Abstract
In clinical practice, antifungal therapy may be switched from fluconazole to voriconazole; such sequential use poses the potential for drug interaction due to cytochrome P450 2C19 (CYP2C19)-mediated inhibition of voriconazole metabolism. This open-label, randomized, two-way crossover study investigated the effect of concomitant fluconazole on voriconazole pharmacokinetics in 10 subjects: 8 extensive metabolizers and 2 poor metabolizers of CYP2C19. The study consisted of 4-day voriconazole-only and 5-day voriconazole-plus-fluconazole treatments, separated by a 14-day washout. Voriconazole pharmacokinetics were determined by noncompartmental analyses. A physiologically based pharmacokinetic model was developed in Simcyp (Simcyp Ltd., Sheffield, United Kingdom) to predict the magnitude of drug interaction should antifungal therapy be switched from fluconazole to voriconazole, following various simulated lag times for the switch. In CYP2C19 extensive metabolizers, fluconazole increased the maximum plasma concentration and the area under the plasma concentration-time curve (AUC) of voriconazole by 57% and 178%, respectively. In poor metabolizers, however, voriconazole pharmacokinetics were unaffected by fluconazole. The simulations based on pharmacokinetic modeling predicted that if voriconazole was started 6, 12, 24, or 36 h after the last dose of fluconazole, the voriconazole AUC ratios (sequential therapy versus voriconazole only) after the first dose would be 1.51, 1.41, 1.28, and 1.14, respectively. This suggests that the remaining systemic fluconazole would result in a marked drug interaction with voriconazole for ≥24 h. Although no safety issues were observed during coadministration, concomitant use of fluconazole and voriconazole is not recommended. Frequent monitoring for voriconazole-related adverse events is advisable if voriconazole is used sequentially after fluconazole.
INTRODUCTION
The pharmacokinetics of the triazole antifungal voriconazole have been widely studied (7, 19, 20). The drug is primarily metabolized by hepatic cytochrome P450 (CYP) enzymes 2C19 (CYP2C19) and CYP3A4, with in vitro data indicating only minimal involvement of CYP2C9 in its metabolism (3). Genetic polymorphism in CYP2C19 probably accounts for a substantial part of the intersubject variability in voriconazole pharmacokinetics (22, 29, 30). Voriconazole metabolism may also be influenced by the concomitant administration of other drugs interacting with CYP isozymes (11, 25), including the structurally related antifungal agent fluconazole (12).
Voriconazole and fluconazole have similar mechanisms of action, and use of the combination of both drugs does not appear to offer an advantage over use of the single agents (23); therefore, it is unlikely that they would be administered together as combination therapy. However, voriconazole may be used sequentially after fluconazole in clinical practice. This situation is most likely to arise in hematology patients who are receiving fluconazole prophylaxis for the prevention of invasive Candida infections and who subsequently develop invasive aspergillosis, for which voriconazole is regarded the treatment of choice (18, 26, 28). To a lesser extent, it may also occur in patients who are treated for invasive Candida infections later confirmed to be caused by fluconazole-resistant strains. The rationale for a therapeutic switch from fluconazole to voriconazole is supported by in vitro and in vivo data showing good activity of voriconazole against many fluconazole-resistant Candida strains, even though there is some azole cross-resistance (14, 15, 21). In Europe, voriconazole is specifically approved for the treatment of fluconazole-resistant, serious invasive Candida infections (2).
Fluconazole is a potent CYP2C19 inhibitor (27), and its systemic presence could inhibit voriconazole metabolism and potentially increase the frequency of voriconazole-related adverse events. In the treatment of serious and life-threatening fungal infections requiring a switch from fluconazole to voriconazole, it may not be feasible to delay voriconazole therapy to allow sufficient time for fluconazole washout. The elimination half-life of fluconazole is approximately 30 h in adults (16), and fluconazole levels with the potential for inhibiting CYP2C19 activity may therefore be expected for some period of time following fluconazole discontinuation.
To examine these issues, we studied the pharmacokinetics of voriconazole when coadministered with fluconazole to assess the maximal impact of fluconazole-mediated CYP2C19 inhibition on voriconazole levels. A physiologically based pharmacokinetic (PBPK) modeling approach was then employed to predict the extent/magnitude of the drug interaction in situations where voriconazole therapy is initiated after various lag times following fluconazole discontinuation.
MATERIALS AND METHODS
Study design.
