Abstract
We evaluated the efficacy of a single intravenous dose peramivir for treatment of influenza B virus infection in ferrets and cynomolgus macaques in the present study. A single dose of peramivir (60 mg/kg of body weight) given to ferrets on 1 day postinfection with influenza B virus significantly reduced median area under the curve (AUC) virus titers (peramivir, 8.3 log10 50% tissue culture infective doses [TCID50s]·day/ml; control, 10.7 log10 TCID50s·day/ml; P < 0.0001). Furthermore, nasal virus titers on day 2 postinfection in ferrets receiving a single injection of peramivir (30 mg/kg) and AUCs of the body temperature increase in ferrets receiving a single injection of peramivir (30 and 60 mg/kg) were lower than those in ferrets administered oral oseltamivir phosphate (30 and 60 mg/kg/day twice daily for 3 days). In macaques infected with influenza B virus, viral titers in the nasal swab fluid on days 2 and 3 postinfection and body temperature after a single injection of peramivir (30 mg/kg) were lower than those after oral administration of oseltamivir phosphate (30 mg/kg/day for 5 days). The two animal models used in the present study demonstrated that inhibition of viral replication at the early time point after infection was critical in reduction of AUCs of virus titers and interleukin-6 production, resulting in amelioration of symptoms. Our results shown in animal models suggest that the early treatment with a single intravenous injection of peramivir is clinically recommended to reduce symptoms effectively in influenza B virus infection.
INTRODUCTION
Peramivir is an influenza neuraminidase (NA) inhibitor for intravenous administration, and it has been introduced into clinical practice for adult and child patients in Japan. Peramivir has potent activity against various influenza A and B viruses, including highly pathogenic avian influenza (HPAI) viruses and pandemic (H1N1) 2009 influenza virus in in vitro and in vivo mouse models (2, 6, 7, 10). Phase II clinical trials of intravenous peramivir in Japanese patients with seasonal influenza demonstrated the efficacy and safety of a single intravenous treatment (24). On the basis of these results, the United States Food and Drug Administration issued an Emergency Use Authorization for intravenous peramivir exclusively for patients hospitalized due to influenza associated with pandemic (H1N1) 2009 virus (4).
Influenza B virus causes seasonal influenza virus infection and clinical symptoms indistinguishable from those caused by influenza A virus, though pandemic influenza A virus infection attracts more attention than does influenza B virus infection (26, 29, 30). Influenza B virus was isolated more frequently and for a longer period after antiviral therapy than influenza A virus (21). Furthermore, influenza B virus epidemics did not diminish after the pandemic of 2009 H1N1 influenza A virus (34). Therefore, treatment for influenza B virus infection as well as for influenza A virus infection is important. NA inhibitors are major antiviral agents of influenza B virus infection because the available M2 inhibitors, amantadine and rimantadine, are not active against influenza B viruses since the amino acid sequences of the influenza B virus M2 protein differ from those of the influenza A virus M2 protein (31). Nonetheless, some studies have claimed that an NA inhibitor, oseltamivir, was less effective in treating influenza B virus infection than influenza A virus infection with regard to duration of fever, irrespective of patient age or the timing of administration of the first dose (21, 32). The difference would be explained by higher 50% inhibitory concentration (IC50) values of oseltamivir against the NA activity of influenza B viruses than those against the NA activity of influenza A viruses (33). In addition, influenza B viruses with low susceptibility to NA inhibitors were isolated from immunocompromised children treated with oseltamivir or zanamivir (11, 12). Therefore, further development and evaluation of clinical efficacy of antiviral drugs against influenza B virus are required.
The appearance of influenza B virus is variable season by season compared with influenza A virus, which tends to appear almost every year (20), and preexisting immunity against influenza B virus in humans may make evaluation of drug effects complicated. Due to the shortage of human patients infected with influenza B virus and of preexisting immunity in clinical trials, studies using immunologically naïve animals would offer an experimental approach for testing the efficacies of antiviral drugs against influenza B virus. Although mice are naturally resistant to most seasonal human influenza viruses, unless the viruses are adapted, ferrets are susceptible to human influenza virus, including various influenza A and B viruses, without adaptation (14, 16, 17, 19, 28). Human influenza viruses infect the upper respiratory tract in ferrets and cause clinical symptoms, including fever, nasal congestion, anorexia, and sneezing. Therefore, ferrets are often used for examining the efficacies of antiviral agents in treatment and prevention of influenza virus infection (9, 25, 35). Nonhuman primates are also susceptible to unadapted human influenza viruses (17). Since the clinical symptoms of infection with influenza B viruses observed in cynomolgus macaque closely reflect the signs of disease observed in humans (23), we investigated the therapeutic efficacy of a new NA inhibitor, peramivir, against influenza B virus infection using not only ferrets but also cynomolgus macaques.
In the present study, the efficacy of a single intravenous administration of peramivir was compared with that of multiple oral administrations of oseltamivir phosphate in ferret and macaque models of influenza B virus infection. We demonstrated that a single intravenous injection of peramivir was more effective for reduction of viral titers and amelioration of symptoms in ferrets and cynomolgus macaques after influenza B virus infection than administration of oseltamivir phosphate. These results suggest that peramivir has potential as a treatment against influenza B virus infection.
MATERIALS AND METHODS
Compounds.
Peramivir was synthesized by BioCryst Pharmaceuticals (Birmingham, AL). Oseltamivir phosphate was purchased from Sequoia Research Products (Oxford, United Kingdom), and oseltamivir carboxylic acid was purchased from Toronto Research Chemicals (Ontario, Canada).
Viruses and cells.
Influenza B viruses B/SendaiH/1051/2007 and B/Kadoma/1/2005 were kindly provided by Sendai Medical Center and Osaka Prefectural Institute of Public Health, respectively. Madin-Darby canine kidney (MDCK) cells were obtained from the American Type Culture Collection (Manassas, VA) and were grown in minimum essential medium (MEM; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen) and 100 μg/ml kanamycin sulfate (Invitrogen) in a humidified atmosphere of 5% CO2 at 37°C.
Ferrets.
Approximately 9- to 11-month-old female ferrets (Japan SLC Inc., Shizuoka, Japan) were used. All ferret studies were conducted under applicable laws and guidelines and after approval from the Shionogi Animal Care and Use Committee. Under anesthesia, at least 1 week before virus inoculation, a data logger (DS1921H-F5; Maxim Integrated Products, Inc., Sunnyvale, CA) was implanted in the peritoneal cavity of each ferret to monitor body temperature every 15 min. The absence of influenza B virus-specific antibody in sera was confirmed before experiments using the hemagglutination inhibition (HI) test.
Cynomolgus macaques.
