Abstract
Antiviral medications with activity against influenza viruses are important in controlling influenza. We compared intravenous peramivir, a potent neuraminidase inhibitor, with oseltamivir in patients with seasonal influenza virus infection. In a multinational, multicenter, double-blind, double-dummy randomized controlled study, patients aged ≥20 years with influenza A or B virus infection were randomly assigned to receive either a single intravenous infusion of peramivir (300 or 600 mg) or oral administration of oseltamivir (75 mg twice a day [b.i.d.] for 5 days). To demonstrate the noninferiority of peramivir in reducing the time to alleviation of influenza symptoms with hazard model analysis and a noninferiority margin of 0.170, we planned to recruit 1,050 patients in South Korea, Japan, and Taiwan. A total of 1,091 patients (364 receiving 300 mg and 362 receiving 600 mg of peramivir; 365 receiving oseltamivir) were included in the intent-to-treat infected population. The median durations of influenza symptoms were 78.0, 81.0, and 81.8 h in the groups treated with 300 mg of peramivir, 600 mg of peramivir, and oseltamivir, respectively. The hazard ratios of the 300- and 600-mg-peramivir groups compared to the oseltamivir group were 0.946 (97.5% confidence interval [CI], 0.793, 1.129) and 0.970 (97.5% CI, 0.814, 1.157), respectively. Both peramivir groups were noninferior to the oseltamivir group (97.5% CI, <1.170). The overall incidence of adverse drug reactions was significantly lower in the 300-mg-peramivir group, but the incidence of severe reactions in either peramivir group was not different from that in the oseltamivir group. Thus, a single intravenous dose of peramivir may be an alternative to a 5-day oral dose of oseltamivir for patients with seasonal influenza virus infection.
INTRODUCTION
Influenza epidemics occur during the winter months in temperate climates. Data from epidemiologic studies during the 2009 influenza A virus (H1N1) pandemic indicated that antiviral agents, especially neuraminidase inhibitors (NAIs) such as oseltamivir and zanamivir, are important for treating patients with influenza (8, 24, 25, 26).
However, these drugs are associated with some unresolved problems. In particular, oral or inhaled administration may be unfeasible for patients in poor health (14, 21); the efficacy of these drugs in severe cases has not been fully established; and the development of NAI-resistant viruses, particularly influenza A/H1N1 viruses resistant to oseltamivir, is increasingly recognized (4, 7, 15). Consequently, new drugs are needed (6).
Peramivir is a NAI that inhibits influenza virus proliferation (1, 2). In a previous controlled, double-blind study, peramivir was found to significantly reduce the duration of influenza symptoms, without safety concerns, from that with a placebo after a single administration at doses of 300 and 600 mg (12). In a parallel trial, we investigated the efficacy and safety of peramivir administered over multiple days at 300 mg or 600 mg/day to patients with high-risk factors for severe disease (13).
Because a single intravenous dose can improve compliance and reliably provide stable pharmacokinetics regardless of the patient's condition, peramivir promises to be an important anti-influenza agent if it shows a level of efficacy comparable to that of the standard anti-influenza treatment. Oseltamivir, the leading anti-influenza agent, has been evaluated mostly in otherwise healthy adults with uncomplicated influenza whose treatment was initiated within 48 h of symptom onset. We therefore compared a single intravenous dose of peramivir with multiple doses of oseltamivir for patients aged 20 years or older with influenza A or B virus infection.
During the 2008-2009 season, most influenza A/H1N1 viruses (Russian strain) carried the H274Y neuraminidase (NA) mutation, resulting in decreased susceptibility to oseltamivir. Therefore, we also evaluated the efficacy of peramivir against oseltamivir-resistant viruses.
MATERIALS AND METHODS
Study design.
Our study was a multicenter, double-blind, randomized, controlled study with dynamic allocation using the minimization method and was conducted in 146 medical institutions in Japan, South Korea, and Taiwan from November 2008 to April 2009. This period was before the emergence of the 2009 pandemic A/H1N1 (pH1N1) influenza virus. The study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines.
Patients.
Patients aged 20 years or older with influenza A or B virus infection who met the following inclusion criteria were enrolled: availability for treatment within 48 h of onset of influenza symptoms, fever with an axillary temperature of ≥38.0°C, at least two moderate to severe symptoms among seven symptoms (headache, muscle or joint pain, feverishness or chills, fatigue, cough, sore throat, and nasal stuffiness) due to influenza, and a rapid antigen test (RAT) result positive for influenza. The onset of influenza symptoms was defined as the time of the first increase of ≥1°C from the patient's normal body temperature or the occurrence of at least one of the seven symptoms listed above. The RAT kits used in the study were the RapidTesta FLU II and FLU Stick kits (Sekisui Medical), the Espline Influenza A&B kit (Fujirebio), and the Capilia FLU A+B kit (Tauns). Exclusion criteria were impaired respiratory function, a history of congestive cardiac failure, poorly controlled diabetes mellitus, immunosuppressive therapy (immunosuppressants, antitumor agents, etc.) or an immunodeficiency disorder such as AIDS, renal disorder (estimated creatinine clearance, <50 ml/min), ischemic heart disease or serious arrhythmia, a corrected QT interval (QTc) of ≥480 ms or bradycardia (heart rate, <40 beats per minute [bpm]), clinically significant disorders that required hospitalization, and infection requiring systemic antimicrobial treatment.
Prior to the enrollment of each patient in the study, the investigator or subinvestigator provided him/her with written patient information (reviewed and approved by the institutional review board [IRB] at each institution) and gave a detailed explanation in order to obtain voluntary written informed consent.
Procedures.
Using a minimization method, patients were randomly assigned in a 1:1:1 ratio to receive peramivir at a dose of 300 or 600 mg (Shionogi, Osaka, Japan) or oseltamivir with a balance of the composite symptom score (≤14 or ≥15), current smoking behavior (yes or no), country, and influenza virus type revealed by a RAT for the diagnosis of influenza. Peramivir was administered as a single intravenous infusion of 60 to 100 ml over 15 to 60 min. Oseltamivir was administered orally at a dose of 75 mg twice daily for 5 days. Blinding was maintained by the double-dummy technique using two placebos identical to peramivir and oseltamivir. The concomitant use of the antipyretic acetaminophen was allowed, but other antipyretics, antivirals, and antimicrobials were not permitted.
All patients returned to the investigational site for protocol-required assessments at days 1 (baseline), 2 (optional), 3, 8, and 14. Laboratory tests were performed on days 1 (baseline), 3, and 8 and included a hematological examination (white blood cell count, differential, hemoglobin concentration, hematocrit, red blood cell count, and platelet count), blood biochemistry examination (aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, γ-glutamyltransferase, alkaline phosphatase, creatine phosphokinase, total bilirubin, direct bilirubin, total protein, albumin, blood urea, creatinine, uric acid, glucose, sodium, potassium, chloride, magnesium, calcium, phosphorus, and HbA1c [only at day 1]), and urinalysis (bilirubin, protein, glucose, ketone bodies, urobilinogen, occult blood, and sediment). Patients assessed their own influenza symptoms and daily living activities using an Influenza Symptom Severity scale (ISS) (0, none [normal]; 1, mild [of little concern]; 2, moderate [very uncomfortable]; 3, severe [intolerable]) for seven symptoms (cough, sore throat, headache, nasal stuffiness, feverishness or chills, muscle or joint pain, and fatigue) and a visual analogue scale (Influenza Impact Well-Being Score [IIWS]) ranging from 0 (unable to perform one's usual activities at all) to 10 (able to perform all usual activities fully) (17). The questionnaire for this assessment was translated into three languages (Japanese, Korean, and Chinese). Symptoms were assessed with the ISS twice daily (morning and evening) from entry to day 8 and once daily (in the evening) from days 9 to 14. Activities were assessed with the IIWS once daily (evening) from entry to day 14. The results were recorded in a patient diary. Body temperature was measured four times daily (morning, noon, evening, and bedtime) from day 1 to day 3 of treatment and twice daily (morning and evening) from day 4 to 14, and results were recorded in a patient diary.