This was an open-label, randomized, two-way crossover study involving 10 healthy volunteers conducted in Singapore. Subjects were included if they were male, were aged 21 to 55 years, had a body mass index of 18 to 30 kg/m2, and had a normal resting 12-lead electrocardiogram. Subjects were excluded if they had any clinically significant disease, allergy, or abnormality; were taking or had taken any prescribed or over-the-counter medication (except acetaminophen) within 3 weeks of starting the study; had received any experimental drug within 4 months of starting the study; had evidence of drug abuse or excessive use of alcohol or tobacco; had donated blood within the previous 8 weeks; had positive human immunodeficiency virus, hepatitis B virus, or hepatitis C virus serology; or had known hypersensitivity to azoles. All subjects gave their written informed consent prior to their participation.
The study was divided into two treatments, a 4-day period of voriconazole-only treatment and a 5-day period of voriconazole-plus-fluconazole combination treatment, separated by a 14-day washout phase. The order in which subjects underwent these two treatments was assigned randomly. All treatments were administered orally in a fasted state. Voriconazole was given as a loading dose of 400 mg orally twice daily (every 12 h) on day 1, followed by 200 mg every 12 h on days 2 and 3 and a single 200-mg dose on day 4. Fluconazole was administered as a single loading dose of 400 mg orally on day 1, followed by single doses of 200 mg every 24 h on days 2 to 5. Water (240 ml) was used for the administration of both agents. During the combination treatment phase, fluconazole was administered immediately after the voriconazole dose.
A blood sample was taken at the screening visit to determine the CYP2C19 genotype status of each subject using previously validated methods (33); the testing was conducted by Genaissance Pharmaceuticals, Morrisville, NC. All adverse events and their likely relationship to treatment were recorded throughout the study period. Physical examination, laboratory safety tests, vital signs, and 12-lead electrocardiogram measurements were used to evaluate safety.
Voriconazole pharmacokinetics.
Serial plasma samples for voriconazole pharmacokinetics were collected predosing and at 1, 2, 3, 4, 6, 8, 10, 12, 16, 24, 36, and 48 h after dosing on day 4. Plasma samples were assayed for voriconazole by a previously validated method using automated solid-phase extraction, followed by liquid chromatographic tandem mass spectrometric analysis (1). The lower and upper limits of quantification were 10 and 3,000 ng/ml, respectively.
Voriconazole pharmacokinetic parameters were determined by noncompartmental analyses. Maximum observed plasma concentration (Cmax), time to first occurrence of Cmax (Tmax), area under the plasma concentration-time curve between 0 and 12 h (AUC12), area under the plasma concentration-time curve from time zero to the time of the last measurable concentration (AUCt), and area under the curve to infinity (AUCinf) were determined on day 4 of each treatment period. Cmax and Tmax were obtained directly from recorded data, and AUC12 and AUCt were calculated using the linear trapezoidal rule. AUCinf was calculated using the equation AUCt + (Ct*/kel), where Ct* was the last measurable concentration and kel was the apparent terminal-elimination-phase rate constant. All pharmacokinetic parameter values were calculated using WinNonlin Professional (version 3.2) software (Pharsight, Sunnyvale, CA).
Statistical analyses.
Pharmacokinetic parameters for voriconazole calculated on day 4 of each treatment period were used as endpoints for the statistical analysis. AUCinf, AUCt, AUC12, and Cmax were subjected to analysis of variance appropriate for the two-period, two-treatment crossover design. Tmax was summarized descriptively. Parameters were calculated as mean values with 90% confidence intervals (CIs), ranges, and coefficients of variation (CVs). Each parameter was calculated separately for subjects found to be extensive metabolizers (EMs) or poor metabolizers (PMs) of voriconazole and was compared between voriconazole alone and the voriconazole-plus-fluconazole combination. All statistical analyses were performed using SAS (version 8.02) software (SAS Institute Inc., Cary, NC).
Pharmacokinetic (Simcyp) modeling and simulations.
PBPK modeling and simulations of drug interaction were performed using Simcyp (Simcyp population-based absorption, distribution, metabolism, and elimination [ADME] simulator, version 9.03; Simcyp Ltd., Sheffield, United Kingdom). A Simcyp model for voriconazole was developed using the physicochemical properties, in vitro data, and clinical pharmacokinetic parameters obtained mainly from the in-house database (Table 1). The recombinant enzyme kinetics inputs (maximum reaction velocity [Vmax] and Michaelis-Menten constant [Km]) were means of the values from multiple sources that included in-house data (Pfizer, data on file) and data from published literature (3, 9).