Approximately 2- to 5-year-old female cynomolgus macaques from the Philippines (Ina Research Inc., Ina, Japan) were used for challenge experiments. Macaque studies were conducted under applicable laws and guidelines and after approval from the Shiga University of Medical Sciences Animal Experiment Committee and Biosafety Committee. Under anesthesia, at least 1 week before virus inoculation, a telemetry probe (TA10CTA-D70; Data Sciences International, St. Paul, MN) was implanted in the peritoneal cavity of each macaque to monitor body temperature every 15 min. The absence of influenza B virus-specific antibody in sera was confirmed before experiments using the HI test. Individual macaques are distinguished by identification numbers. The macaques used in this study did not carry herpes B virus, hepatitis E virus, Mycobacterium tuberculosis, Shigella spp., Salmonella spp., or Entamoeba histolytica.
NA inhibition assay.
Whole viruses inactivated by NP-40 were used as a source of NA activity. 2′-(4-Methylumbelliferyl)-α-d-N-acetylneuraminic acid (MUNANA; Sigma-Aldrich, St. Louis, MO) was used as substrate. The virus, a compound, and MUNANA (final concentration, 10 mM) were mixed in reaction buffer and incubated at 37°C for 30 min. The fluorometric intensity of 4-methylumbelliferone released from MUNANA was measured, the percent inhibition at each concentration of drug was determined, and the IC50 was determined. The results are reported as the average of three experiments.
PK analysis.
Female ferrets and cynomolgus macaques (three animals per group) were given peramivir (30 mg/kg of body weight) intravenously or oseltamivir phosphate (30 mg/kg) orally. Blood samples were collected from 0.083 h to 24 h after dosing and centrifuged to obtain plasma. The concentrations of peramivir and oseltamivir carboxylate in plasma were determined by liquid chromatography-tandem mass spectrometry (LC/MS/MS). Pharmacokinetic (PK) parameters were calculated, and modeling was performed using WinNonlin software (version 4.0; Pharsight Corp., Mountain View, CA).
Antiviral study in a ferret model.
Under anesthesia, ferrets were inoculated intranasally with B/Kadoma/1/2005 (300 tissue culture infective doses [TCID50s]) in 200 μl of phosphate-buffered saline (PBS). Four and six ferrets were used in treated groups and an untreated control group, respectively. Six groups were compared in the study using ferrets, as follows: (i) peramivir (30 mg/kg) in saline was administered once intravenously 1 day after infection (P30×1). (ii) Peramivir (60 mg/kg) in saline was administered once intravenously 1 day after infection (P60×1). (iii) Peramivir (30 mg/kg/day) in saline was administered once a day for 3 days from day 1 to day 3 after infection (P30×3). (iv) Oseltamivir phosphate (30 mg/kg/day) in 0.5% methylcellulose solution (MC) was administered orally twice a day for 3 days from day 1 to day 3 (O30×3). (v) Oseltamivir phosphate (60 mg/kg/day) in 0.5% MC was administered orally twice a day for 3 days from day 1 to day 3 (O60×3). The first administration of peramivir and oseltamivir phosphate was performed on day 1 postinfection (p.i.). (vi) Control animals inoculated with virus but not treated with any reagents were orally administered 0.5% MC twice a day from day 1 to day 3.
To monitor virus replication in nasal cavities, nasal washes were collected from infected ferrets on days 1 to 4 p.i. Collected samples were stored at a temperature below −80°C until use. For virus titration, serial dilutions of nasal washes were inoculated onto confluent MDCK cells in 96-well plates. After a 1-h incubation, the suspension was removed and the cells were cultured in MEM including 0.5% bovine serum albumin (BSA; Sigma-Aldrich) and 3 μg/ml trypsin. The plates were incubated at 37°C in 5% CO2 for 3 days. The presence of cytopathic effects (CPEs) was determined under a microscope, and viral titers were calculated as log10 TCID50/ml. When no CPE was observed using undiluted viral solution, it was defined as the undetectable level, which was considered to be lower than 1.4 log10 TCID50s/ml. Inflammatory cells in nasal washes were counted microscopically in a hemocytometer. The protein concentration in cell-free nasal washes was measured by using a protein reagent (Wako Pure Chemicals, Osaka, Japan). Body temperature was expressed by calculating the average temperatures during nighttime (10 p.m. to 8 a.m.) to avoid the effect of anesthesia and was compared with that before virus inoculation.
Antiviral study in a cynomolgus macaque model.
Under anesthesia, cynomolgus macaques were inoculated intranasally with B/SendaiH/1051/2007 (2 × 105 TCID50s) in 1 ml of PBS. Three macaques were used in each group. Four groups were compared in the macaque study, as follows: (i) peramivir (30 mg/kg) in saline was administered intravenously once immediately after virus inoculation, on day 0 (P30-d0). (ii) Peramivir (30 mg/kg) in saline was administered intravenously on day 1 p.i. (P30-d1). (iii) Oseltamivir phosphate (30 mg/kg/day) in 0.5% MC was administered orally once a day for 5 days from day 0 to day 4 (O30×5). The first administration of oseltamivir phosphate was performed immediately after virus inoculation. (iv) MC (0.5%) was orally administered from day 0 to day 4 to control animals inoculated with virus but not treated with any reagents.
On day 0 before virus inoculation and days 1 to 12 after virus inoculation, the macaques were anesthetized and then nasal swab samples were collected with two cotton sticks (TE8201; Eiken Chemical, Ltd., Tokyo, Japan), and the sticks were subsequently immersed in 1 ml of PBS containing 0.1% BSA and penicillin-streptomycin. Blood samples were collected for measuring anti-HA antibody responses in sera. Collected samples were stored at a temperature below −80°C until use. Virus titers in nasal swab fluid were determined using MDCK cells as described above. Levels of inflammatory cytokines and chemokines (interleukin-6 [IL-6], tumor necrosis factor alpha [TNF-α], and monocyte chemotactic protein 1 [MCP-1]) in the nasal swab fluid were assessed using a cytometric bead assay (Becton Dickinson and Company, Franklin Lakes, NJ) according to the manufacturer's instructions. Results of assays were analyzed using FCAP array software (Becton Dickinson and Company). Body temperature was expressed by calculating the averages of the highest and lowest temperatures over 1 day, and the body temperature after virus inoculation was compared with that before virus inoculation.
HI assay.
Sera were treated with receptor-destroying enzyme (RDEII; Denka Seiken, Tokyo, Japan). Serially diluted sera were mixed with 4 hemagglutination (HA) units of virus antigen for 1 h at room temperature. The mixture was then incubated with 0.5% chicken red blood cells for 30 min at room temperature. The hemagglutination inhibition (HI) titers were expressed as reciprocals of the highest dilution of serum samples that completely inhibited hemagglutination.