A nasal swab from one naris and a single throat swab were collected at days 1 (baseline), 2 (optional), 3, and 8. All samples were taken from the same sites throughout the study. These samples were each transported in 3 ml of viral transport medium to a central laboratory and were divided for typing and gene sequencing using PCR (0.3 ml), virus titration (0.8 ml), and an NA enzyme inhibitory assay (0.6 ml). Viral titers were calculated as log10 50% tissue culture infective doses (TCID50) per milliliter of viral transport medium, according to the Spearman-Karber equation. Madin-Darby canine kidney (MDCK) cells were infected in triplicate with 0.1 ml of a 10-fold dilution series of samples (ranging from no dilution to 1:107) in serum-free medium containing 3 μg/ml trypsin. Virus was adsorbed for 1 h, and cells were washed twice to remove unadsorbed virus and residual peramivir. MDCK cells were then incubated at 37°C under 5% CO2 for 6 days. Following this incubation period, the appearance of cytopathic effect (CPE) on cell monolayers was scored using light microscopy, and the final titer was expressed as TCID50/ml. When no CPE was observed using undiluted viral solution, this was defined as an undetectable level. We defined the undetectable level as 100.5 TCID50/ml. NA enzyme inhibitory assays were performed on isolated virus using a standard fluorometric assay (19). The 50% inhibitory concentration (IC50) was calculated by plotting the percentage of inhibition of NA activity versus the inhibitor concentration. A laboratory strain, A/PR/8/34, from the American Type Culture Collection, was also used as a standard strain in the NA inhibitory assay. The reliability of each assay was confirmed by the observation that the IC50 of peramivir ranged from 0.2 to 2 nM for the standard strain. Results are reported as means ± standard deviations (SD) for three independent experiments. The sequences of the NA gene in A (H1N1) viruses isolated from patients on day 1 (baseline) were analyzed. cDNA was generated using viral RNA as a template and a PrimeScript II 1st-strand cDNA synthesis kit according to the manufacturer's instructions (Takara Bio Inc.). The DNA fragment of a portion of the NA region was amplified from the cDNA with TaKaRa Ex Taq and PCR primers (forward, 5′-GAATTGGCTCCAAAGGAGATG-3′; reverse, 5′-GGGACGCGGGTTGTCACCGA-3′). The PCR products were purified, sequenced with a BigDye Terminator (version 3.1) cycle sequencing kit according to the manufacturer's instructions (Applied Biosystems), and analyzed on a DNA sequencer. Amino acid substitutions at positions 222, 234, 274, and 294 (N2 numbering) of the NA gene were investigated.
The plasma peramivir concentration was determined on days 1 (just before completion of infusion), 2, and 3. When possible, the plasma peramivir concentration was also determined at any time after the end of infusion on day 1. Blood samples were continuously collected from a subset of patients. The plasma peramivir concentration was measured as described previously (12). The lower limit for the quantification of peramivir in plasma was 1.00 ng/ml.
Study outcomes.
The primary efficacy endpoint was the time to alleviation of influenza symptoms. Alleviation of influenza symptoms was considered to occur at the first time point when all seven influenza symptoms (cough, sore throat, headache, nasal stuffiness, feverishness or chills, muscle or joint pain, and fatigue) were rated as 0 (none) or 1 (mild) for at least 21.5 h. In addition, the following secondary endpoints were assessed: (i) change from baseline in the composite symptom score, (ii) proportion of patients whose body temperature returned to normal (<37.0°C), (iii) time to resumption of usual activities (defined as the first time point when the IIWS score was 10 [able to perform all usual activities fully]), (iv) incidence of influenza-related complications (sinusitis, otitis media, bronchitis, and pneumonia), and (v) time-weighted change from baseline in the virus titer.
Safety was evaluated by assessing the incidence of adverse events and adverse drug reactions. Severity was graded according to the Division of AIDS table for grading the severity of adult and pediatric adverse events, and grades 1, 2, and 3 or higher corresponded to “mild,” “moderate,” and “severe,” respectively.
Statistical analysis.
The primary efficacy analysis population was the intent-to-treat infected (ITTI) population, which included all patients who had positive results on the RAT and received the study drug. Given the nature of the noninferiority study, patients who were not treated as assigned were included in the analyses according to the actual treatment received, allowing a more conservative interpretation of results from the noninferiority test.
The duration of influenza, the primary endpoint, was analyzed using a Cox proportional-hazards model with the following covariates: the composite symptom score at baseline, current smoking behavior (yes or no), country (Japan, South Korea, or Taiwan), influenza virus type identified by RAT (type A, B, or A and B), sex (male or female), the presence of coexisting disease at baseline that was considered by the physician to be medically important and/or affecting evaluation (yes or no), and treatment with any drugs from the onset of influenza to randomization (yes or no). The factors of sex, coexisting disease at baseline, and receipt of drugs before randomization were added as covariates prior to unblinding, because the blind review revealed that these factors may have affected the duration of influenza. The other covariates were used as minimization factors to ensure balance in randomization. Patients without alleviation of influenza symptoms were treated as censored data. The 97.5% confidence intervals (CI) for the hazard ratios of the 300- and 600-mg-peramivir groups compared to the oseltamivir group were calculated. Noninferiority to the oseltamivir group was indicated if the upper confidence limit was less than 1.170. All statistical tests were performed at a two-sided significance level of 0.05 using the Bonferroni adjustment for multiplicity. In addition, for each group, a Kaplan-Meier curve was plotted for the duration of influenza in order to calculate the median and 95% CI. In this study, the noninferiority test was designed to show that peramivir was not inferior to oseltamivir by more than half of the difference between oseltamivir and the placebo in terms of the log hazard ratio, and the corresponding noninferiority margin was selected. The hazard ratio of oseltamivir versus the placebo was estimated to be 0.73 on the basis of the results of three previously reported studies (9, 16, 22). Accordingly, the noninferiority margin was calculated to be −0.157 [calculated as 0.5 × log(0.73)] in terms of the log hazard ratio and 0.170 {calculated as [exp(−0.157) − 0.73]/0.73} in terms of the hazard ratio of peramivir versus oseltamivir.
Regarding the secondary endpoints, body temperature was summarized by calculating the summary statistics at each time point for each group and comparing these statistics for the different groups at each time point with the van Elteren test, including randomization factors as covariates. For the time to resumption of activities, a Kaplan-Meier curve was plotted for each group to calculate the median and its 95% CI, and an analysis was performed using a Cox proportional-hazards model with the randomization factors as covariates to estimate the differences between the groups. All statistical tests of these secondary endpoints were performed at a two-sided significance level of 0.05.
The time-weighted changes in virus titer for the different groups were compared with the van Elteren test, which was stratified by randomization factors. Patients with a positive virus titer at screening were included in the analysis. The virus titer was summarized by calculating the summary statistics at each time point for each group and comparing these statistics for the different groups with the van Elteren test, which was stratified by randomization factors.
The target sample size of 1,050 patients (350 patients per group × 3 groups) was calculated to provide a power of 0.80 to detect a difference with a two-sided significance level of 0.025 in the noninferiority test with a noninferiority margin of 0.170 (peramivir versus oseltamivir). This calculation was based on the assumptions that the hazard ratios of peramivir and oseltamivir versus the placebo were 0.67 and 0.73, respectively, and that the duration of influenza was 73 h.
For the safety evaluation, reported adverse events and adverse drug reactions were summarized according to the MedDRA preferred terms (version 11.1) in order to calculate the number of occurrences, number of affected patients, incidence, and 95% CI for each treatment group. The Clopper-Pearson method was used to calculate the CI of the percentage. In addition, the incidences in the different groups were compared using Fisher's exact test.
All statistical analyses were performed using SAS, version 9.1 for Windows. Statistics were reported to one decimal place beyond the number of decimal places present in the original endpoint.
RESULTS
Study population.