Table 1.
Summary of input parameters for voriconazole used in the Simcyp model
| Parametera | Input valueb | ||
|---|---|---|---|
| Physicochemical properties | |||
| Mol wt (g/mol) | 349 | ||
| Log P | 1.8 | ||
| Compound type | Monoprotic base | ||
| pKa | 1.76 | ||
| Fraction unbound | 0.42 | ||
| Absorption | |||
| Absorption type | First order | ||
| Fraction absorbed | 0.96 | ||
| Absorption rate constant (1/h) | 1.44 | ||
| Caco-2 cell permeation (10−6 cm/s) | 28.10 | ||
| Distribution | |||
| Distribution model | PBPK | ||
| Vss (liters/kg) | 1.079 | ||
| Prediction method | Poulin and Theil (method 1) | ||
| Elimination | |||
| In vitro metabolic system | Recombinant | ||
| Pathway | Pathway 1 | Pathway 1 | Pathway 2 |
| Enzyme | CYP2C19 | CYP3A4 | CYP3A4 |
| Vmax (pmol/min/pmol) | 1.19 | 0.31 | 0.10 |
| Km (μM) | 3.5 | 15.0 | 11.0 |
P, partition coefficient; pKa, acid dissociation constant; Vss, volume of distribution at steady state; Vmax, maximum reaction velocity; Km, Michaelis-Menten constant.
For fluconazole, standard inputs available within Simcyp were utilized. The inhibition constant (Ki) of fluconazole for the CYP2C19 enzyme used for simulating drug-drug interaction was 2.1 μM (31). The literature reports Ki values for fluconazole-mediated CYP3A4 inhibition ranging from 1.9 to 63 μM; therefore, a mean value of 25.2 μM was used as the input for Simcyp modeling (4, 8, 10, 31, 34).
Each simulation was performed for 100 subjects (10 trials × 10 subjects). The virtual population had a body weight of 70 kg, with ages ranging from 18 to 65 years, and included both sexes. The dose, dosing interval, and dosing duration of voriconazole and fluconazole in the simulation were identical to those used in the clinical study. Accordingly, voriconazole alone was dosed every 12 h for 4 days (first two doses of 400 mg and subsequent five doses of 200 mg each), or voriconazole was coadministered with fluconazole (first fluconazole dose of 400 mg and subsequent doses of 200 mg each every 24 h). For simulating sequential dosing, fluconazole dosing remained the same, while voriconazole administration was initiated at times of 6, 12, and 24 h following the last dose of fluconazole.
RESULTS
Study population.
Five subjects (mean age, 29 years; age range, 24 to 43 years) received voriconazole alone followed by voriconazole plus fluconazole, and five (mean age, 24 years; age range, 22 to 27 years) received voriconazole plus fluconazole followed by voriconazole alone. The mean weights in the two groups were 70 kg (range, 63 to 77 kg) and 65 kg (range, 56 to 77 kg), respectively, and the mean heights were 174 cm (range, 170 to 178 cm) and 170 cm (range 161 to 177 cm), respectively. All subjects were Asian and male. Eight subjects were EMs, and two were PMs (four EMs and one PM in each treatment group).
Pharmacokinetic data.
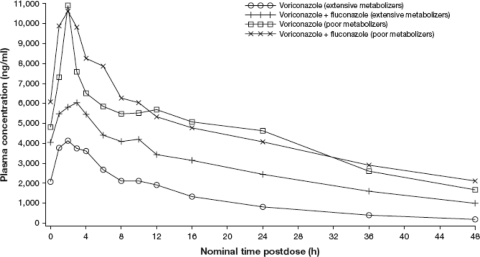
Figure 1 shows the mean plasma voriconazole concentration-time profile by metabolizer type and by treatment group. As expected, voriconazole concentrations in PMs were markedly higher than those in EMs. The Cmax and AUC12 of voriconazole in PMs were approximately 2.7- and 2.5-fold higher, respectively, than those in EMs (Table 2).
Fig. 1.
Mean plasma concentrations of voriconazole over time in extensive and poor metabolizers given voriconazole alone and voriconazole with concomitant fluconazole.
Table 2.