Statistical analysis.
Virus titers, cytokine levels, and the body temperature of each animal were calculated as the area under the curve (AUC) by the trapezoidal method. Differences of AUCs for virus titers, cytokine levels, and body temperature change were analyzed by Dunnett's multiple-comparison method. Dunnett's test was also carried out for comparison of the virus titers, body temperature, body weight, and the number of inflammatory cells on each day. The efficacy of peramivir was compared with that of oseltamivir by using Student's t test. Statistical analysis was performed using the statistical analysis software SAS (version 9.2) for Windows (SAS Institute, Cary, NC). P values below 0.05 were considered statistically significant.
Sequence analysis of NA genes.
Viral RNA was isolated directly from nasal swab fluid of infected macaques or nasal wash fluid of infected ferrets by using an RNeasy minikit (Qiagen, Dusseldorf, Germany). The NA region of influenza virus was amplified by PCR using a OneStep reverse transcription-PCR kit (Qiagen) and specific primers. The primers for amplification of the NA included a forward primer (5′-ATGCTACCTTCAACTGTACAAAC-3′ or 5′-CCTAAGAACACAAGAAAGTGCC-3′) and a reverse primer (5′-GCCAGCAATAAAACCATAGAATG-3′ or 5′-CACAGGTGTTGATATGGCTCTGTAA-3′). The amplified DNA was sequenced in an Applied Biosystems 3730xl DNA analyzer by the Takara sequencing service. The sequences of the NA region derived from isolated viruses were compared with those of the inoculation viruses, and amino acid substitutions were analyzed.
RESULTS
Sensitivity of influenza B virus NA to NA inhibitors in vitro.
Initially, we examined the sensitivity of B/Kadoma/1/2005 and B/SendaiH/1051/2007 neuraminidases to peramivir and oseltamivir carboxylate in vitro (Table 1). Since virus sensitivity to NA inhibitors in vivo is not always correlated with that analyzed in cell culture, we determined enzyme IC50s (27). The IC50s of peramivir against the NA activity of B/Kadoma/1/2005 and B/SendaiH/1051/2007 were 1.78 ± 0.08 nM and 4.26 ± 0.17 nM, respectively. On the other hand, the IC50s of oseltamivir carboxylate against the NA activity of B/Kadoma/1/2005 and B/SendaiH/1051/2007 were 5.93 ± 0.51 nM and 13.1 ± 0.50 nM, respectively. These values were comparable to previous results, which showed that the IC50s of peramivir against the NA activity of other strains of influenza B virus were between 0.60 and 10.8 nM and the IC50s of oseltamivir carboxylate were from 5.0 to 24.3 nM (3). Regarding these two influenza B viruses, the inhibitory activity of peramivir was more potent than that of oseltamivir carboxylate (P < 0.001).
Table 1.
Sensitivity of influenza B virus to NA inhibitors
| Strain | Mean IC50 ± SD (nM)a |
|
|---|---|---|
| Peramivir | Oseltamivir carboxylate | |
| B/Kadoma/1/2005 | 1.78 ± 0.08 | 5.93 ± 0.51 |
| B/SendaiH/1051/2007 | 4.26 ± 0.17 | 13.1 ± 0.50 |
Each data point represents the mean ± SD of at least three determinations.
Pharmacokinetics of peramivir and oseltamivir carboxylate in ferrets and cynomolgus macaques.
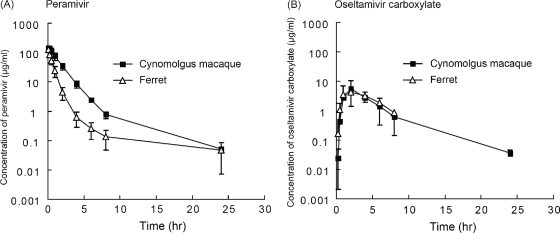
Pharmacokinetic analysis of peramivir in ferrets and cynomolgus macaques showed rapid uptake into the circulation following intravenous injection (Fig. 1). After administration of 30 mg/kg, peramivir concentrations in ferrets and macaques, on average, were 126 μg/ml and 147 μg/ml, respectively, at 0.083 h and then decreased to 0.14 μg/ml and 0.76 μg/ml, respectively, at 8 h. The maximum concentrations in plasma (Cmax) were calculated to be 194 μg/ml in ferrets and 160 μg/ml in macaques (Table 2). The AUC of the plasma concentration of peramivir after injection of 30 mg/kg in the macaques (234 μg·h/ml) was higher than that observed in ferrets injected with 30 mg/kg peramivir (89.1 μg·h/ml). On the other hand, mean times to the maximum concentration (Tmax) of oseltamivir carboxylate were 2.67 h and 3 h in ferrets and macaques, respectively. The AUC of the plasma concentration of oseltamivir carboxylate after injection of 30 mg/kg in ferrets (23.7 μg·h/ml) was almost equivalent to that in macaques (25.4 μg·h/ml).
Fig. 1.
Pharmacokinetic analysis in ferrets and cynomolgus macaques. Female ferrets and cynomolgus macaques (three per group) were given peramivir (30 mg/kg) intravenously (A) or oseltamivir phosphate (30 mg/kg) orally (B). Blood samples were collected at the indicated time points, and concentrations of peramivir and oseltamivir carboxylate were analyzed by LC/MS/MS. Means ± standard deviations are shown in semilogarithmic plots.
Table 2.
Summary of pharmacokinetic parameters for peramivir and oseltamivir carboxylate in plasma
| Drug | Animal | Route | Dose (mg/kg) | Mean ± SD for indicated parametera |
||
|---|---|---|---|---|---|---|
| Cmax (μg/ml)b | Tmax (h) | AUC0-∞c (μg·h/ml) | ||||
| Peramivir | Ferret | Intravenous | 30 | 194 ± 56 | NAd | 89.1 ± 21.9 |
| Cynomolgus macaque | 160 ± 23 | NA | 234 ± 45 | |||
| Oseltamivir carboxylate | Ferret | Oral | 30 | 4.38 ± 0.68 | 2.67 ± 1.15 | 23.7 ± 2.7 |
| Cynomolgus macaque | 6.44 ± 3.18 | 3.0 ± 1.0 | 25.4 ± 2.0 | |||
Means and SDs of data from three animals are shown.
Cmax was calculated as the concentration at time zero for the intravenous dose.
AUC0-∞, area under the concentration-time curve from 0 h to infinity.
NA, not applicable.
