Abstract
Antiretroviral drugs cross from maternal plasma to breast milk and from breast milk to the infant in different concentrations. We measured concentrations of nelfinavir and its active metabolite (M8) in maternal plasma and breast milk from women and in dried blood spots collected from their infants at delivery and postnatal weeks 2, 6, 14, and 24 in the Kisumu Breastfeeding Study, Kisumu, Kenya. Nelfinavir-based antiretroviral regimens given to mothers as prevention of mother-to-child HIV transmission (PMTCT) do not expose the breast-feeding infant to biologically significant concentrations of nelfinavir or M8.
INTRODUCTION
Maternal antiretroviral medications taken during breast-feeding as prevention of mother-to-child HIV transmission (PMTCT) reduce HIV viral load in the breast milk of nursing mothers (5). Antiretrovirals cross from maternal plasma to breast milk and from breast milk to the infant in different concentrations (4, 5, 7, 8, 9, 10). The Kisumu Breastfeeding study (KiBS) was a phase IIb open-label single-arm PMTCT trial of maternal triple-antiretroviral regimens administered from 34 weeks' gestation to 6 months postpartum while infants exclusively breast-fed. The study was conducted in Kisumu, Kenya, with women and their infants enrolled between July 2003 and November 2006 (11). We demonstrated in a prior KiBS substudy that nevirapine, lamivudine, and zidovudine were present in the breast milk of lactating mothers prescribed those medications and that nevirapine and lamivudine were transferred to infants via breast- feeding in biologically significant concentrations, as evidenced by the emergence of resistance to lamivudine and nevirapine, respectively, among strains of HIV infecting the infants (7, 12). However, breast-feeding HIV-infected infants from the same substudy whose mothers received nelfinavir did not develop protease inhibitor resistance (12). The aim of this study was to describe concentrations of nelfinavir and its active metabolite, hydroxy-t-butylamidenelfinavir (M8), in maternal plasma, breast milk, and infant dried blood spots collected during the administration of nelfinavir to nursing mothers.
MATERIALS AND METHODS
A subset of mothers participating in KiBS with a CD4 count of >250 cells/μl received nelfinavir mesylate (1,250 mg twice daily; Viracept, Hoffman-La Roche Ltd, Germany) and zidovudine-lamivudine (300 mg/150 mg twice daily; Combivir, GlaxoSmithKline, United Kingdom) (11). Maternal plasma, breast milk, and infant dried blood spots were collected at delivery and postnatal weeks 2, 6, 14, and 24 from a nonrandom subset of sequentially enrolled subjects participating in a breast milk substudy (7). Nelfinavir was dispensed in pill bottles with MEMS (medication event monitoring system) caps (Aardex, Ltd., Union City, CA), which were used to determine the dosing times for the last maternal antiretroviral dose prior to sampling; timing was confirmed by pharmacy staff. Prior to analysis, plasma and breast milk specimens were stored at −70°C and dried blood spots were stored at −20°C. Included in this analysis were mother-infant pairs where maternal plasma, breast milk, and infant dried blood spot specimens were available.