Pharmacokinetic parameters for voriconazole
| Parameter | Voriconazole |
Voriconazole + fluconazole |
Ratio (%) of means (voriconazole plus fluconazole vs voriconazole)a |
90% CI of ratio of means |
||
|---|---|---|---|---|---|---|
| Mean | CV (%) | Mean | CV (%) | |||
| Extensive metabolizers (n = 8) | ||||||
| AUC12 (ng·h/ml)a | 31,024 | 45.6 | 55,538 | 18.7 | 179.0 | 140.8–227.5 |
| AUCt (ng·h/ml)a | 51,483 | 59.4 | 128,648 | 22.9 | 249.9 | 187.6–332.8 |
| AUCinf (ng·h/ml)a | 53,140 | 62.0 | 140,199c | 24.7c | 277.7 | 198.0–389.6 |
| Cmax (ng/ml)a | 4,019 | 48.4 | 6,316 | 27.2 | 157.1 | 119.5–206.7 |
| C12 (ng/ml) | 1,930.7 | 66.2 | 3,441.0 | 20.5 | ||
| Tmax (h)b | 2.0 | 37.8 | 2.6 | 34.9 | ||
| Poor metabolizers (n = 2) | ||||||
| AUC12 (ng·h/ml)a | 77,108 | 4.7 | 91,209 | 4.3 | ||
| AUCt (ng·h/ml)a | 206,000 | 0 | 218,888 | 4.5 | ||
| AUCinf (ng·h/ml)a | 245,927 | 3.4 | NCd | NC | ||
| Cmax (ng/ml)a | 10,889 | 6.5 | 11,449 | 1.9 | ||
| C12 (ng/ml) | 5,682.0 | 5.9 | 5,355.5 | 8.0 | ||
| Tmax (h)b | 2.0 | 0 | 1.5 | 47.1 | ||
Geometric means.
Arithmetic mean.
n = 6.
NC, not calculated.
Coadministration of fluconazole had a different effect on voriconazole pharmacokinetics in EMs and PMs. In EMs, plasma concentrations of voriconazole were increased when coadministered with fluconazole compared with voriconazole alone, while in PMs, the concentration-time profiles were similar during both treatments (Fig. 1). In EMs, Cmax and AUC12 values increased significantly when fluconazole was coadministered with voriconazole compared with when voriconazole was administered alone (Table 2). In PMs, the pharmacokinetic parameters were similar with the two treatments (Table 2).
Coadministration of fluconazole reduced the intersubject variability of voriconazole Cmaxs and AUCs in EMs (Table 2). In this group, the coefficient of variations in voriconazole pharmacokinetic parameters ranged from 18.7 to 34.9% for voriconazole plus fluconazole, whereas they ranged from 37.8 to 62.0% for voriconazole alone.
Simcyp modeling and simulations.
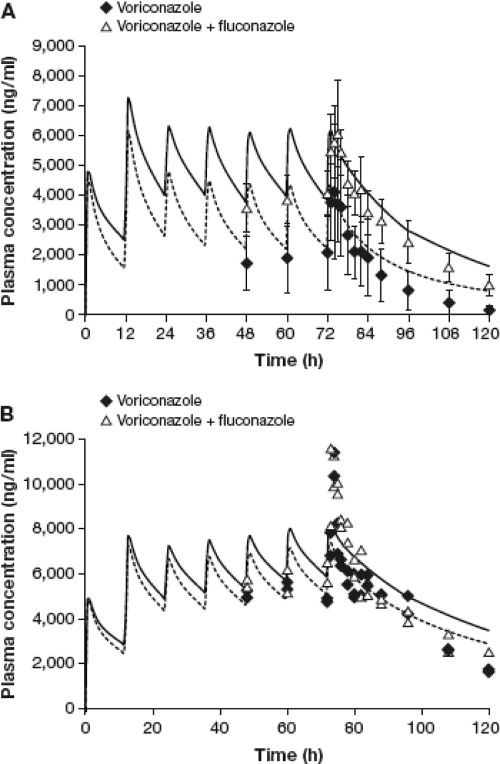
The in vitro data and clinical pharmacokinetic parameters were used to build a PBPK model for voriconazole using Simcyp. Figure 2 shows the predicted plasma concentrations for voriconazole in EMs and PMs using a dosing regimen identical to that used in the clinical study. For EMs, these simulations predicted ratios (voriconazole plus fluconazole versus fluconazole alone) of 2.65 (95% CI, 1.33 to 5.85) and 1.40 (95% CI, 1.14 to 2.20) for voriconazole AUCt and Cmax, respectively. However, no significant effect was found in PMs. These results are in agreement with those observed in the clinical study (Table 2) and confirm the validity of the model.