We have confirmed the low toxicity of peramivir in cynomolgus macaques. When peramivir was administered daily by intravenous infusion to macaques at a dose of 720 mg/kg/day for 30 days, which was the maximum feasible dose, no toxic findings were evident according to clinical observations, body weights, levels of food consumption, hematology and urinalysis results, organ weights, or histopathology findings (data not shown).
Efficacy of peramivir against influenza B virus infection in ferrets.
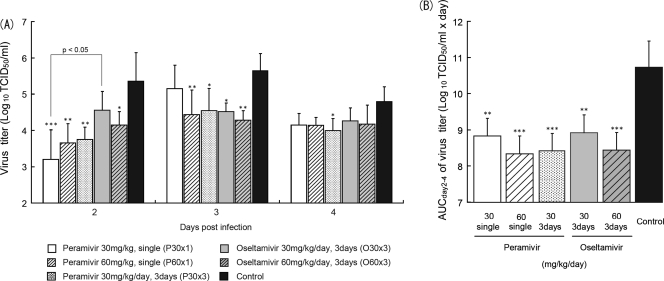
To evaluate the efficacy of peramivir and oseltamivir against influenza B virus in vivo, peramivir or oseltamivir phosphate was administered on day 1 p.i. into ferrets infected intranasally with B/Kadoma/1/2005 (300 TCID50s/ferret), the titer of which in the nasal samples of ferrets was the highest among the various clinical isolates examined (data not shown) and which induced fever in ferrets; thereafter, virus titers in nasal washes were examined. No viruses were detected in nasal washes of 6 control ferrets on day 1 p.i., the maximum virus titer in the control group was observed on day 3 p.i., and then the virus titer declined (Fig. 2 A). According to these results in control ferrets, we focused the analysis on days 2, 3, and 4 p.i. to examine the effects of peramivir and oseltamivir on virus replication in the early phase after infection. On day 2 p.i., virus titers in all groups treated with peramivir were significantly reduced compared with the titer in the control group (P < 0.01), but no significant difference on days 3 and 4 p.i. was observed in the group treated with peramivir (30 mg/kg) once (P30×1). On day 3 p.i., a significant reduction in virus titers was also observed in groups P60×1 and P30×3 compared with the control group. A significant reduction in virus titers on day 2 p.i. was observed in the group treated with oseltamivir (60 mg/kg/day group O60×3) compared with the control group (P < 0.05) but not in group O30×3. In addition, on day 3 p.i., virus titers in the two groups treated with oseltamivir phosphate were significantly lower than the titer in the control group (P < 0.05). Comparing the efficacy between peramivir and oseltamivir, the virus titer on day 2 p.i. in group P30×1 was significantly lower than that in group O30×3 (P < 0.05).
Fig. 2.
Virus titers in nasal washes of infected ferrets treated with peramivir or oseltamivir phosphate. The nasal cavities of ferrets were inoculated with B/Kadoma/1/2005. Nasal washes were collected for virus titration on days 1 to 4 p.i. (A) Each plot represents the mean ± standard deviation of at least 4 animals. On day 1 p.i., no virus was detected in nasal washes except those from one ferret treated with a single administration of 30 mg/kg of peramivir. A significant reduction in virus titers is observed in groups administered peramivir once (30 mg/kg; P30×1) on day 2 p.i., peramivir once (60 mg/kg; P60×1) on days 2 and 3 p.i., and peramivir three times (30 mg/kg/day; P30×3) on days 2 to 4 p.i. in comparison with the control group. Groups orally administered oseltamivir phosphate three times (30 and 60 mg/kg/day; O30×3 and O60×3) also show statistically significant reductions in virus titers on day 3 p.i. and days 2 and 3 p.i., respectively, in comparison with the control group. A significant difference is observed between groups P30×1 and O30×3 on day 2 p.i. Asterisks indicate statistically significant differences, as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) Virus titer AUCs were calculated using the trapezoidal rule on the basis of means of the virus titers (TCID50/ml) on days 2 to 4. Statistically significant reductions in virus titer AUCs are observed in treated groups compared with the control group (**, P < 0.001; ***, P < 0.0001).
To examine the effects of peramivir against virus replication during infection, the AUCs of the viral titers from days 2 to 4 p.i. were calculated. Virus titer AUCs of the groups treated with peramivir and oseltamivir phosphate were significantly reduced in a dose-dependent manner (Fig. 2B). In addition, the virus titer AUC in group P60×1 was comparable to the virus titer AUCs in groups P30×3 and O60×3. These results indicated that a single intravenous injection of peramivir had potent antiviral activity in the early phase after virus inoculation and that the efficacy of a single intravenous injection of peramivir against influenza B virus was comparable to that of oseltamivir phosphate administered for 3 days in the ferret model.
To monitor the emergence of resistant variants after peramivir treatment and during oseltamivir phosphate treatment, we determined NA gene sequences of virus obtained from nasal wash fluid on day 4 p.i. A virus isolated from group O30×3 showed a mutation resulting in an amino acid change at position 31 (L to I) of the NA gene. This mutation is located around the transmembrane domain and not associated with reported resistance or decreased susceptibility to NA inhibitors (1, 8). In the other ferrets, no NA amino acid changes were detected in viruses from the nasal wash fluid (data not shown).
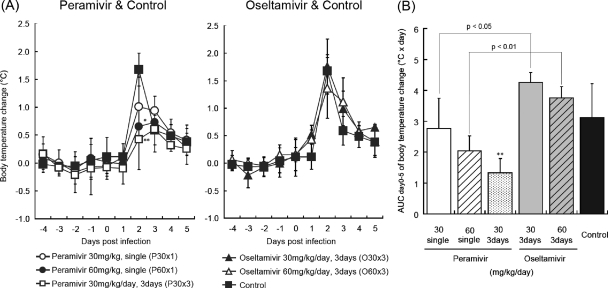
We examined the effects of peramivir and oseltamivir on body temperature in ferrets after inoculation with B/Kadoma/1/2005. A body temperature higher than that before the inoculation was observed for 4 days in the control group (Fig. 3A). On day 2 p.i., groups P60×1 and P30×3 showed a significantly lower body temperature than the control group (P < 0.05). Group P30×1 showed a slight but not significant reduction in body temperature on day 2 p.i. In contrast, two groups administered oseltamivir phosphate showed no apparent reduction in body temperature. AUCs of the body temperature change of groups P30×1 and P60×1 were lower than those of groups O30×3 and O60×3, respectively (P < 0.05 and P < 0.01, respectively) (Fig. 3B).
Fig. 3.