Nelfinavir and M8 from plasma and breast milk (100 μl) were extracted with 370 μl of acetonitrile containing 270 ng/ml of internal standard (nelfinavir-d3; Toronto Research Chemicals Inc., Toronto, Canada). After a brief centrifugation to precipitate suspended proteins, the liquid was transferred to a 96-well plate and evaporated to almost dryness in a vacuum concentrator, after which 100 μl of buffer A (0.57 ml glacial acetic acid–0.40 ml formic acid–0.40 ml ammonium hydroxide in 1 liter of water) was then added. The solvent was again evaporated to almost dryness in a vacuum concentrator, and another aliquot of 100 μl of buffer A was added. The sample plate was then sealed and stored at −20°C for future analysis by liquid chromatography-mass spectrometry. Six-millimeter-diameter punches from infant dried blood spot samples were extracted with 370 μl of acetonitrile containing 270 ng/ml of internal standard and sonicated for 30 min at room temperature, and the liquid was removed to a 96-well plate that was then treated in like manner. Standard samples of known concentrations for both nelfinavir and M8 (Toronto Research Chemicals Inc.) were spiked into the appropriate matrices and treated in like manner for the construction of the standard curve used in quantification. Standard and quality control samples for blood spot analysis were created as described by Mei et al. (6). To construct the standard curves, 6-mm-diameter punches from the standard dried blood spot samples were extracted with 370 μl of acetonitrile containing 270 ng/ml of internal standard and sonicated for 30 min at room temperature, and the liquid was removed to a 96-well plate also containing unknown samples and treated as described above. Nelfinavir and M8 are fairly insoluble in aqueous buffers, so recovery from breast milk and plasma was approximately twice as efficient (with 25% variability) as that from purely aqueous medium. Recovery from dried blood spots was enhanced by approximately 50% when acetonitrile was used as the elution buffer, while matrix effects were somewhat less.
Five microliters of the final processed sample was injected onto a 2- by 30-mm Ascentis C8 reverse-phase column (Supelco Inc., Bellefonte, PA) connected to a Shimadzu (Durham, NC) Prominence high-performance liquid chromatography (HPLC) system. An HPLC method consisting of a 3-min gradient from 5% to 90% buffer B (0.57 ml glacial acetic acid–0.40 ml formic acid–0.40 ml ammonium hydroxide in 1 liter of acetonitrile), followed by isocratic runs of 1 min at 90% buffer B and then 3 min at 5% buffer B at a flow rate of 0.5 ml/min, was used. A model 3200 QTrap mass spectrometer (AB Sciex, Foster City, CA) working in the positive multiple-reaction monitoring mode was used to monitor the transitions for the compounds of interest. For nelfinavir, m/z 568.3 → 135.1 and 568.3 → 330.2 were monitored, and for M8, m/z 584.2 → 135.0 and 584.2 → 330.2 were monitored. The limit of quantification (LOQ) for plasma and breast milk was 10 ng/ml, and that for dried blood spots was 5 ng/ml. Assay of infant dried blood spots was discontinued after 28 specimens due to the predominance of samples at less than the LOQ. Inter- and intraday variability for both analytes and each matrix was less than 10% at the LOQ. The linear range for this assay was 0.01 ng/ml to 3,000 ng/ml.
The median nelfinavir and M8 concentrations in plasma and breast milk were calculated for each study visit. The ratio between matched maternal breast milk and plasma concentrations (breast milk/plasma ratio) was calculated for each period. We excluded from the calculation of the breast milk/plasma ratio specimens where the maternal breast milk or plasma concentrations were less than the LOQ. Linear regression and Spearman-rank correlation statistics for trends were used to estimate the change in nelfinavir and M8 concentrations in maternal plasma and breast milk after the most recent nelfinavir dose. Analyses were performed using the SAS software program, version 9.2 (SAS Institute, Cary, NC).
RESULTS
Of the 212 mothers receiving nelfinavir, 26 mothers were in the breast milk substudy, for whom we had 114 stored plasma samples and 104 breast milk samples. These 26 women delivered 27 infants (one mother had twins), which were included as well. At study entry, the median maternal age was 24 years (interquartile range [IQR], 21 to 26) and the median maternal CD4 count was 444 cells/μl (IQR, 362 to 611). Median nelfinavir concentrations in maternal plasma and breast milk peaked at postnatal week 2 (Table 1). Median M8 concentrations peaked in plasma at postnatal weeks 2 and 6. Median breast milk M8 concentrations were less than the LOQ at three of five visits and were no greater than 12 ng/ml at the other two visits (Table 1). The median maternal breast milk/plasma ratio for nelfinavir was 0.12 (IQR, 0.08 to 0.17), and that for M8 was 0.03 (IQR, 0.02 to 0.05). Concentrations of nelfinavir and M8 were less than the LOQ for 20 (71%) of 28 infant dried blood spots tested. Median nelfinavir concentrations were less than the LOQ (range, below LOQ to 30 ng/ml) and M8 concentrations were less than the LOQ (range, below LOQ to 32 ng/ml) for infant dried blood spots.