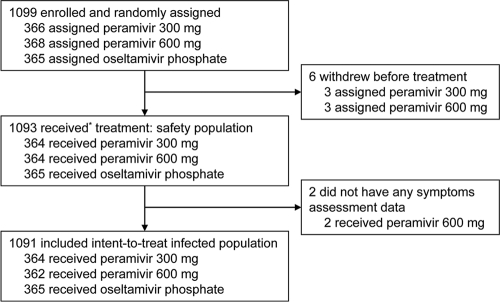
A total of 1,099 patients were randomly allocated to treatments (366 to receive 300 mg peramivir, 368 to receive 600 mg peramivir, and 365 to receive oseltamivir [Fig. 1 ]). All patients were confirmed to be RAT positive prior to entry. Six patients who dropped out before treatment and two patients with no posttreatment efficacy data were excluded from all analyses. One patient who was randomized to the 600-mg-peramivir group mistakenly received 300 mg peramivir and was thus included in the 300-mg-peramivir group. Therefore, 1,091 patients (364 receiving 300 mg peramivir, 362 receiving 600 mg peramivir, and 365 receiving oseltamivir) were included in the ITTI population, the primary efficacy analysis population. The three treatment groups did not differ significantly with respect to any baseline characteristics (Table 1).
Fig. 1.
Study profiles. *, One patient assigned to the 600-mg-peramivir group received 300 mg peramivir. This patient's data were included in the analyses according to the treatment actually administered.
Table 1.
Baseline characteristics for the intent-to-treat infected population
| Characteristica | Value for group receiving: |
||
|---|---|---|---|
| Peramivir |
Oseltamivir (n = 365) | ||
| 300 mg (n = 364) | 600 mg (n = 362) | ||
| Region/countryb | |||
| Japan | 247 (67.9) | 249 (68.8) | 246 (67.4) |
| Taiwan | 81 (22.3) | 79 (21.8) | 84 (23.0) |
| South Korea | 36 (9.9) | 34 (9.4) | 35 (9.6) |
| Male sex | 180 (49.5) | 198 (54.7) | 184 (50.4) |
| Age (yr) | |||
| Mean ± SD | 34.9 ± 11.7 | 35.9 ± 12.0 | 34.6 ± 11.7 |
| Range | 20–78 | 20–78 | 20–80 |
| Wt (kg) | |||
| Mean ± SD | 61.50 ± 13.04 | 62.69 ± 13.05 | 61.59 ± 13.09 |
| Range | 39.5–120.0 | 33.4–104.7 | 40.0–140.1 |
| Smokingb | 113 (31.0) | 111 (30.7) | 112 (30.7) |
| Coexisting disease at baseline | 127 (34.9) | 146 (40.3) | 132 (36.2) |
| Receipt of drugs from onset of influenza to randomization | 206 (56.6) | 212 (58.6) | 211 (57.8) |
| Influenza vaccination | 64 (17.6) | 56 (15.5) | 63 (17.3) |
| Duration of influenza | |||
| 0-12 h | 33 (9.1) | 24 (6.6) | 30 (8.2) |
| >12-24 h | 129 (35.4) | 117 (32.3) | 131 (35.9) |
| >24-36 h | 94 (25.8) | 114 (31.5) | 107 (29.3) |
| >36-48 h | 108 (29.7) | 106 (29.3) | 95 (26.0) |
| >48 h | 0 (0.0) | 1 (0.3) | 2 (0.5) |
| Composite symptom score (mean ± SD)b | 12.5 ± 3.4 | 12.5 ± 3.3 | 12.5 ± 3.2 |
| Body temp (°C) (mean ± SD) | 38.53 ± 0.49 | 38.48 ± 0.49 | 38.56 ± 0.52 |
| Result of rapid antigen testb | |||
| A | 335 (92.0) | 333 (92.0) | 338 (92.6) |
| B | 27 (7.4) | 29 (8.0) | 25 (6.8) |
| A and B | 2 (0.5) | 0 (0.0) | 2 (0.5) |
| Influenza virus subtype | |||
| A/H1 | 197 (54.1) | 200 (55.2) | 201 (55.1) |
| A/H1, H3 | 0 (0.0) | 0 (0.0) | 1 (0.3) |
| A/H3 | 112 (30.8) | 108 (29.8) | 108 (29.6) |
| A/− | 21 (5.8) | 15 (4.1) | 17 (4.7) |
| B | 21 (5.8) | 26 (7.2) | 23 (6.3) |
| Unknown | 13 (3.6) | 13 (3.6) | 15 (4.1) |
Unless otherwise indicated, each value is the number (percentage) of patients with the characteristic.
Randomization ensured balance for this factor.
A/H1N1 virus was isolated at baseline from 598 patients, and the base sequence of the NA gene was identified in 428 patients. The H274Y mutation (tyrosine instead of histidine at position 274 of the NA gene) was identified in 427 of 428 virus samples, and both R222Q and V234Y were identified in all samples. As shown in Table 2, the median IC50 for the A/H1N1 subtype at baseline was 100 nM (the upper limit of the assay) for oseltamivir and 21.59 nM for peramivir. However, the IC50 for the A/H1N1 virus without H274Y at baseline was 0.661 nM for oseltamivir and 0.414 nM for peramivir.
Table 2.
IC50 in NA inhibition assays at baseline for the intent-to-treat infected populationa
| Influenza virus subtype (n) and treatment group | IC50 (nM) |
|
|---|---|---|
| Mean ± SD | Median (range)b | |
| A/H1 (593) | ||
| Peramivir | 22.25 ± 4.37 | 21.59 (0.41–100.00) |
| Oseltamivir | 87.70 ± 16.38 | 100.00 (0.66–100.00) |
| Zanamivir | 1.35 ± 0.18 | 1.34 (0.97–3.41) |
| A/H3 (323) | ||
| Peramivir | 0.83 ± 0.17 | 0.82 (0.45–2.13) |
| Oseltamivir | 0.63 ± 0.17 | 0.62 (0.27–1.84) |
| Zanamivir | 1.97 ± 0.37 | 1.91 (1.46–5.93) |
| B (70) | ||
| Peramivir | 3.51 ± 0.39 | 3.58 (2.18–4.33) |
| Oseltamivir | 16.53 ± 2.30 | 16.77 (8.77–22.33) |
| Zanamivir | 9.74 ± 1.10 | 9.79 (5.92–12.17) |
The reliability of each assay was confirmed by the observation that the IC50 of peramivir ranged from 0.2 to 2 nM for the standard strain (A/PR/8/34).
Minimum to maximum IC50. The upper limit of the IC50 was 100.0 nM.
Efficacy based on clinical symptoms.
The median times to alleviation of symptoms were 78.0 (95% CI, 68.4, 88.6), 81.0 (95% CI, 72.7, 91.5), and 81.8 (95% CI, 73.2, 91.1) h in the 300-mg-peramivir, 600-mg-peramivir, and oseltamivir groups, respectively (Table 3). The hazard ratios of the 300-mg- and 600-mg-peramivir groups compared to the oseltamivir group were 0.946 (97.5% CI, 0.793, 1.129) and 0.970 (95% CI, 0.814, 1.157), respectively. The upper limits of both 97.5% CIs for the hazard ratios were less than the prespecified noninferiority margin. Both peramivir groups demonstrated noninferiority to oseltamivir. The effect was consistent in subgroup analysis according to the influenza virus subtype (A/H1N1, A/H3N2, and B).
Table 3.