Body temperature changes in ferrets. On day 0, the nasal cavities of ferrets were inoculated with B/Kadoma/1/2005. The ferrets' body temperatures were recorded every 15 min by data loggers implanted into their peritoneal cavities. (A) The body temperatures were calculated from the average temperatures during nighttime (10 p.m. to 8 a.m.), and the body temperature after virus inoculation was compared with that before virus inoculation. A significant reduction of average temperature is observed in groups P60×1 and P30×3 on day 2 p.i. (*, P < 0.05; ** P < 0.01) in comparison with the control group (left graph). No significant difference is observed in a comparison between groups treated with oseltamivir and a control group (right graph). (B) AUCs of body temperature change were calculated by use of the trapezoidal rule on the basis of the means of the body temperature change on days 0 to 5. Group P30×3 shows a statistically significant reduction in body temperature change AUC compared with the control group (**, P < 0.01). Significant differences are observed between groups P30×1 and O30×3 and between groups P60×1 and O60×3.
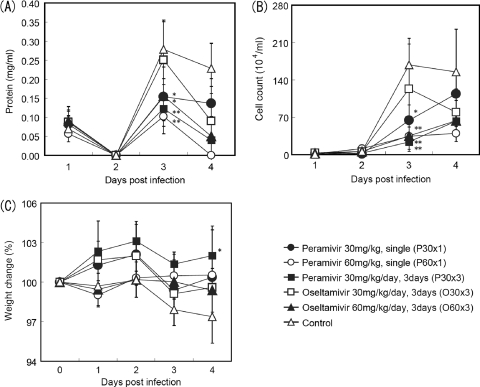
To assess the effects of peramivir and oseltamivir on inflammation in nasal cavities of infected ferrets, the protein concentration and the number of inflammatory cells in nasal wash fluid were measured. The protein concentration and the number of inflammatory cells on day 3 p.i. were reduced in all groups treated with peramivir and group O60×3 but not in group O30×3 (Fig. 4A and B). In addition, body weights were measured as one of the clinical signs. A significant difference in the average percent body weight (weight on day 4/weight on day 0) between group P30×3 (102.0%) and the untreated control group (97.4%) was observed (P < 0.05) (Fig. 4C). While the nasal signs (nasal discharge, sneezing, and mouth breathing) induced by influenza B virus infection in the control group were relatively mild, ferrets treated with peramivir or oseltamivir phosphate exhibited few or no nasal signs (data not shown).
Fig. 4.
Effects of peramivir and oseltamivir phosphate treatment on nasal inflammatory cell count, protein concentration, and weight change of ferrets infected with B/Kadoma/1/2005. The means ± SDs of 4 ferrets (6 ferrets for the untreated control group) are shown in the graphs. P values are calculated by comparison with the control group. (A) Protein concentration in nasal washes (*, P < 0.05; **, P < 0.01); (B) total number of inflammatory cells in nasal washes (*, P < 0.01; **, P < 0.001); (C) body weight change. Percent body weight was calculated on the basis of the body weight measured on the indicated days compared with the weight before inoculation with virus (*, P < 0.05).
Efficacy of a single intravenous injection of peramivir against influenza B virus in cynomolgus macaques.
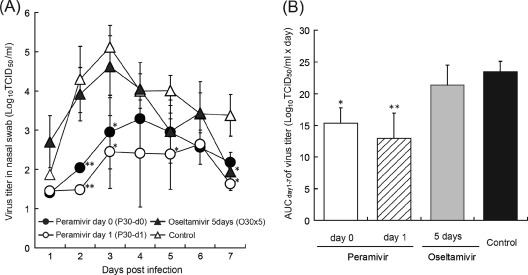
We have reported that cynomolgus macaques were susceptible to influenza B virus, which replicated in the upper respiratory tract. B/SendaiH/1051/2007 especially caused fever with a loss of appetite and body weight more clearly than B/Kadoma/1/2005 (23). Therefore, cynomolgus macaques were intranasally inoculated with B/SendaiH/1051/2007 (2 × 105 TCID50s), and 30 mg/kg of peramivir was administered intravenously once immediately after virus inoculation (P30-d0) (Fig. 5) or 24 h after virus inoculation (P30-d1). Oseltamivir phosphate was administered at 30 mg/kg/day orally once a day for 5 days (O30×5) as a comparison. When the virus titers in nasal swab fluid were measured from days 1 to 12 p.i., the maximum virus titer in the control group was observed on day 3 p.i., and then the virus titer declined after day 4 p.i. (Fig. 5A). On days 2 and 3 p.i., virus titers in all groups treated with peramivir were significantly reduced compared with the titer in the control group (P < 0.01). On days 5 and 7 p.i., a significant reduction in virus titers was also observed in group P30-d1. In contrast, no apparent reduction of virus titers was observed in the early phase after virus inoculation in group O30×5, and the virus titer on day 7 p.i. in group O30×5 was significantly lower than that in the control group (P < 0.05). Comparing peramivir and oseltamivir on day 2 p.i., virus titers in groups P30-d0 and P30-d1 were significantly lower than the titer in group O30×5 (P < 0.05 and P < 0.01, respectively). On day 3 p.i., the virus titer in group P30-d1 was also significantly lower than that in group O30×5 (P < 0.05).
Fig. 5.
Virus titers in the nasal swab fluid of infected cynomolgus macaques treated with peramivir or oseltamivir phosphate. The nasal cavities of macaques were inoculated with B/SendaiH/1051/2007. Nasal swab samples were collected for virus titration on days 1 to 12 p.i. (A) Virus titers in nasal swab samples collected on the indicated days after virus inoculation are indicated as means ± SDs of 3 animals. No virus was detected in nasal swab fluid of two macaques in the control group on day 1 p.i., two macaques on day 1, and one macaque on days 2, 6, and 7 in the group administered peramivir on day 0 (P30-d0) and two macaques on days 1, 2, and 7 in the group administered peramivir on day 1 (P30-d1). No virus was detected in any of the macaques on day 8 p.i. When virus was not detected in samples, the mean viral titers are calculated as an undetectable level, considered to be 101.4 log TCID50s/ml. Significant differences were observed in groups P30-d0 and P30-d1 in comparison with the control group on days 1 and 2 and days 1, 2, 5, and 7 p.i., respectively. The group administered oseltamivir phosphate orally five times (30 mg/kg/day; O30×5) also showed a statistically significant reduction in virus titers on day 7 in comparison with the control group. Significant differences were observed between groups P30-d0 and O30×5 on day 2 p.i. (P = 0.025) and between groups P30-d1 and O30×5 on day 2 p.i. (P = 0.004) and on day 3 p.i. (P = 0.036). (B) Virus titer AUC was calculated by use of the trapezoidal rule on the basis of the mean of the virus titers (TCID50/ml) on days 1 to 7. Groups P30-d0 and P30-d1 showed statistically significant reductions in virus titer AUC compared with the control group (*, P < 0.05; **, P < 0.01). A significant difference was observed between groups P30-d1 and O30×5 (P = 0.036).