Fig. 2.
Simulated mean plasma concentrations of voriconazole dosed alone or with concomitant fluconazole in extensive metabolizers (A) and poor metabolizers (B). Data points represent mean ± SD (n = 8) concentrations for extensive metabolizers and individual data (n = 2) for poor metabolizers. Dotted and solid curves represent mean simulated plasma concentrations of voriconazole when dosed alone and with concomitant fluconazole, respectively.
Further simulations were carried out to evaluate the effect of sequential dosing of voriconazole following termination of fluconazole therapy. The doses of both drugs remained the same as in the clinical study, with the exception that voriconazole was initiated sequentially at various time intervals (6, 12, 24, and 36 h) after the last dose of fluconazole. Accordingly, fluconazole was given at a dose of 400 mg on day 1, followed by 200 mg every 24 h on days 2 to 5, and voriconazole was initiated sequentially at doses of 400 mg every 12 h on day 5, followed by 200 mg every 12 h on subsequent days. The pharmacokinetic parameters of interest from these simulations were the Cmax and AUC values for voriconazole after the first loading dose of 400 mg, since this represents double the maintenance dose of 200 mg and could result in higher voriconazole concentrations due to fluconazole-mediated interaction. The predicted Cmax and AUC values for voriconazole and their ratios (voriconazole after fluconazole versus voriconazole alone) are presented in Table 3. The predicted Cmaxs were comparable for both treatments; however, the predicted AUC values for voriconazole were higher when this antifungal was given sequentially after fluconazole than when voriconazole was given alone. For instance, the voriconazole AUC ratio was 1.51 when voriconazole was started 6 h after the last dose of fluconazole, indicating a significant drug interaction. This effect diminished as the lag time for sequential dosing was increased, and at 12 and 24 h, the AUC ratios were 1.41 and 1.28, respectively. Furthermore, the mean effect was reduced to less than 20% (AUC ratio, 1.14) when the lag time for sequential dosing was increased to 36 h. Overall, the simulation results suggest that the inhibitory effect of fluconazole on voriconazole metabolism would last for at least 24 h after the last dose of fluconazole.
Table 3.
Model-predicted pharmacokinetic parameters for the first dose of voriconazole, when given sequentially after fluconazole at various lag times after the last fluconazole dose
| Lag time for starting voriconazole (h) | Pharmacokinetic parametera | Model-predicted value (mean) |
Ratio (95% CI)b | |
|---|---|---|---|---|
| Voriconazole (reference) | Voriconazole + fluconazole (test) | |||
| 6 | Cmax | 4,458 | 4,716 | 1.06 (1.02–1.14) |
| AUC | 60,326 | 84,118 | 1.51 (1.21–1.85) | |
| 12 | Cmax | 4,507 | 4,746 | 1.05 (1.01–1.14) |
| AUC | 58,685 | 77,122 | 1.41 (1.15–1.73) | |
| 24 | Cmax | 4,625 | 4,749 | 1.03 (1.00–1.09) |
| AUC | 57,899 | 70,022 | 1.28 (1.09–1.53) | |
| 36 | Cmax | 4,593 | 4,776 | 1.04 (1.00–1.13) |
| AUC | 58,964 | 67,218 | 1.14 (1.02–1.41) | |
Units for Cmax are ng/ml, and those for AUC are ng·h/ml.
Ratio (95% CI) for test/reference.
Tolerability and safety.
There were no serious adverse events or discontinuations during the study. All 10 subjects had at least one adverse event, with the majority (25/27) being treatment related. Most treatment-related events (26/27) were mild (n = 15) or moderate (n = 11) in intensity. The most common adverse events were photophobia, abnormal vision, headache, alopecia, and diarrhea. The numbers of subjects experiencing an adverse event were similar in both treatment arms, although slightly fewer adverse events were reported during treatment with voriconazole plus fluconazole (14 versus 11 treatment-related adverse events). No apparent clinically significant concerns were identified in safety laboratory tests, vital signs, and electrocardiogram evaluations.