Time to alleviation of symptoms for the intent-to-treat infected population
| Population and treatment (n) | Median time to alleviation (h) (95% CI) | Hazard ratio (97.5% CI)a |
|---|---|---|
| Overall | ||
| Peramivir | ||
| 300 mg (364) | 78.0 (68.4, 88.6) | 0.946 (0.793, 1.129)b |
| 600 mg (362) | 81.0 (72.7, 91.5) | 0.970 (0.814, 1.157)b |
| Oseltamivir (365) | 81.8 (73.2, 91.1) | |
| A/H1 | ||
| Peramivir | ||
| 300 mg (197) | 80.2 (69.3, 90.6) | 0.854 (0.672, 1.085) |
| 600 mg (200) | 83.6 (72.7, 101.9) | 0.927 (0.730, 1.176) |
| Oseltamivir (201) | 88.8 (73.1, 102.2) | |
| A/H3 | ||
| Peramivir | ||
| 300 mg (112) | 69.9 (54.4, 97.1) | 1.039 (0.745, 1.448) |
| 600 mg (108) | 70.6 (47.7, 91.9) | 0.958 (0.687, 1.335) |
| Oseltamivir (108) | 75.1 (63.4, 92.6) | |
| B | ||
| Peramivir | ||
| 300 mg (21) | 55.3 (43.9, 86.4) | 0.445 (0.202, 0.982) |
| 600 mg (26) | 92.8 (57.4, 116.1) | 0.706 (0.341, 1.460) |
| Oseltamivir (23) | 92.7 (70.2, 138.5) |
Hazard ratios compared to the oseltamivir group were estimated using Cox proportional-hazards models, which were adjusted for current smoking behavior, composite symptom score at baseline, country/region, influenza virus type, sex, complications, and previous therapy.
Both peramivir groups were noninferior to the oseltamivir group, with a noninferiority margin of 0.170.
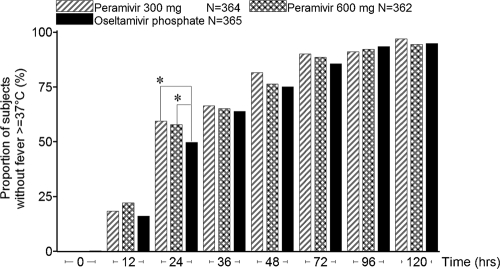
The proportion of patients whose body temperatures returned to normal 24 h after treatment was significantly higher in the 300-mg- and 600-mg-peramivir groups (59.3% [213/359 patients] and 57.9% [209/361], respectively) than in the oseltamivir group (49.7% [181/364]) (two-sided P values, 0.0272 and 0.0326, respectively) (Fig. 2).
Fig. 2.
Proportions of patients in the intent-to-treat infected population reporting normal temperatures. Asterisks indicate significant differences (P < 0.05) between peramivir and oseltamivir as determined by the Mantel-Haenszel test, which was stratified by current smoking behavior, composite symptom score at baseline, country/region, and influenza virus type.
The median times to resumption of usual activity were 155.7, 195.5, and 171.3 h in the 300-mg-peramivir, 600-mg-peramivir, and oseltamivir groups, respectively. Analysis using a Cox proportional-hazards model found no significant difference between either peramivir group and the oseltamivir group.
Analysis of the incidence of physician-diagnosed influenza-related complications using Fisher's exact test found no significant difference between either peramivir group and the oseltamivir group. (There was 1 case [0.3%] of sinusitis in the 300-mg-peramivir group, 1 case [0.3%] in the 600-mg-peramivir group, and 4 cases [1.1%] in the oseltamivir group. One case [0.3%] of otitis media occurred in the 600-mg-peramivir group. For bronchitis, there were 6 cases [1.6%] in the 300-mg-peramivir group, 6 cases [1.7%] in the 600-mg-peramivir group, and 6 cases [1.6%] in the oseltamivir group. There were 3 cases [0.8%] of pneumonia in the 300-mg-peramivir group, 1 case [0.3%] in the 600-mg-peramivir group, and 2 cases [0.5%] in the oseltamivir group.)
Virological efficacy.
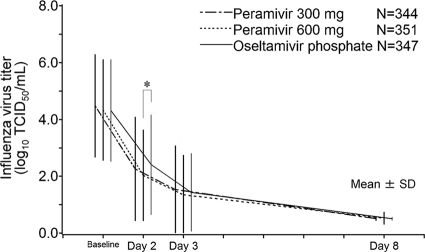
The mean virus titer (log10 TCID50/ml) over time and the time-weighted change from baseline are shown in Fig. 3 and Table 4, respectively. The time-weighted changes from baseline in the two peramivir groups were similar and numerically greater than that in the oseltamivir group. In the A/H3N2-infected subpopulation, the time-weighted change from baseline in the 300-mg-peramivir group was greater than that in the oseltamivir group (P, 0.0386 at day 2 and 0.0218 at day 3). The proportions of virus-positive patients on days 2, 3, and 8 were 74.6% (150/201), 47.9% (162/338), and 1.2% (4/323), respectively, in the 300-mg-peramivir group, 68.8% (132/192), 45.3% (158/349), and 1.5% (5/338), respectively, in the 600-mg-peramivir group, and 82.1% (160/195), 49.9% (171/343), and 0.9% (3/331), respectively, in the oseltamivir group, decreasing over time in all groups. In each of the peramivir groups, the proportions of virus-positive patients were lower than those in the oseltamivir group, especially for the 600-mg-peramivir group on day 2 (P, 0.0038 by the Mantel-Haenszel test).
Fig. 3.
Mean influenza virus titers (log10 TCID50/ml) and SD over time in the intent-to-treat infected population. The data analyzed were from the subset of patients who were positive for the influenza virus at baseline. Virus titers below the lower limit of quantification were set to 0.5. The asterisk indicates a significant difference (P < 0.05) between peramivir and oseltamivir as determined by the van Elteren test, which was stratified by current smoking behavior, composite symptom score at baseline, country/region, and influenza virus type.
Table 4.
Time-weighted change from baseline in virus titer for the intent-to-treat infected population
| Population and time interval | Value for group receiving: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Peramivir (300 mg) |
Peramivir (600 mg) |
Oseltamivir |
||||||
| No. of patients tested | Change in virus titera | Pb | No. of patients tested | Change in virus titera | Pb | No. of patients tested | Change in virus titera | |
| Overall | ||||||||
| From day 1 to day 2 | 201 | −1.10 ± 0.90 | 0.4278 | 192 | −1.08 ± 0.82 | 0.2252 | 195 | −1.04 ± 0.84 |
| From day 1 to day 3 | 338 | −1.71 ± 1.21 | 0.1337 | 349 | −1.71 ± 1.10 | 0.1778 | 343 | −1.63 ± 1.11 |
| From day 1 to day 8 | 323 | −2.97 ± 1.53 | 0.0674 | 338 | −2.91 ± 1.44 | 0.2066 | 331 | −2.82 ± 1.49 |
| A/H1 | ||||||||
| From day 1 to day 2 | 115 | −1.18 ± 0.95 | 0.6244 | 117 | −1.15 ± 0.90 | 0.4678 | 111 | −1.06 ± 0.97 |
| From day 1 to day 3 | 190 | −1.79 ± 1.26 | 0.5092 | 198 | −1.81 ± 1.19 | 0.5204 | 195 | −1.71 ± 1.22 |
| From day 1 to day 8 | 182 | −3.20 ± 1.55 | 0.1735 | 191 | −3.17 ± 1.42 | 0.4007 | 187 | −3.04 ± 1.57 |
| A/H3 | ||||||||
| From day 1 to day 2 | 58 | −1.23 ± 0.68 | 0.0386 | 54 | −1.12 ± 0.52 | 0.3129 | 60 | −1.01 ± 0.60 |
| From day 1 to day 3 | 106 | −1.87 ± 0.91 | 0.0218 | 105 | −1.68 ± 0.71 | 0.2434 | 107 | −1.58 ± 0.76 |
| From day 1 to day 8 | 102 | −2.86 ± 1.28 | 0.0644 | 103 | −2.57 ± 1.05 | 0.5459 | 103 | −2.48 ± 1.01 |
| B | ||||||||
| From day 1 to day 2 | 13 | −0.82 ± 1.02 | 0.1612 | 10 | −1.13 ± 0.93 | 0.8342 | 16 | −1.21 ± 0.73 |
| From day 1 to day 3 | 21 | −1.68 ± 1.26 | 0.1891 | 25 | −2.08 ± 1.13 | 0.6794 | 23 | −2.08 ± 1.00 |
| From day 1 to day 8 | 20 | −3.46 ± 1.31 | 0.1128 | 24 | −3.92 ± 1.61 | 0.8778 | 23 | −3.99 ± 1.24 |
Expressed as log10 TCID50/ml. Values are means ± SD.