When the statistical analysis was performed on the basis of the AUCs of the viral titers from day 1 to 7 p.i., the virus titer AUCs of the groups treated with peramivir were significantly lower than the virus titer AUC of the control group (P < 0.05), while that of the group treated with oseltamivir was not (Fig. 5B). Furthermore, the virus titer AUC of group P30-d1 was significantly lower than that of group O30×5 (P < 0.05). We determined NA gene sequences of virus obtained from nasal swab fluid on day 5 p.i. Viruses isolated from macaques treated with peramivir and oseltamivir showed no mutation in NA amino acid sequences (data not shown).]
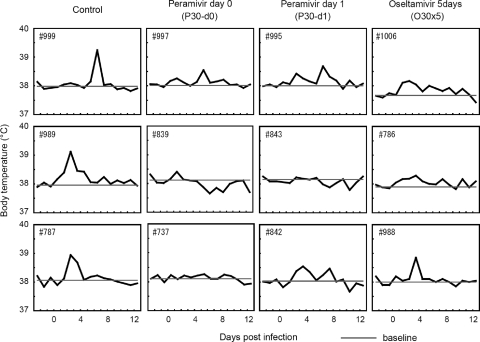
Body temperatures were compared among the treated and untreated groups. Body temperatures higher than those before the virus inoculation were observed in the untreated control group for 1 to 4 days (Fig. 6). The body temperatures of macaques treated with peramivir were substantially lower than those of macaques in the control group, whereas no significant suppression in body temperature was observed in any of the treated macaques using statistical analysis on the basis of the AUC of body temperature change (data not shown). However, the averages of the maximal temperature rises in groups P30-d0 (mean ± standard deviation [SD] = 0.33 ± 0.15°C) and P30-d1 (0.43 ± 0.31°C) after infection were significantly lower than the rise in the control group (1.13 ± 0.21°C) (P < 0.05). In contrast, no significant suppression of maximal temperature rise was observed in group O30×5 (0.60 ± 0.26°C) compared with the control group.
Fig. 6.
Body temperature in cynomolgus macaques treated with peramivir. On day 0, the nasal cavities of macaques were inoculated with B/SendaiH/1051/2007. Macaques were treated as described in the legend to Fig. 5. The macaques' body temperatures were recorded every 15 min by telemetry probes implanted into their peritoneal cavities. The plotted temperature changes were calculated from the average of the highest and lowest temperatures during 1 day, and the body temperature after virus inoculation was compared with that before virus inoculation. Baseline indicates the average temperature of the macaque before inoculation with virus.
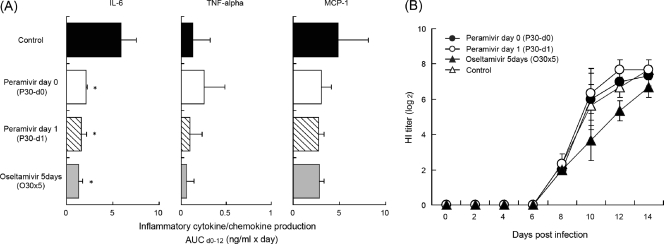
To examine the effects of peramivir and oseltamivir on immune responses in infected macaques, production of inflammatory cytokines and chemokines in nasal swab fluid was measured (Fig. 7A). The levels of cytokine and chemokine secretion were increased after infection. However, the kinetics of cytokine and chemokine production varied in individual macaques. Therefore, levels of cytokines and chemokines were expressed as AUCs from day 1 to 12, and statistical analysis was conducted on the basis of the AUC (Fig. 7A). Levels of IL-6 production in groups P30-d0, P30-d1, and O30×5 were significantly lower than those in the control group. On the other hand, no significant effect on TNF-α and MCP-1 production was observed in treated groups compared with the control group.
Fig. 7.
Immune responses in cynomolgus macaques treated with peramivir. Cynomolgus macaques were inoculated with B/SendaiH/1051/2007 on day 0 and treated as described in the legend to Fig. 5. The levels of cytokines/chemokines in the fluid of nasal swabs from infected cynomolgus macaques were measured by the bead array assay. (A) Cytokine/chemokine AUC was calculated by use of the trapezoidal rule on the basis of the mean of the levels of cytokine/chemokine (pg/ml) on days 1 to 12. The IL-6 level AUC in groups treated with peramivir or oseltamivir phosphate was reduced significantly compared with that in the control group (*, P < 0.01). (B) Sera were collected on the indicated days after infection with B/SendaiH/1051/2007. Diluted sera were incubated with 4 HA units of virus antigen for HI tests. HI titers are expressed as reciprocals of the highest dilution at which samples completely inhibited hemagglutination.
To serologically confirm influenza B virus infection and to compare the effects of the reagents on production of anti-HA antibodies, sera were collected on the indicated days after virus inoculation. HI activities against B/SendaiH/1051/2007 were observed in the sera of groups treated with peramivir or oseltamivir phosphate on day 8 p.i. and increased until day 14 p.i. (Fig. 7B). These results indicate that peramivir and oseltamivir did not inhibit the production of serum anti-HA antibodies against B/SendaiH/1051/2007 in cynomolgus macaques.
DISCUSSION
Influenza B virus causes seasonal influenza even after newly emerging H1N1 influenza A virus infection became pandemic in 2009 and the number of Russian H1N1 virus cases decreased (34). Since influenza B virus infection causes significant morbidity like influenza A virus does (26, 29, 30), antiviral agents against influenza B virus are required. The efficacy of peramivir against influenza B virus in vitro was more potent than that of oseltamivir and zanamivir (5, 15). Therefore, in the present study, we examined the efficacy of peramivir against influenza B virus in vivo using ferrets and macaques that were susceptible to infection with a number of unadapted human influenza virus isolates and that showed clinical symptoms. Since the absence of HI activity in sera was confirmed before experiments and animals were maintained under the specific-pathogen-free condition in the present study, use of animals immunologically naïve against influenza B virus might support the interpretation that the change of virus titers in the early stage of infection was mainly caused by antiviral agents and not by memory immune responses against influenza B virus, which might exist in many humans. In addition, animal models enable examination of effective time points for initiation of treatments after virus exposure, since it might be difficult to know when patients were infected with virus in clinical studies. These points might be critical if differences in the effects among antiviral agents are modest.