Table 1.
Median concentrations of nelfinavir and its active metabolite, hydroxyl-t-butylamidenelfinavir (M8), in maternal breast milk and plasma by postpartum week in the Kisumu Breastfeeding Study, Kenya, 2004 to 2007a
| Parameter | Value at delivery or postpartum wkd |
||||
|---|---|---|---|---|---|
| Delivery | Wk 2 | Wk 6 | Wk 14 | Wk 24 | |
| Median time (h) since last dose of nelfinavir until plasma drawnb (IQR) | 3.6 (2.4-5.0) | 5.2 (3.7-6.9) | 5.3 (3.3-7) | 6.6 (4.6-7.9) | 7.6 (6.7-8.8) |
| Nelfinavir concn [ng/ml] (IQR) in: | |||||
| Maternal plasmac | 2,004 (1,083–3,391) | 3,593 (1,920–4,165) | 2,490 (1,687–3,463) | 1,976 (1,194–2,985) | 1,990 (1,248–2,720) |
| Breast milk | 83 (62–253) | 358 (200–472) | 286 (210–543) | 233 (105–442) | 180 (79–260) |
| M8 concn [ng/ml] (IQR) in: | |||||
| Maternal plasma | 214 (104–337) | 514 (280–641) | 516 (243–737) | 425 (181–597) | 405 (185–781) |
| Breast milk | <LOQ (<LOQ) | 13 (<LOQ–21) | 12 (<LOQ–32) | <LOQ (<LOQ–19) | <LOQ (<LOQ–17) |
The LOQ for nelfinavir and M8 in both maternal plasma and breast milk is 10 ng/ml. This table includes concentrations where the time since last dose was less than 24 h. Time since last dose was >24 h for four plasma and four breast milk specimens and was not available for three plasma and seven breast milk specimens.
Time of obtaining breast milk was typically within 10 to 30 min of plasma draw.
For comparative purposes, the target plasma trough concentration of nelfinavir is 800 ng/ml.
Samples analyzed are as follows: at delivery, 20 plasma samples and 11 breast milk samples; at week 2, 26 plasma samples and 23 breast milk samples; at week 6, 26 plasma samples and 24 breast milk samples; at week 14, 24 plasma samples and 24 breast milk samples; at week 24, 11 plasma samples and 11 breast milk samples.
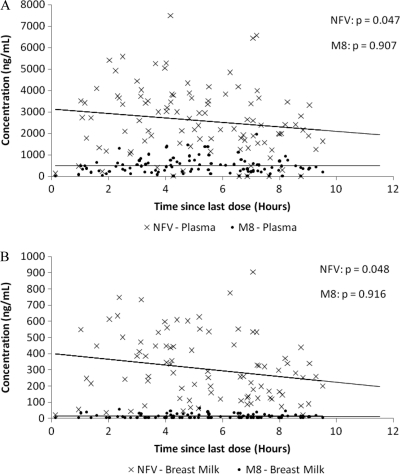
After the most recent nelfinavir dose, nelfinavir concentrations in the plasma and breast milk trended downward, but M8 concentrations were stable over the ensuing 12-h dose interval (Fig. 1A and B). During the 12-h interval after the most recent nelfinavir dose, the maternal breast milk/plasma ratio did not vary for either nelfinavir (P = 0.55) or M8 (P = 0.64).
Fig. 1.