Determined by the van Elteren test, which was stratified by current smoking behavior, composite symptom score at baseline, and country/region.
Safety.
The incidence of adverse drug reactions (14.0%, 18.1%, and 20.0% in the 300-mg-peramivir, 600-mg-peramivir, and oseltamivir groups, respectively [Table 5 ]) was significantly lower in the 300-mg-peramivir group and nonsignificantly lower in the 600-mg-peramivir group than in the oseltamivir group. Because peramivir required intravenous access, we were concerned that intravenous administration would result in adverse effects at the injection site. As a result of the study, there were two cases of injection site anesthesia in the 300-mg-peramivir group and one case of injection site irritation in the oseltamivir group. None of these adverse events were considered to be due to test drugs (instead, they were due to blood sampling after day 2 or to intramuscular administration of a concomitant drug).
Table 5.
Summary of adverse events and adverse drug reactions (safety population)
| Parametera | Value for group |
||
|---|---|---|---|
| Peramivir |
Oseltamivir (n = 365) | ||
| 300 mg (n = 364) | 600 mg (n = 364) | ||
| AEs | |||
| Total | |||
| No. of AEs | 272 | 288 | 297 |
| No. (% [95% CI]) of patients with ≥1 | 170 (46.7 [41.5, 52.0]) | 174 (47.8 [42.6, 53.1]) | 178 (48.8 [43.5, 54.0]) |
| P | 0.6040 | 0.8242 | |
| Mild | |||
| No. of mild AEs | 90 | 90 | 95 |
| No. (%) of patients with ≥1 | 69 (19.0) | 66 (18.1) | 74 (20.3) |
| Moderate | |||
| No. of moderate AEs | 161 | 166 | 177 |
| No. (%) of patients with ≥1 | 119 (32.7) | 116 (31.9) | 121 (33.2) |
| Severe | |||
| No. of severe AEs | 21 | 32 | 25 |
| No. (%) of patients with ≥1 | 19 (5.2) | 30 (8.2) | 24 (6.6) |
| ADRs | |||
| Total | |||
| No. of ADRs | 80 | 99 | 104 |
| No. (% [95% CI]) of patients with ≥1 | 51 (14.0 [10.6, 18.0]) | 66 (18.1 [14.3, 22.5]) | 73 (20.0 [16.0, 24.5]) |
| P | 0.0382 | 0.5718 | |
| Mild | |||
| No. of mild ADRs | 40 | 42 | 48 |
| No. (%) of patients with ≥1 | 29 (8.0) | 32 (8.8) | 40 (11.0) |
| Moderate | |||
| No. of moderate ADRs | 37 | 47 | 47 |
| No. (%) of patients with ≥1 | 29 (8.0) | 34 (9.3) | 37 (10.1) |
| Severe | |||
| No. of severe ADRs | 3 | 10 | 9 |
| No. (%) of patients with ≥1 | 3 (0.8) | 10 (2.7) | 9 (2.5) |
| No. (%) of patients with the following AEb: | |||
| Neutrophil count decreased | 39 (10.7) | 38 (10.4) | 34 (9.3) |
| Diarrhea | 24 (6.6) | 30 (8.2) | 27 (7.4) |
| Protein present in urine | 17 (4.7) | 16 (4.4) | 22 (6.0) |
| Blood glucose increased | 11 (3.0) | 14 (3.8) | 12 (3.3) |
| Urine positive for WBCs | 14 (3.8) | 8 (2.2) | 16 (4.4) |
| Nausea | 8 (2.2) | 8 (2.2) | 20 (5.5) |
| Vomiting | 2 (0.5) | 6 (1.6) | 15 (4.1) |
| No. (%) of patients with the following ADRb: | |||
| Diarrhea | 14 (3.8) | 20 (5.5) | 19 (5.2) |
| Neutrophil count decreased | 9 (2.5) | 14 (3.8) | 13 (3.6) |
| Nausea | 2 (0.5) | 7 (1.9) | 16 (4.4) |
AEs, adverse events; ADRs, adverse drug reactions; WBCs, white blood cells. P values were calculated by intergroup comparison between the peramivir and oseltamivir groups using Fisher's exact test.
Data are shown only for AEs or ADRs that occurred at a frequency of >3% in any of the three groups.
Serious adverse events occurred in four patients receiving 300 mg peramivir (myalgia, bronchitis, influenza with acute exacerbation, and pneumonia) and two patients receiving oseltamivir (pneumonia and vomiting). Of these, only vomiting in the oseltamivir group was considered to be an adverse drug reaction.
Most adverse events were mild or moderate in severity. The incidences of severe adverse events were 5.2%, 8.2%, and 6.6% in the 300-mg-peramivir, 600-mg-peramivir, and oseltamivir groups, respectively. There was no difference in the incidence of severe adverse events or adverse drug reactions among the treatment groups. The most common severe adverse events were a prolonged QT interval and a decreased neutrophil count. Prolonged QT intervals were reported by 5, 8, and 10 patients in the 300-mg-peramivir, 600-mg-peramivir, and oseltamivir groups, respectively. Because a separate, thorough QT/QTc study showed that peramivir had no effect on the QT interval (unpublished data), the prolonged QT interval in our current study may have been due to variation in the QT interval during the course of infection. Decreases in neutrophil counts were observed in four, nine, and nine patients in the 300-mg-peramivir, 600-mg-peramivir, and oseltamivir groups, respectively. In many of these patients, the lowest neutrophil count was observed on day 3. The grade and duration of the decreased neutrophil counts observed in the previous phase II study (12) and the current phase III study are summarized in Tables 6 and 7. The incidences of decreased neutrophil counts in the peramivir groups were similar to that in the oseltamivir group. A decrease in the neutrophil count also occurred in patients receiving a placebo in the phase II study (Table 6). The number of days that elapsed until recovery to grade 1 indicated that recovery tended to be at least as rapid in the peramivir group as in the oseltamivir group (Table 7).
Table 6.
Summary of postbaseline minimum neutrophil count by grade (safety population)
| Grade (no. of neutrophils/μl) | No. (%) of patients |
|||
|---|---|---|---|---|
| Phase II studya |
Phase III studyb |
|||
| Peramivir (n = 198) | Placebo (n = 100) | Peramivir (n = 723) | Oseltamivir (n = 363) | |
| Grade 0 (≥1,300/μl) | 151 (76.3) | 89 (89.0) | 557 (77.0) | 288 (79.3) |
| Grade 1 (≥1,000 and <1,300/μl) | 29 (14.6) | 7 (7.0) | 93 (12.9) | 41 (11.3) |
| Grade 2 (≥750 and <1,000/μl) | 14 (7.1) | 3 (3.0) | 60 (8.3) | 25 (6.9) |
| Grade 3 (≥500 and <750/μl) | 3 (1.5) | 1 (1.0) | 10 (1.4) | 7 (1.9) |
| Grade 4 (<500/μl) | 1 (0.5) | 0 (0.0) | 3 (0.4) | 2 (0.6) |
A placebo-controlled randomized study (12).
An oseltamivir-controlled randomized study (our present study).
Table 7.
Summary of time (duration) to recovery from grade 2 or more-severe neutropenia to grade 1 or less (safety population)
| Duration (days) of ≥grade 2 neutropeniaa | No. (%) of patients |
|||
|---|---|---|---|---|
| Phase II studyb |
Phase III studyc |
|||
| Peramivir (n = 198) | Placebo (n = 100) | Peramivir (n = 723) | Oseltamivir (n = 363) | |
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2–3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4–9 | 0 (0.0) | 0 (0.0) | 66 (90.4) | 25 (73.5) |
| 10–14 | 14 (77.8) | 2 (50.0) | 4 (5.5) | 6 (17.6) |
| ≥15 | 4 (22.2) | 2 (50.0) | 3 (4.1) | 1 (2.9) |
| No recovery | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (5.9)d |
| Total | 18 | 4 | 73 | 34 |
Calculated as (date of recovery to grade 1 or less-severe neutropenia) − (date of onset of grade 2 or more-severe neutropenia) + 1.
A placebo-controlled randomized study (12).