In ferrets infected with human influenza B virus, viral titers and virus titer AUCs were decreased with a single intravenous administration of peramivir and repeated oral administration of oseltamivir phosphate compared with those in the controls (Fig. 2). However, a single administration of 60 mg/kg of peramivir attenuated fever by about 1°C, while no change in fever was observed in the groups treated with oseltamivir phosphate (Fig. 3). The difference of virus titers between ferrets treated with peramivir and those treated with oseltamivir phosphate was observed on day 2 p.i. This suggested that viral propagation in an early phase after virus infection contributed to the severity of symptoms, including fever, and that suppression of virus replication in the early phase of infection was crucial to ameliorate symptoms in the ferret model.
To further evaluate the therapeutic efficacy of peramivir against influenza B virus, we next compared the inhibitory effects of a single intravenous administration of peramivir with those of repeated oral administration of oseltamivir phosphate using cynomolgus macaques. Virus titers, virus titer AUC, body temperature, and the IL-6 level AUC in nasal swabs of macaques treated with peramivir were lower than those for the control group (Fig. 5 to 7). These findings were concordant with those of our previous study, which showed that the levels of IL-6 in the nasal swabs were correlated with both virus replication and symptoms (23). These results observed in macaques and ferrets suggest that virus propagation in the nasal cavity in the early phase of infection is related to body temperature during infection.
On the other hand, the virus titer on day 7 p.i. and the IL-6 level AUC in nasal swabs of macaques treated with oseltamivir phosphate were significantly lower than those of the control group, but body temperature and virus titer AUCs were not. It was thought that reduction of virus titers in the late phase might cause a low IL-6 level on days 7 to 8 p.i., resulting in a decrease in the IL-6 level AUC (data not shown). However, it did not affect suppression of fever in macaques treated with oseltamivir phosphate, since the virus titer in the early phase was not suppressed. These results also support the importance of suppression on virus replication in the early phase of infection to reduce symptoms of influenza and the significance of early treatment with antiviral agents.
Although oseltamivir phosphate has been administered twice a day in humans, we administered oseltamivir phosphate once a day for 5 days in the present macaque study for the following reasons. First, since anesthesia was necessary for drug administration and it decreased body temperature, anesthesia twice a day would make evaluation of the macaques' body temperature change and feeding of food to macaques difficult. Second, there were no significant differences in the duration of virus shedding and total symptom scores after treatment between oseltamivir phosphate given once and oseltamivir phosphate given twice a day in the previous study, although a group treated with oseltamivir once daily showed a higher virus titer AUC than did a group treated twice daily (13). Third, we confirmed that the Cmax of oseltamivir carboxylate in plasma of macaques was 6,440 ± 3,180 ng/ml after oral administration of the 30-mg/kg dose of oseltamivir phosphate, which was 1,570 times higher than the enzyme inhibition IC50 (4.1 ng/ml, or 13.1 nM) against B/SendaiH/1051/2007 (Tables 1 and 2). In addition, the plasma concentration of oseltamivir carboxylate after 24 h (36 ± 8.0 ng/ml) was still 9 times higher than the IC50 (4.1 ng/ml) (Fig. 1 and Table 1). Therefore, single-daily-dose administration of oseltamivir in the macaque model was expected to show pharmacological effects from the aspect of pharmacokinetics. Furthermore, we did not detect mutations in NA genes in nasal samples from macaques after treatment with oseltamivir once a day or after treatment with peramivir, though substitution of an amino acid was detected in a nasal wash sample from a ferret treated with oseltamivir twice daily. Nonetheless, the low effectiveness of oseltamivir against influenza B virus was demonstrated not only by the virus titer in nasal swab fluid but also by the symptoms in the macaque model. These results partially coincide with those of clinical studies that showed a longer duration of virus shedding and fever in patients treated with oseltamivir phosphate in influenza B virus infection than in influenza A virus infection (21, 22, 32). In contrast, in the macaque model, intravenous administration of a 30-mg/kg dose of peramivir can achieve a Cmax of up to 160 μg/ml, which is 100,000 times higher than the IC50 (1.4 ng/ml, or 4.26 nM) against B/SendaiH/1051/2007 (Fig. 1; Tables 1 and 2). The concentration of peramivir in plasma dropped quickly after injection, but the level of peramivir at 24 h postdosing (52.4 ng/ml) was still 37 times higher than the IC50 (1.4 ng/ml). Therefore, the high concentration of peramivir in plasma during the 24 h after injection is thought to be effective to reduce virus replication in the early phase of infection.
In our previous study, HI and neutralization activities in sera of macaques were detected 6 to 8 days after infection and coincided with decreasing virus titers in swab samples (23). In the present study, HI activities against B/SendaiH/1051/2007 were observed in sera of macaques treated with peramivir or oseltamivir phosphate after day 8 p.i. at levels comparable to those in sera from untreated macaques. These results indicate that peramivir and oseltamivir did not inhibit production of anti-HA antibodies against B/SendaiH/1051/2007 in cynomolgus macaques. It is thought that the titers of anti-HA antibodies against B/SendaiH/1051/2007 in treated cynomolgus macaques may be sufficient to protect them from challenge with the same virus strain, since macaques possessing similar or lower HI titers of sera after vaccination with an H7 vaccine were protected from infection with a different H7 strain in the previous experiment (18).
In the present study, we demonstrated that peramivir injected once intravenously had beneficial effects on the viral titers and symptoms of ferrets and cynomolgus macaques with influenza B virus infection. The effects observed with peramivir treatment seem to be superior to those observed with once-daily oseltamivir phosphate treatment in cynomolgus macaque when oseltamivir was given once daily. Therefore, single intravenous administration of peramivir could be an alternative to oseltamivir to treat patients with acute influenza B virus infection.
ACKNOWLEDGMENTS
We thank Hidekazu Nishimura for providing B/SendaiH/1051/2007, Tetsuo Kase for providing B/Kadoma/1/2005, Hideaki Tsuchiya, Norio Okahara, and Takahiro Nakagawa for animal care, Shinya Omoto and Shinobu Kawauchi-Miki for valuable discussions, and Hiroko Iwasaki and Mayumi Kakui for help with enzyme assays.
All work reported here was financially supported by Shionogi Co., Ltd.
Footnotes
Published ahead of print on 15 August 2011.