DISCUSSION
The results of this clinical study suggest that concomitant administration of fluconazole and voriconazole significantly increases plasma voriconazole concentrations in CYP2C19 EMs. Although the sample size was small for PMs, the pharmacokinetics of voriconazole appeared to be unaffected by fluconazole in that population. This observation is not surprising, since the innate metabolic activity of CYP2C19 is already impaired in PMs and administration of a CYP2C19 inhibitor is unlikely to cause a further reduction in enzyme activity. On the basis of these observations, concomitant administration of these two antifungals should be avoided. Despite the small sample size and the fact that the study was conducted in healthy volunteers, the overall conclusions should be applicable to the larger patient population. The agreement between the observed pharmacokinetic parameters and those predicted by the PBPK model while simulating the clinical study provided adequate justification for the use of this model. PBPK simulations of sequential use suggested that the influence of fluconazole on systemic levels of voriconazole would persist for at least 24 h after the last dose of fluconazole.
Fluconazole is categorized as a potent inhibitor of CYP2C9 and CPY2C19, as well as a moderate inhibitor of CYP3A4. Fluconazole's effect on voriconazole pharmacokinetics is probably due to its inhibition of CPY2C19, since voriconazole metabolism is mainly mediated by CYP2C19, followed by CYP3A4, with CYP2C9 appearing to play only a minimal role (3, 9). Omeprazole is another potent competitive inhibitor of CYP2C19 (Ki, 3.1 μM), but it has less of an effect on CYP3A4 (Ki, 84 μM) (6). In a previous study, concomitant omeprazole increased the average AUC of voriconazole by about 40% (32); however, the magnitude of this increase does not require adjustments in the voriconazole dose when coadministered with omeprazole (17). In contrast, fluconazole inhibits CYP2C19 and CYP3A4 with Kis of 2.1 μM and 25.2 μM (mean of the values reported in the literature), respectively (4, 8, 10, 31, 34), and hence, it is not unexpected that its overall effect on voriconazole levels is greater than that of omeprazole.
Interestingly, in this population of healthy Asian volunteers, the considerable intersubject variability in voriconazole pharmacokinetics was reduced when voriconazole was coadministered with fluconazole compared with the variability in pharmacokinetics when voriconazole was administered alone. This presumably occurred because the inhibition of CYP2C19 by fluconazole caused EM subjects to behave in a similar fashion to PM subjects in this respect. Nevertheless, the systemic exposure to voriconazole when given along with fluconazole observed in EMs did not exceed that observed in PMs. In clinical practice, voriconazole is administered to both EMs and PMs at an identical dose. On the basis of these results, it is tempting to deduce that concomitant fluconazole could be used as a booster to achieve therapeutic levels of voriconazole with doses lower than those currently recommended, to allow once-daily voriconazole dosing, or to reduce the intersubject variability in voriconazole pharmacokinetics. Although this approach seems attractive, there are several factors that require additional consideration. First, the optimum dose of fluconazole needed to boost voriconazole levels is currently unknown. Second, fluconazole alters the shape of the concentration-time curve and markedly increases voriconazole trough concentrations, which have been linked to a higher incidence of voriconazole-associated toxicity (24). Third, an additional microbiologic benefit with the concomitant use of both drugs is very unlikely (23), especially if the patient has not responded to prior fluconazole therapy. Finally, there is also a greater likelihood that patients will experience drug-drug interactions and adverse events with both agents combined than with either agent alone. Concomitant administration of voriconazole with fluconazole is therefore not recommended at present until additional dose-finding studies are conducted to evaluate the efficacy and safety of specific voriconazole-plus-fluconazole dosing regimens in the relevant therapeutic settings.
No clinically significant safety concerns were noted during coadministration of voriconazole and fluconazole in healthy volunteers in the present study. However, elevated voriconazole plasma levels are associated with an increased likelihood of visual side effects and possible increases in hepatic enzymes (5, 24). Therapeutic drug monitoring of voriconazole suggests that trough concentrations greater than 6 μg/ml may be associated with a higher incidence of adverse events (13). A fluconazole-mediated increase in voriconazole levels would put patients at risk of exceeding this threshold. The PBPK model predicted that in a sequential use scenario, fluconazole would continue to have a substantial effect on voriconazole pharmacokinetics for at least 24 h after fluconazole discontinuation. However, it should be noted that voriconazole accumulates after multiple dosing (7) and that the plasma voriconazole levels seen on the first day of fluconazole-voriconazole sequential therapy are expected to be below those seen at steady state. This would diminish some of the risks associated with a fluconazole-mediated increase in voriconazole levels during sequential use in clinical practice. However, it is prudent to regularly monitor patients for voriconazole-associated adverse events during overlapping exposure to fluconazole, especially if the affected patients receive additional concomitant drugs that inhibit CYP isozymes involved in voriconazole metabolism. This is not an unlikely scenario, given the often serious underlying conditions in patients with systemic fungal infection, requiring the administration of multiple comedications.