An oseltamivir-controlled randomized study (our present study).
Patients were categorized as “not recovered” because follow-up was discontinued.
Pharmacokinetics.
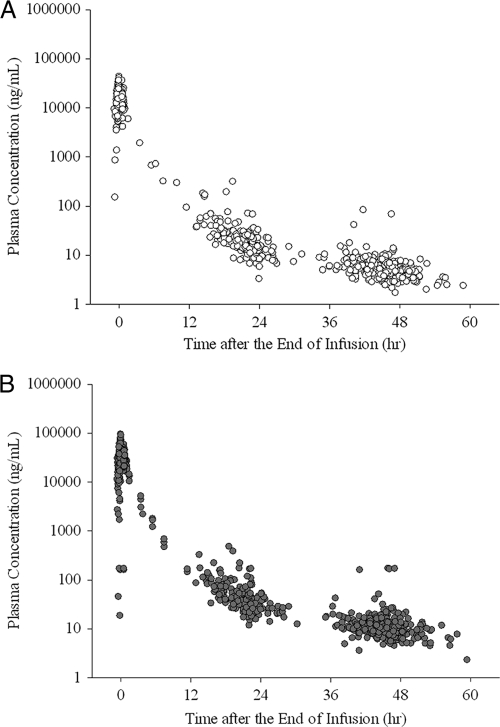
The median duration of infusion was 0.47 h (range, 0.25 to 1.18 h). At the end of infusion, the median plasma peramivir concentrations were 21,800 ng/ml (range, 4,010 to 43,500 ng/ml) (n = 328) in the 300-mg-peramivir group and 43,100 ng/ml (range, 18.6 to 94,900 ng/ml) (n = 317) in the 600-mg-peramivir group. The median plasma peramivir concentrations from 18 to 24 h after the end of infusion in the two groups were 17.4 ng/ml (range, 3.31 to 315 ng/ml) (n = 153) and 33.0 ng/ml (range, 11.8 to 483 ng/ml) (n = 136), respectively. The median plasma peramivir concentrations from 36 to 48 h after the end of infusion were 5.34 ng/ml (range, 1.71 to 83.3 ng/ml) (n = 302) and 10.6 ng/ml (range, 3.59 to 51.1 ng/ml) (n = 291), respectively. The time-plasma peramivir concentration plot is shown in Fig. 4.
Fig. 4.
Plasma peramivir concentrations after administration of 300 mg (top) or 600 mg (bottom) peramivir. The conversion factor between the plasma concentration (in nanograms per milliliter) and the IC50 (nanomolar concentration) was as follows: 1 ng/ml = 2.84 nM.
DISCUSSION
In this study, we compared the efficacy and safety of peramivir with those of oseltamivir (the most widely used anti-influenza drug) in patients with seasonal influenza virus infection. For the primary endpoint, the time to alleviation of symptoms, the noninferiority of 300 mg and 600 mg peramivir to oseltamivir was demonstrated. In addition, peramivir significantly decreased the number of patients with fever (a secondary endpoint) on the day following administration from that with oseltamivir, indicating a rapid peramivir effect. In terms of safety, the incidence of adverse drug reactions was lower in the peramivir groups than in the oseltamivir group and was significantly lower in the 300-mg-peramivir group.
During the 2008-2009 influenza season, when this study was conducted, influenza A/H1N1 viruses (Russian strain) with the H274Y mutation were detected worldwide (5, 7, 15). In our current study as well, the H274Y mutant was isolated from nearly 100% of patients infected with the H1N1 virus. Given the possibility that oseltamivir may have been ineffective in the study population (approximately 50% of patients were infected with the less sensitive H274Y mutant of A/H1N1), the lack of a placebo group may have undermined the significance of the study. Kawai et al. found that the clinical efficacy of oseltamivir against the H1N1 virus with H274Y was reduced, especially among children (10, 11). In clinical trials with laninamivir (CS-8958) in the same season, this drug provided a shorter duration of influenza than did oseltamivir in the H1N1 subpopulation in a pediatric trial, but not in an adult trial (20, 23). These results suggest that no reduction in the efficacy of oseltamivir against oseltamivir-resistant virus infection was obvious in adult patients. In addition, the median duration of influenza in the oseltamivir group (81.8 h) in our current study was comparable to that in past clinical studies conducted in seasons when oseltamivir-sensitive strains were predominant (70.0 to 87.4 h for oseltamivir versus 93.3 to 116.5 h for the placebo), suggesting that the duration of influenza in our current study was within the range of year-to-year variation (9, 16, 22). Therefore, the clinical efficacy of oseltamivir was considered to have been maintained in this study involving adults, and the sensitivity of the study was thus ensured.
This study did not have a placebo group, and we could not confirm the clinical and virological efficacy of peramivir against the resistant A/H1N1 influenza virus that was widespread in the 2008-2009 season. All A/H1N1 viruses isolated in this study had both R222Q and V234Y mutations, which permit the evolution of H274Y-resistant virus by sustaining surface NA expression, and the pathogenicity of the H274Y mutant virus was similar to that of the oseltamivir-sensitive A/H1N1 virus (3, 5). The 2009 pandemic A/H1N1 (pH1N1) viruses that prevailed after the period of the present study remain mostly oseltamivir sensitive, and the introduction of H274Y into the pH1N1 virus causes a large drop in total surface NA expression activity. Pizzorno et al. reported that the I222V mutation in the pH1N1 virus increases concern about the potential emergence and sustained communal transmission of resistant H274Y virus, and they emphasized the importance of continuous monitoring of antiviral resistance in clinical samples as well as the need to develop new drugs (18). The H274Y mutant A/H1N1 virus reportedly exhibits cross-resistance to peramivir. The median (minimum to maximum) IC50s (limit of quantification, 100 nM) for the A/H1N1 virus in the present study were 21.59 (0.41 to 100.00) nM for peramivir and 100.00 (0.66 to 100.00) nM for oseltamivir, showing that sensitivity to peramivir was less affected than sensitivity to oseltamivir. Because the plasma peramivir concentration at the end of infusion of 300 or 600 mg was more than 20,000 ng/ml (ca. 60,000 nM) and much higher than the IC50, peramivir is expected to be effective even in patients infected with the resistant A/H1N1 virus (7, 13). Further study will be needed to clarify whether peramivir provides a clinical benefit to patients with the resistant virus that harbors the H274Y mutation.
In subgroups of patients infected with other subtypes (A/H3N2 and B), peramivir was as effective as oseltamivir. The times to alleviation of symptoms were similar in the two peramivir groups and the oseltamivir group for the subgroup of patients infected with influenza virus A/H3N2; they were significantly shorter (the upper limit of the 97.5% CI for the hazard ratio was lower than 1 [Table 3]) in the 300-mg-peramivir group and nonsignificantly shorter in the 600-mg-peramivir group than in the oseltamivir group for the subgroup of patients infected with influenza B virus. Thus, the consistent efficacy of a single intravenous dose of peramivir may provide reliable outcomes in the practical treatment of influenza.
Our results show that peramivir is generally safe and is expected to be consistently effective after a single intravenous administration at a dose of 300 or 600 mg, regardless of the viral subtype, including A/H1N1, A/H3N2, and B influenza viruses. In addition, treatment with peramivir can be completed with a single intravenous dose, thus ensuring good compliance. These results highlight the usefulness of single-dose intravenous peramivir as an effective therapy for patients with seasonal influenza virus infection.
ACKNOWLEDGMENTS
This clinical trial was funded by Shionogi & Co., Ltd. (Osaka, Japan) and Green Cross Corporation (Yongin, South Korea), which participated in the Korean Study. We express our sincere gratitude to the patients for their participation, to the physicians who conducted the clinical trial in accordance with the protocol, and to all those who acted as coworkers and monitors, for their dedicated work.
The members of the project steering committee of the S-021812 Clinical Study Group were S. Kohno, M.-Y. Yen, H.-J. Cheong, N. Hirotsu, T. Ishida, J. Kadota, M. Mizuguchi, and H. Kida. The members of the writing committee, who take responsibility for the content and accuracy of the paper, were S. Kohno, H. Kida, M. Mizuguchi, J. Shimada, and Masafumi Seki (Nagasaki University, Nagasaki, Japan).