REFERENCES
- 1. Air G. M., et al. 1990. Antigenic, sequence, and crystal variation in influenza B neuraminidase. Virology 177:578–587 [DOI] [PubMed] [Google Scholar]
- 2. Babu Y. S., et al. 2000. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J. Med. Chem. 43:3482–3486 [DOI] [PubMed] [Google Scholar]
- 3. Bantia S., et al. 2001. Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob. Agents Chemother. 45:1162–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Birnkrant D., Cox E. 2009. The Emergency Use Authorization of peramivir for treatment of 2009 H1N1 influenza. N. Engl. J. Med. 361:2204–2207 [DOI] [PubMed] [Google Scholar]
- 5. Boivin G., Goyette N. 2002. Susceptibility of recent Canadian influenza A and B virus isolates to different neuraminidase inhibitors. Antiviral Res. 54:143–147 [DOI] [PubMed] [Google Scholar]
- 6. Boltz D. A., et al. 2008. Intramuscularly administered neuraminidase inhibitor peramivir is effective against lethal H5N1 influenza virus in mice. Antiviral Res. 80:150–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chand P., et al. 1997. Design and synthesis of benzoic acid derivatives as influenza neuraminidase inhibitors using structure-based drug design. J. Med. Chem. 40:4030–4052 [DOI] [PubMed] [Google Scholar]
- 8. Collins P. J., et al. 2008. Crystal structures of oseltamivir-resistant influenza virus neuraminidase mutants. Nature 453:1258–1261 [DOI] [PubMed] [Google Scholar]
- 9. Govorkova E. A., et al. 2007. Efficacy of oseltamivir therapy in ferrets inoculated with different clades of H5N1 influenza virus. Antimicrob. Agents Chemother. 51:1414–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Govorkova E. A., Leneva I. A., Goloubeva O. G., Bush K., Webster R. G. 2001. Comparison of efficacies of RWJ-270201, zanamivir, and oseltamivir against H5N1, H9N2, and other avian influenza viruses. Antimicrob. Agents Chemother. 45:2723–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gubareva L. V., Matrosovich M. N., Brenner M. K., Bethell R. C., Webster R. G. 1998. Evidence for zanamivir resistance in an immunocompromised child infected with influenza B virus. J. Infect. Dis. 178:1257–1262 [DOI] [PubMed] [Google Scholar]
- 12. Hatakeyama S., et al. 2007. Emergence of influenza B viruses with reduced sensitivity to neuraminidase inhibitors. JAMA 297:1435–1442 [DOI] [PubMed] [Google Scholar]
- 13. Hayden F. G., et al. 1999. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA 282:1240–1246 [DOI] [PubMed] [Google Scholar]
- 14. Herlocher M. L., et al. 2001. Ferrets as a transmission model for influenza: sequence changes in HA1 of type A (H3N2) virus. J. Infect. Dis. 184:542–546 [DOI] [PubMed] [Google Scholar]
- 15. Hurt A. C., et al. 2006. Neuraminidase inhibitor-resistant and -sensitive influenza B viruses isolated from an untreated human patient. Antimicrob. Agents Chemother. 50:1872–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hurt A. C., et al. 2010. Assessing the viral fitness of oseltamivir-resistant influenza viruses in ferrets, using a competitive-mixtures model. J. Virol. 84:9427–9438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Itoh Y., et al. 2009. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Itoh Y., et al. 2010. Subcutaneous inoculation of a whole virus particle vaccine prepared from a non-pathogenic virus library induces protective immunity against H7N7 highly pathogenic avian influenza virus in cynomolgus macaques. Vaccine 28:780–789 [DOI] [PubMed] [Google Scholar]
- 19. Jackson S., et al. 2009. Reassortment between avian H5N1 and human H3N2 influenza viruses in ferrets: a public health risk assessment. J. Virol. 83:8131–8140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kawai N., Ikematsu H., Iwaki N., Hirotsu N., Kashiwagi S. 2006. Prevalence of influenza B during the 2004-2005 season in Japan. Clin. Infect. Dis. 43:1226–1228 [DOI] [PubMed] [Google Scholar]
- 21. Kawai N., et al. 2007. Longer virus shedding in influenza B than in influenza A among outpatients treated with oseltamivir. J. Infect. 55:267–272 [DOI] [PubMed] [Google Scholar]
- 22. Kawai N., et al. 2006. A comparison of the effectiveness of oseltamivir for the treatment of influenza A and influenza B: a Japanese multicenter study of the 2003-2004 and 2004-2005 influenza seasons. Clin. Infect. Dis. 43:439–444 [DOI] [PubMed] [Google Scholar]
- 23. Kitano M., et al. 2010. Establishment of a cynomolgus macaque model of influenza B virus infection. Virology 407:178–184 [DOI] [PubMed] [Google Scholar]
- 24. Kohno S., Kida H., Mizuguchi M., Shimada J. 2010. Efficacy and safety of intravenous peramivir for treatment of seasonal influenza virus infection. Antimicrob. Agents Chemother. 54:4568–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kubo S., Tomozawa T., Kakuta M., Tokumitsu A., Yamashita M. 2010. Laninamivir prodrug CS-8958, a long-acting neuraminidase inhibitor, shows superior anti-influenza virus activity after a single administration. Antimicrob. Agents Chemother. 54:1256–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee N., et al. 2009. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J. Infect. Dis. 200:492–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matrosovich M., Matrossovich T., Carr J., Roberts N. A., Klenk H.-D. 2003. Overexpression of the alpha-2,6-sialyltransferase in MDCK cells increases influenza virus sensitivity to neuraminidase inhibitors. J. Virol. 77:8418–8425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Munster V. J., et al. 2009. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science 325:481–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Newland J. G., et al. 2003. Encephalitis associated with influenza B virus infection in 2 children and a review of the literature. Clin. Infect. Dis. 36:e87–e95 [DOI] [PubMed] [Google Scholar]
- 30. Peltola V., Ziegler T., Ruuskanen O. 2003. Influenza A and B virus infections in children. Clin. Infect. Dis. 36:299–305 [DOI] [PubMed] [Google Scholar]
- 31. Pinto L. H., Lamb R. A. 2006. The M2 proton channels of influenza A and B viruses. J. Biol. Chem. 281:8997–9000 [DOI] [PubMed] [Google Scholar]
- 32. Sugaya N., et al. 2007. Lower clinical effectiveness of oseltamivir against influenza B contrasted with influenza A infection in children. Clin. Infect. Dis. 44:197–202 [DOI] [PubMed] [Google Scholar]
- 33. Tashiro M., et al. 2009. Surveillance for neuraminidase-inhibitor-resistant influenza viruses in Japan, 1996-2007. Antivir. Ther. 14:751–761 [DOI] [PubMed] [Google Scholar]
- 34. World Health Organization 2010. Influenza activity in the world. Global alert and response. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/disease/influenza/update/en/index.html [Google Scholar]
- 35. Yun N. E., et al. 2008. Injectable peramivir mitigates disease and promotes survival in ferrets and mice infected with the highly virulent influenza virus, A/Vietnam/1203/04 (H5N1). Virology 374:198–209 [DOI] [PMC free article] [PubMed] [Google Scholar]