In conclusion, the results of this study show that concomitantly administered fluconazole significantly increases plasma levels of voriconazole. Therefore, concomitant use of fluconazole and voriconazole is not recommended until it has been further evaluated in clinical trials. Frequent monitoring for voriconazole-related adverse events is advisable if voriconazole is used sequentially after fluconazole, especially when voriconazole is initiated within 24 h of the last fluconazole dose.
ACKNOWLEDGMENTS
This study was sponsored by Pfizer Inc. Editorial support was provided by Dominik Wolf of Parexel and was funded by Pfizer Inc.
We thank Maurice Dickins (Pfizer) and Sharon Ripp (Pfizer) for their assistance with the pharmacokinetic modeling and simulations.
B. Damle and M. V. Varma are full-time employees of Pfizer Inc. N. Wood was a full-time employee of Pfizer Inc. at the time that the pharmacokinetic study was conducted.
Footnotes
Published ahead of print on 29 August 2011.
REFERENCES
- 1. Andrews E., et al. 2008. Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co-administered in healthy female subjects. Br. J. Pharmacol. 65:531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. European Medicines Agency 2009. Vfend: EPAR–product information (last updated January 2011). European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000387/human_med_001135.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d125 Accessed 11 March 2011 [Google Scholar]
- 3. Hyland R., Jones B. C., Smith D. A. 2003. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab. Dispos. 31:540–547 [DOI] [PubMed] [Google Scholar]
- 4. Iatsimirskaia E., et al. 1997. Metabolism of rifabutin in human enterocyte and liver microsomes: kinetic parameters, identification of enzyme systems, and drug interactions with macrolides and antifungal agents. Clin. Pharmacol. Ther. 61:554–562 [DOI] [PubMed] [Google Scholar]
- 5. Imhof A., Schaer D. J., Schanz U., Schwarz U. 2006. Neurological adverse events to voriconazole: evidence for therapeutic drug monitoring. Swiss Med. Wkly. 136:739–742 [DOI] [PubMed] [Google Scholar]
- 6. Ko J.-W., Sukhova N., Thacker D., Chen P., Flockhart D. A. 1997. Evaluation of omeprazole and lansoprazole as inhibitors of cytochrome P450 isoforms. Drug Metab. Dispos. 25:853–862 [PubMed] [Google Scholar]
- 7. Lazarus H. M., Blumer J. L., Yanovich S., Schlamm H., Romero A. 2002. Safety and pharmacokinetics of oral voriconazole in patients at risk of fungal infection: a dose escalation study. J. Clin. Pharmacol. 42:395–402 [PubMed] [Google Scholar]
- 8. Maurice M., et al. 1992. Effects of imidazole derivatives on cytochromes P450 from human hepatocytes in primary culture. FASEB J. 6:752–758 [DOI] [PubMed] [Google Scholar]
- 9. Murayama N., Imai N., Nakane T., Shimizu M., Yamazaki H. 2007. Roles of CYP3A4 and CYP2C19 in methyl hydroxylated and N-oxidized metabolite formation from voriconazole, a new anti-fungal agent, in human liver microsomes. Biochem. Pharmacol. 73:2020–2026 [DOI] [PubMed] [Google Scholar]
- 10. Nielsen T. L., Rasmussen B. B., Flinois J. P., Beaune P., Brosen K. 1999. In vitro metabolism of quinidine: the (3S)-3-hydroxylation of quinidine is a specific marker reaction for cytochrome P-4503A4 activity in human liver microsomes. J. Pharmacol. Exp. Ther. 289:31–37 [PubMed] [Google Scholar]
- 11. Nivoix Y., et al. 2008. The enzymatic basis of drug-drug interactions with systemic triazole antifungals. Clin. Pharmacokinet. 47:779–792 [DOI] [PubMed] [Google Scholar]
- 12. Niwa T., Shiraga T., Takagi A. 2005. Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol. Pharm. Bull. 28:1805–1808 [DOI] [PubMed] [Google Scholar]
- 13. Pascual A., et al. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201–211 [DOI] [PubMed] [Google Scholar]