The members of the investigator group of the S-021812 Clinical Study Group in Japan were Hironi Makita, Makita Hospital; Norio Sakai, Hokushin-Naikaichoka Clinic; Hiroshi Hachinohe, Aoba Medical Clinic; Takehiko Kokubun, Teine ENT Clinic; Nobuyuki Ura, Teine Keijinkai Clinic; Shin Tsutahara, Sapporo Naika Clinic; Kaoko Ikeda, Nakajima Hospital; Yasuhiko Munakata, Taihaku Sakura Hospital; Mayumi Eiro, Social Insurance Nihonmatsu Hospital; Hajime Ishii, Kashinoki Internal Medicine Clinic; Takeshi Nawa, Hitachi General Hospital; Naka Araki, Iryohojin Keiyukai Moriya Keiyu Hospital; Tomotaka Kaizan, Iryohojin Keiyukai Ina Clinic; Takenori Okada, National Hospital Organization Utsunomiya National Hospital; Akihiko Okano, Okano Clinic; Makiko Fujikawa, Ayumi Clinic; Motohide Kaneko, Kaneko Internal Medicine Clinic; Tomoya Kouguchi, Ootakanomori Hospital; Yoshiaki Ishizuya, Kuriyama Central Hospital; Yasuko Murakawa, Clinic Kashiwanoha; Mamoru Nakamura, Yatsu Royal Clinic; Shin Totokawa, Gyotoku Flower Street Clinic; Manabu Yamamoto, Adachi Kyosai Hospital; Mitumi Ikeda, Nishio Hospital; Akinori Yamashita, Yamashita Clinic; Tsuyoshi Yamaguchi, Yamaguchi Clinic; Tetsuya Tsurumachi, Tsurumachi Clinic; Keiko Kono, Kono Medical Clinic; Hisakuni Sekino, Sekino Hospital; Sumio Aizawa, Nakano Egota Hospital; Yoshinori Akimoto, Kichijyoji Minami Hospital; Tsuyoshi Yamato, Kouwa Clinic; Shin Suzuki, Suzuki Internal & Circulatory Medical Clinic; Toru Isono, Isono Clinic; Hiroyoshi Kanemitu, Musashikoganei Clinic; Ikkyou Kawa, Ootsuka Kitaguchi Clinic; Yoshihiko Maezawa, Maezawa Clinic; Yuuko Miyazono, Miyazono Internal Medicine Clinic; Hiroshi Toyoda, Kamiyouga Setagaya Street Clinic; Makio Wada, Wada Internal Medical Clinic; Takehide Fumimori, Avex Building Clinic; Haruhisa Kuroda, Kuroda Medical Clinic; Hiroaki Shirai and Toshiaki Ohto, Iryohojin Koganeibashi Sakura Clinic; Kiyomitsu Miyachi, Keigu Clinic; Nobuo Hirotsu, Hirotsu Clinic; Kouichi Miyashita, Fukui General Hospital; Kenji Yamada, Yamada Clinic; Yasunaga Noda, Sakura Clinic; Syuichi Morikawa, Clinic Horikawa of Shakai Iryouhoujin Nishijin Kenkoukai; Toshiki Nishi, Nishi Clinic; Masaharu Kitada, Iryohojin Kowakai Kitada-iin; Emi Kose, Sato Hospital; Akimasa Bandou, Bandou Clinic; Motokazu Kato, Kishiwada City Hospital; Mari Tashiro, Ishikawa Clinic; Sadahiro Senpuku, Senpuku Clinic; Michio Yagi, Osaka Pharmacology Clinical Research Hospital; Hitoshi Yasumoto, Showa Hospital Saibikai; Yoshikazu Irie, Iryouhoujinn Shoutoukai Irie Hospital; Yuji Nakatani, Nakatani Hospital; Satoko Wada, Wada Clinic; Tomohide Yoshimura, Yoshimura Clinic; Hiroki Hara, Kurashiki Daiichi Hospital; Masayoshi Kawanishi, Kaneda Hospital; Susumu Yagi, Yagi Naika Clinic; Soichiro Hozawa, Hiroshima Allergy & Respiratory Clinic; Teiichi Nishio, Medical Corporation Ichijukai Nishio Hospital; Masaharu Kinoshita, Nagata Hospital; Shinichi Tanaka, Shin Yukuhashi Hospital; Kazunori Takahashi, Bunyukai Hara Hospital; Toru Rikimaru, Fukuokaken Saiseikai Futsukaichi Hospital; Takashi Irie, Irie Internal Medicine, Pediatrics Clinic; Tetsuji Akiyama, Fukuokakieikai Hospital; Ken Inoue, Seishinkai Inoue Hospital; Kyota Higashi, Medical Co. Chiyukai Fukuoka Wajiro Hospital; Masahiko Seki, Seki Clinic; Toru Umezu, Iryouhoujin Houmankai Umezu Medical Clinic; Nobukuni Yoshida, Momochihama Clinic Twins Momochi Zaitakushinryozyo; Munemitu Yamamoto, Hakataeki-Higashi Clinic; Shinichi Osaki, Osaki Internal Medicine, Respiratory Clinic; Shigeki Hatama, Hatama Internal Medicine Clinic; Junji Shibata, Medical Corporation Shibata-Clinic; Yuji Harada, Harada Clinic; Kouichi Fukuda, Fukuda General Internal Clinic; Kohji Hashiguchi, Japanese Red Cross Nagasaki Genbaku Hospital; Yoshihiro Yamamoto, Nagasaki University Hospital; Yoji Futsuki, Nagasaki Municipal Hospital; Yuichi Inoue, Isahaya Health Insurance General Hospital; Yasuhito Higashiyama, Hokusho Central Hospital; Hironobu Koga, Miyazaki Clinic; Yasumasa Doutsu, Iryouhoujin Kouseikai Hospital; Toshie Onodera, Mie Memorial Hospital; Eiji Yamagata, Goto Clinic; Takashige Miyazaki, Miyazaki Clinic, Oita City; Issei Tokimatsu, Oita University Hospital; Yoichi Karimata, Healthcare Corporation Taiyoukai Karimata Medical Clinic; Hiroshi Sakugawa, Medical Corporation Kariyushi Heart-Life Hospital; Masamichi Gushiken, Healthcare Corporation Doushinkai Gushiken Cardiovascular Medical Clinic; Hiroshi Nakamura, Healthcare Corporation H·S·R Nakamura Clinic; and Kiyoyuki Tokuyama, Healthcare Corporation Seishinkai Tokuyama Clinic.
The members of the investigator group in Taiwan were Ming-Yuan Chou, Cheng Hsin Rehabilitation Medical Center; Bor-Shen Hu, Taipei City Hospital, Heping Branch; Muh-Yong Yen, Taipei City Hospital, Renai Branch; Teng-Ho Wang, Taipei City Hospital, Zhongxiao Branch; Jia-Fong Lue, Taipei City Hospital, Zhongxing Branch; Yung-Fong Yen, Taipei City Hospital, Yangming Branch; Tsung-Zu Wu, Taipei City Hospital, Women & Children Branch; Hen-I Lin, Cardinal Tien Hospital; Peter Woo, Cardinal Tien Hospital, Yung Ho Branch; Pa-Chun Wang, Cathay General Hospital; Wen-Han Chang, Mackay Memorial Hospital; Kuang-Chau Tsai, Far Eastern Memorial Hospital; Yuarn-Jang Lee, Taipei Medical University Hospital; Ke-Chang Chang, National Yang-Ming University Hospital; Ko-Hsin Hu, Keelung Hospital, Department of Health; Hsin-Hung Pan, Kuang Tien General Hospital, Dajia Branch; Pu-Sheng Yeh, Min-Sheng General Hospital; Yin-Ching Chuang, Chi Mei Medical Center; Sheng-Chung Tsai, Taipei Hospital, Department of Health; Cheng-Yao Liu, Cathay General Hospital, Sijhih Branch; and Chao-Huei Yang, Kuang Tien General Hospital, Shalu Branch.
