am J Hum Genet. 1976 Jul;28(4):332–338. [PMC free article] [PubMed] [Google Scholar]
- Sakakibara R., Huynh Q. K., Nishida Y., Watanabe T., Wada H. In vitro synthesis of glutamic oxaloacetic transaminase isozymes of rat liver. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1781–1788. doi: 10.1016/s0006-291x(80)80105-7. [DOI] [PubMed] [Google Scholar]
- Schmelzer E., Heinrich P. C. Synthesis of a larger precursor for the subunit IV of rat liver cytochrome c oxidase in a cell-free wheat germ system. J Biol Chem. 1980 Aug 25;255(16):7503–7506. [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore G. C., Carignan P., Raymond Y. In vitro synthesis of a putative precursor to the mitochondrial enzyme, carbamyl phosphate synthetase. J Biol Chem. 1979 May 10;254(9):3141–3144. [PubMed] [Google Scholar]
- Short E. M., Conn H. O., Snodgrass P. J., Campbell A. G., Rosenberg L. E. Evidence for x-linked dominant inheritance of ornithine transcarbamylase deficiency. N Engl J Med. 1973 Jan 4;288(1):7–12. doi: 10.1056/NEJM197301042880102. [DOI] [PubMed] [Google Scholar]
- Sonderegger P., Jaussi R., Christen P. Cell-free synthesis of a putative precursor of mitochondrial aspartate aminotransferase with higher molecular weight. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1256–1260. doi: 10.1016/0006-291x(80)90554-9. [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Hayashi N., Kikuchi G. Cell-free synthesis of rat liver delta-aminolevulinate synthase and possible occurrence of processing of the enzyme protein in the course of its translocation from the cytosol into the mitochondrial matrix. FEBS Lett. 1980 Jun 16;115(1):15–18. doi: 10.1016/0014-5793(80)80716-2. [DOI] [PubMed] [Google Scholar]
- Zimmerman R., Paluch U., Sprinzl M., Neupert W. Cell-free synthesis of the mitochondrial ADP/ATP carrier protein of Neurospora crassa. Eur J Biochem. 1979 Sep;99(2):247–252. doi: 10.1111/j.1432-1033.1979.tb13251.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Neupert W. Transport of proteins into mitochondria. Posttranslational transfer of ADP/ATP carrier into mitochondria in vitro. Eur J Biochem. 1980 Aug;109(1):217–229. doi: 10.1111/j.1432-1033.1980.tb04787.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Paluch U., Neupert W. Cell-free synthesis of cytochrome c. FEBS Lett. 1979 Dec 1;108(1):141–146. doi: 10.1016/0014-5793(79)81196-5. [DOI] [PubMed] [Google Scholar]