Concentrations of nelfinavir (NFV) and its active metabolite, hydroxyl-t-butylamidenelfinavir (M8), after dosing of nelfinavir mesylate (1,250 mg given orally twice daily) in maternal plasma (A) or breast milk (B) in the Kisumu Breastfeeding Study, Kenya, 2004 to 2007. Dashed lines are regression lines for nelfinavir, and solid lines are regression lines for M8. The P values represent the probability that the slope of the line is different from zero as calculated by Spearman-rank correlation statistics for trends. The changes in maternal plasma and breast milk concentrations of nelfinavir over a 12-h dosing interval were statistically significant at a significance level of 0.05.
DISCUSSION
Nelfinavir and its active metabolite (M8) are present in breast milk at low concentrations and are passed on to breast-feeding infants in negligible quantities. Our findings regarding the breast milk/plasma ratio for nelfinavir are consistent with those of other studies, which found this ratio to be 0.06 to 0.24 (4, 8). Studies of nelfinavir have typically calculated maternal breast milk/plasma ratios, which may underestimate the actual proportion of drug transferred to breast milk, and pharmacokinetic studies using a ratio calculated from the area under the curve may better characterize the proportion of drug transferred (1). Regardless, our findings indicate that an infant receives a negligible dose of nelfinavir through breast milk. For instance, a 2-week-old infant at the 50th percentile for weight (3,750 g) taking an average meal of 3.5 oz would receive 0.01 mg/kg nelfinavir per dose or a total daily dose of 0.06 mg/kg, which is <1% of the recommended treatment dose of nelfinavir for an infant of that weight. This dose would decrease as the infant grows and the level of nelfinavir in breast milk declines following the postpartum peak in maternal concentration at 2 weeks postpartum. This is confirmed by our finding that nelfinavir was predominantly undetectable in infants nursed by mothers receiving nelfinavir, and it likely explains the lack of emergence of resistance to protease inhibitors among HIV-infected breast-feeding infants whose mothers are taking nelfinavir (12). The minimum plasma trough concentration of nelfinavir considered therapeutic is 800 ng/ml; the concentrations in infants we observed were much lower (3). For comparison, lamivudine concentrations in breast-fed infants whose mothers are taking lamivudine are just above the detectable range but are near the MIC for HIV, which is the likely explanation for the common emergence of resistance to lamivudine in HIV-infected infants (7, 12). Our study also demonstrates the transfer of M8 into breast milk. However, despite M8's activity against HIV, no biological effect would be expected in the breast-feeding infant due to the low concentration of M8 in breast milk and minimal amount transferred to the breast-feeding child. Others have demonstrated placental transfer of nelfinavir to be low, with median cord blood levels of 70 ng/ml or ∼5% of maternal plasma nelfinavir concentrations, indicating low exposure to nelfinavir among infants in utero as well (2).
Antiretroviral drugs transfer from plasma to breast milk in different concentrations. Lamivudine and nevirapine transfer into breast milk and to breast-feeding infants in quantities that have biological significance (5, 7, 10). This likely explains the emergence of resistance to these drugs in breast-feeding HIV-infected children of mothers who take those drugs (12). Efavirenz has also been demonstrated to transfer to breast-feeding infants in biologically significant concentrations (9). Zidovudine appears to transfer to breast-feeding infants in low quantities (7).
Nelfinavir-based antiretroviral regimens given to mothers as PMTCT do not expose their breast-feeding infants to biologically significant concentrations of nelfinavir or its active metabolite (M8). Therefore, the risk is low that HIV resistance mutations against protease inhibitors will emerge among HIV-infected breast-feeding infants whose mothers are taking nelfinavir. In humans, the transfer of other protease inhibitors from maternal plasma to breast milk and to breast-feeding infants has not been well studied. Design of antiretroviral regimens for pregnant and nursing women should take into account the extent of antiretroviral transfer into breast milk, which may affect the future treatment options for the HIV-infected infant.
ACKNOWLEDGMENTS
We thank the Kisumu Breastfeeding Study participants, the Kisumu Breastfeeding Study Team, the Kenya Medical Research Institute (KEMRI), the KEMRI/CDC Research and Public Health Collaboration, and the Kenyan Ministry of Health.