The members of the investigator group in the Republic of Korea were Seong-Heon Wie, St. Vincent Hospital; Ja Cob Lee, Hallym University Kangnam Sacred Heart Hospital; Jin Won Chung, Chung Ang University Medical Center; Eun-Ju Choo, Soonchunhyang University Bucheon Hospital; Hee Jung Choi, Ewha Womans University Mokdong Hospital; Hee Jung Yoon, Daejeon Eulji University Hospital; Hyo Youl Kim, Wonju-Christian Hospital; Sook In Jung, Chonnam National University Hospital; Yee Gyung Kwak, Inje University Ilsan Paik Hospital; Hee Jin Cheong, Korea University Guro Hospital; Yoon Soo Park, Gachon University Gil Medical Center; Sang-Rok Lee, Cheongju Saint Mary's Hospital; Sun Hee Lee, Pusan University Hospital; Jin-Soo Lee, Inha University Hospital; Jun Jae Bum, Ulsan University Hospital; Seung Soon Lee, Hallym University Sacred Heart Hospital; Sung Kwan Hong, Bundang Cha Hospital; Jae Phil Choi, Seoul Medical Center; Eun Seok Kim, Sam Anyang Hospital; Hyuck Lee, Dong-A University Hospital; Seong Ho Choi, Chung-Ang University Yong-San Hospital; Yeon-Sook Kim, Chungnam National University Hospital; Eu Suk Kim, Dongkuk University International Hospital; Dong Min Kim, Chosun University Hospital; and Dae Won Park, Korea University Ansan Hospital.
S.K. was a medical adviser for the study and a technical advisor to Chugai-Roche, Daiichi Sankyo, and Taisho-Toyama and has received research grants from Shionogi, Chugai-Roche, Daiichi Sankyo, Taisho-Toyama, and GlaxoSmithKline. H.K. was a virological adviser for the study and has received research funding from Shionogi. M.M. was a medical adviser for the study and has received research funding from Shionogi. M.-Y.Y. was a coordinating investigator for the study and has received research funding from Shionogi. H.-J.C. was a coordinating investigator for the study and has received research funding from Green Cross. N.H. was a coordinating investigator for the study and a technical advisor to Shioinogi and has received consulting fees from Shionogi. T.I. was a coordinating investigator for the study. J.K. was a coordinating investigator for the study and a technical advisor to Chugai-Roche, Daiichi Sankyo, Taisho-Toyama, and GlaxoSmithKline and has received research funding from Shionogi, Chugai-Roche, Daiichi Sankyo, Taisho-Toyama, and GlaxoSmithKline. J.S. is employed as a counselor for Shionogi & Co., Ltd.
Footnotes
Published ahead of print on 8 August 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Babu Y. S., et al. 2000. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J. Med. Chem. 43:3482–3486 [DOI] [PubMed] [Google Scholar]
- 2. Bantia S., et al. 2001. Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob. Agents Chemother. 45:1162–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bloom J. D., Gong L. I., Baltimore D. 2010. Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science 328:1272–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dharan N. J., et al. 2010. Antiviral treatment of patients with oseltamivir-resistant seasonal influenza A (H1N1) infection during the 2007–2008 influenza season in the United States. Clin. Infect. Dis. 50:621–622 [DOI] [PubMed] [Google Scholar]
- 5. Dharan N. J., et al. 2009. Infections with oseltamivir-resistant influenza A (H1N1) virus in the United States. JAMA 301:1034–1041 [DOI] [PubMed] [Google Scholar]
- 6. Hayden F. 2009. Developing new antiviral agents for influenza treatment: what does the future hold?. Clin. Infect. Dis. 48:S3–S13 [DOI] [PubMed] [Google Scholar]
- 7. Hurt A. C., et al. 2009. Emergence and spread of oseltamivir-resistant A (H1N1) influenza viruses in Oceania, South East Asia and South Africa. Antiviral Res. 83:90–93 [DOI] [PubMed] [Google Scholar]
- 8. Kamigaki T., Oshitani H. 2009. Epidemiological characteristics and low case fatality rate of pandemic (H1N1) 2009 in Japan. PLoS Curr. 1:RRN1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kashiwagi S., Kudoh S., Watanabe A., Yoshimura I. 2000. Clinical efficacy and safety of selective oral neuraminidase inhibitor oseltamivir in treating acute influenza: placebo controlled double-blind multicenter phase III trial. Kansenshogaku Zasshi 74:1044–1061 (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 10. Kawai N., et al. 2009. Clinical effectiveness of oseltamivir and zanamivir for treatment of influenza A virus subtype H1N1 with the H274Y mutation: a Japanese, multicenter study of the 2007–2008 and 2008–2009 influenza seasons. Clin. Infect. Dis. 49:1828–1835 [DOI] [PubMed] [Google Scholar]
- 11. Kawai N., et al. 2009. Clinical effectiveness of oseltamivir for influenza A(H1N1) virus with H274Y neuraminidase mutation. J. Infect. 59:207–212 [DOI] [PubMed] [Google Scholar]
- 12. Kohno S., Kida H., Mizuguchi M., Shimada J., for the S-021812 Clinical Study Group 2010. Efficacy and safety of intravenous peramivir for treatment of seasonal influenza virus infection. Antimicrob. Agents Chemother. 54:4568–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kohno S., et al. 2011. Intravenous peramivir for treatment of influenza A and B virus infection in high-risk patients. Antimicrob. Agents Chemother. 55:2803–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Medeiros R., et al. 2007. Failure of zanamivir therapy for pneumonia in a bone-marrow transplant recipient infected by a zanamivir-sensitive influenza A (H1N1) virus. Antivir. Ther. 12:571–576 [PubMed] [Google Scholar]
- 15. Moscona A. 2009. Global transmission of oseltamivir-resistant influenza. N. Engl. J. Med. 360:953–956 [DOI] [PubMed] [Google Scholar]
- 16. Nicholson K. G., et al. 2000. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet 355:1845–1850 [DOI] [PubMed] [Google Scholar]
- 17. Osborne R. H., Hawthorne G., Panicolaou M., Wegmueller Y. 2000. Measurement of rapid changes in health outcomes in people with influenza symptoms. J. Outcomes Res. 4:15–30 [Google Scholar]
- 18. Pizzorno A., Bouhy X., Abed Y., Boivin G. 2011. Generation and characterization of recombinant pandemic influenza A(H1N1) viruses resistant to neuraminidase inhibitors. J. Infect. Dis. 203:25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Potier M., Mameli L., Belislem M., Dallaire L., Melanxon S. B. 1979. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-α-d-N-acetylneuraminate) substrate. Anal. Biochem. 94:287–296 [DOI] [PubMed] [Google Scholar]
- 20. Sugaya N., Ohashi Y. 2010. Long-acting neuraminidase inhibitor laninamivir octate (CS-8958) versus oseltamivir as treatment for children with influenza virus infection. Antimicrob. Agents Chemother. 54:2575–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor W. R., et al. 2008. Oseltamivir is adequately absorbed following nasogastric administration to adult patients with severe H5N1 influenza. PLoS One 3:e3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Treanor J. J., et al. 2000. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. JAMA 283:1016–1024 [DOI] [PubMed] [Google Scholar]
- 23. Watanabe A., Chang S.-C., Kim M. J., Chu D. W., Ohashi Y., for the MARVEL Study Group 2010. Long-acting neuraminidase inhibitor laninamivir octate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin. Infect. Dis. 51:1167–1175 [DOI] [PubMed] [Google Scholar]
- 24. World Health Organization February 2010, revision date WHO guidelines for pharmacological management of pandemic influenza A(H1N1) 2009 and other influenza viruses. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/resources/publications/swineflu/h1n1_guidelines_pharmaceutical_mngt.pdf [PubMed] [Google Scholar]
- 25. Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza 2010. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N. Engl. J. Med. 362:1708–1719 [DOI] [PubMed] [Google Scholar]
- 26. Zarychanski R., et al. 2010. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ 182:257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]