- 14. Pemán J., et al. 2006. Voriconazole in the management of nosocomial invasive fungal infections. Ther. Clin. Risk Manag. 2:129–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pfaller M. A., Messer S. A., Hollis R. J., Jones R. N., Diekema D. J. 2002. In vitro activities of ravuconazole and voriconazole compared with those of four approved systemic antifungal agents against 6,970 clinical isolates of Candida spp. Antimicrob. Agents Chemother. 46:1723–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pfizer Inc 2010. Diflucan—U.S. prescribing information (revised January 2010). Pfizer Inc., New York, NY [Google Scholar]
- 17. Pfizer Inc 2010. Vfend—U.S. prescribing information (revised June 2010). Pfizer Inc., New York, NY [Google Scholar]
- 18. Prentice A. G., et al. 2008. Guidelines on the management of invasive fungal infection during therapy for haematological malignancy. BCSH guidelines home page. Pfizer Inc., New York, NY [Google Scholar]
- 19. Purkins L., et al. 2002. Pharmacokinetics and safety of voriconazole following intravenous-to oral-dose escalation regimens. Antimicrob. Agents Chemother. 46:2546–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Purkins L., Wood N., Greenhalgh K., Allen M. J., Oliver S. D. 2003. Voriconazole, a novel wide-spectrum triazole: oral pharmacokinetics and safety. Br. J. Clin. Pharmacol. 56(Suppl.1):10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruhnke M., Schmidt-Westhausen A., Trautmann M. 1997. In vitro activities of voriconazole (UK-109,496) against fluconazole-susceptible and-resistant Candida albicans isolates from oral cavities of patients with human immunodeficiency virus infection. Antimicrob. Agents Chemother. 41:575–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scholz I., et al. 2009. Pharmacokinetics, metabolism and bioavailability of the triazole antifungal agent voriconazole in relation to CYP2C19 genotype. Br. J. Clin. Pharmacol. 68:906–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Segal B. H., Steinbach W. J. 2007. Combination antifungals: an update. Expert Rev. Anti Infect. Ther. 5:883–892 [DOI] [PubMed] [Google Scholar]
- 24. Tan K., Brayshaw N., Tomaszewski K., Troke P., Wood N. 2006. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J. Clin. Pharmacol. 46:235–243 [DOI] [PubMed] [Google Scholar]
- 25. Theuretzbacher U., Ihle F., Derendorf H. 2006. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin. Pharmacokinet. 45:649–663 [DOI] [PubMed] [Google Scholar]
- 26. Thursky K. A., et al. 2008. Recommendations for the treatment of established fungal infections. Intern. Med. J. 38:496–520 [DOI] [PubMed] [Google Scholar]
- 27. Venkatakrishnan K., von Moltke L. L., Greenblatt D. J. 2000. Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin. Pharmacokinet. 38:111–180 [DOI] [PubMed] [Google Scholar]
- 28. Walsh T. J., et al. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327–360 [DOI] [PubMed] [Google Scholar]
- 29. Wang G., et al. 2009. The CYP2C19 ultra-rapid metabolizer genotype influences the pharmacokinetics of voriconazole in healthy male volunteers. Eur. J. Clin. Pharmacol. 65:281–285 [DOI] [PubMed] [Google Scholar]
- 30. Weiss J., et al. 2009. CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J. Clin. Pharmacol. 49:196–204 [DOI] [PubMed] [Google Scholar]
- 31. Wienkers L. C., et al. 1996. Formation of (R)-8-hydroxywarfarin in human liver microsomes. A new metabolic marker for the (S)-mephenytoin hydroxylase, P4502C19. Drug Metab. Dispos. 24:610–614 [PubMed] [Google Scholar]
- 32. Wood, et al. 2003. Effect of omeprazole on the steady-state pharmacokinetics of voriconazole. Br. J. Clin. Pharmacol. 56(Suppl. 1):56–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiao Z.-S., et al. 1997. Differences in the incidence of the CYP2C19 polymorphism affecting the S-methenytoin phenotype in Chinese Han and Bai populations and identification of a new rare CYP2C19 mutant allele. J. Pharmacol. Exp. Ther. 281:604–609 [PubMed] [Google Scholar]
- 34. Yamazaki H., Nakamoto M., Shimizu M., Murayama N., Niwa T. 2010. Potential impact of cytochrome P450 3A5 in human liver on drug interactions with triazoles. Br. J. Clin. Pharmacol. 69:593–597 [DOI] [PMC free article] [PubMed] [Google Scholar]