The authors made the following contributions: P. J. Weidle, C. Zeh, A. Martin, and T. K. Thomas, concept; P. J. Weidle, C. Zeh, A. Martin, R. Lando, F. Angira, J. Osoga, P. Ogindo, and T. K. Thomas, study design; and P. J. Weidle, A. Martin, S. Girde, and T. D. Minniear, analysis. Weidle and Minniear led the writing, though all authors contributed to writing the article.
We have no conflict of interest to report.
The protocol was approved by the Institutional Review Boards of the Kenya Medical Research Institute and the U.S. Centers for Disease Control and Prevention. This article is published with the permission of the director of KEMRI.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the CDC. Use of trade names is for identification purposes only and does not constitute endorsement by the CDC or the Department of Health and Human Services.
Funding was provided by the U.S. Centers for Disease Control and Prevention (CDC). CDC staff participated in the design, data collection, analysis, and interpretation of the data, the writing of the report, and the decision to submit the article for publication.
Footnotes
Published ahead of print on 29 August 2011.
REFERENCES
- 1. Breitzka R. L., Sandritter T. L., Hatzopoulos F. K. 1997. Principles of drug transfer into breast milk and drug distribution in the nursing infant. J. Hum. Lact. 13:155–158 [DOI] [PubMed] [Google Scholar]
- 2. Bryson Y. J., et al. 2008. Pharmacokinetics and safety of nelfinavir when used in combination with zidovudine and lamivudine in HIV-infected pregnant women: pediatric AIDS clinical trials group (PACTG) 353 team. HIV Clin. Trials 9(2):115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burger D. M., et al. 2004. Maintaining the nelfinavir trough concentrations above 0.8 mg/L improves virologic response in HIV-1-infected children. J. Pediatr. 145:403–405 [DOI] [PubMed] [Google Scholar]
- 4. Colebunders R., et al. 2005. The effect of highly active antiretroviral treatment on viral load and antiretroviral drug levels in breast milk. AIDS 19:1912–1915 [DOI] [PubMed] [Google Scholar]
- 5. Giuliano M., et al. 2007. Triple antiretroviral prophylaxis administered during pregnancy and after delivery significantly reduces breast milk viral load. J. Acquir. Immune Defic. Syndr. 44:286–291 [DOI] [PubMed] [Google Scholar]
- 6. Mei J. V., et al. 1998. Radioimmunoassay for monitoring zidovudine in dried blood spot specimens. Clin. Chem. 44:281–286 [PubMed] [Google Scholar]
- 7. Mirochnick M., et al. 2009. Antiretroviral concentrations in breast-feeding infants of mothers receiving highly active antiretroviral therapy. Antimicrob. Agents Chemother. 53:1170–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rezk N. L., et al. 2008. Studies on antiretroviral drug concentrations in breast milk: validation of a liquid chromatography-tandem mass spectrometric method for the determination of 7 anti-human immunodeficiency virus medications. Ther. Drug Monit. 30:611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schneider S., et al. 2008. Efavirenz in human breast milk, mothers', and newborns' plasma. J. Acquir. Immune Defic. Syndr. 48:450–454 [DOI] [PubMed] [Google Scholar]
- 10. Shapiro R. L., et al. 2005. Antiretroviral concentrations in breast-feeding infants of women in Botswana receiving antiretroviral treatment. J. Infect. Dis. 192:720–727 [DOI] [PubMed] [Google Scholar]
- 11. Thomas T. K., et al. 2011. A trial of triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding: the Kisumu Breastfeeding Study, Kenya. PLoS Med. 8(3):e1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeh C., et al. 2011. HIV-1 drug resistance emergence among breastfeeding infants born to HIV-infected mothers taking HAART for prevention of mother-to-child transmission. PLoS Med. 8(3):e1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]