Abstract
Ten blaKPC-2-harboring Pseudomonas aeruginosa isolates from hospitals located in five different Colombian cities have been characterized. Isolates were multidrug resistant, belonged to five different pulsotypes, and possessed naturally chromosome-encoded blaAmpC and blaOXA-50 genes and the acquired blaKPC-2 gene. In most cases, the blaKPC-2 genes were carried by plasmids of different sizes and were associated with Tn4401b or a new structure containing only part of the Tn4401 sequence. This study revealed that several clones of P. aeruginosa producing blaKPC-2 are disseminating in Colombia.
TEXT
Carbapenem resistance in Pseudomonas aeruginosa isolates represents a major threat to the management of nosocomial infections and involves several mechanisms, such as porin modifications, efflux pump overexpression, and acquired carbapenem-hydrolyzing β-lactamases (carbapenemases) (10, 14). Carbapenemases identified in P. aeruginosa are mostly metallo-ß-lactamases (14), but the Ambler class A β-lactamase KPC has also been reported (17). KPC carbapenemases were described initially in a report of a Klebsiella pneumoniae isolate collected in 2001 in North Carolina (20) and have since rapidly emerged and disseminated in the world, in enterobacterial species in particular (4, 12). KPC-producing P. aeruginosa isolates were reported first in 2006 from Colombia (17) and subsequently in Puerto Rico (19), in Trinidad and Tobago (1), in the southern part of the United States (13), and very recently in China (6). The rapid spread of KPC enzymes in Enterobacteriaceae has been linked to the genetic elements carrying the blaKPC-2 gene: plasmids of different sizes harboring a Tn3-like transposon, Tn4401 (4, 11). Nevertheless, other genetic structures have been characterized with different insertion se-quences (IS) upstream of the blaKPC gene, as seen in a P. aeruginosa isolate harboring blaKPC-5 (8, 19). Until now, little has been known about the clonal relationships and genetic background of KPC-producing P. aeruginosa strains. The aim of the present work was to characterize 10 blaKPC-2-harboring P. aeruginosa isolates collected from Colombian hospitals located in different cities scattered throughout the country and sent to the International Center for Medical Research and Training (CIDEIM), Cali, Colombia, between 2006 and 2010.
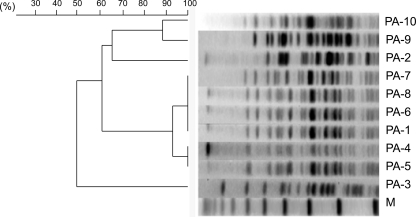
Molecular typing by pulsed-field gel electrophoresis (PFGE) identified five pulsotypes (A to E) among the isolates (Table 1; Fig. 1). Two closely related patterns (A1 and A2), differing by only one band, were identified in isolates from three different wards in the same hospital in Medellin, Colombia. Four isolates belonged to clone A1, and two others belonged to clone A2. The other isolates were unrelated, differing by at least seven bands, thus suggesting heterogeneity among KPC-producing P. aeruginosa strains from Colombia. Four distinct allelic profiles were obtained by multilocus sequence type (MLST) analysis (Table 1) (3): ST308 (isolates PA-1 and PA-4 to -8), ST235 (isolates PA-9 and PA-10), ST1006 (isolate PA-2), and ST1060 (isolate PA-3). These results matched perfectly with the PFGE results. The four sequence types identified were not single- or double-locus variants of each other. Among them, only ST235 had already been identified as a single-locus variant of ST227 and ST230; all three correspond to clonal complex CC11 (7), which has been isolated in several countries and has been shown to be associated with various β-lactamases (5, 7). The dissemination of blaKPC into genetically unrelated P. aeruginosa isolates has already been described in Puerto Rico (19), where seven different clones have been found, highlighting the rapid spread of the blaKPC gene in this area.
Table 1.
Origin, structure of Tn4401, other β-lactamases, plasmid analysis, pulsotype, and sequence type of the P. aeruginosa isolates
| Isolate | Origin in Columbia | Yr of isolation | Site of isolation | Location | PCR and sequencing result (Tn4401 homology) |
blaKPC-2-positive plasmid |
Bacterial isolate characteristic |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Size (kb) | Transformant |
Pulsotype | Sequence type | ||||||||||
| blaKPC-2 | TnpA | ISKPN6 | ISKPN7 | ECa | PAb | |||||||||
| PA-1 | Medellin | 2006 | Bronchial secretion | Tertiary care center | KPC-2 | + | + | + | pCOL | 45 | + | + | A1 | ST308 |
| PA-2 | Bogota | 2006 | Stool | Unknown | KPC-2 | − | + | − | pPA-2 | 13 | + | + | B | ST1006 |
| PA-3 | Barranquiila | 2006 | Bronchial secretion | Intensive care unit | KPC-2 | + | + | + | pPA-3 | 60 | − | − | C | ST1060 |
| PA-4 | Medellin | 2006 | Bone | General wardc | KPC-2 | + | + | + | pPA-4 | 45 | + | + | A2 | ST308 |
| PA-5 | Medellin | 2006 | Surgical wound | General ward | KPC-2 | + | + | + | pPA-5 | 45 | + | + | A2 | ST308 |
| PA-6 | Medellin | 2006 | Surgical wound | General ward | KPC-2 | + | + | + | pPA-6 | 45 | + | + | A1 | ST308 |
| PA-7 | Medellin | 2006 | Urine | General ward | KPC-2 | + | + | + | pPA-7 | 45 | + | + | A1 | ST308 |
| PA-8 | Medellin | 2006 | Tracheal aspirate | Intensive care unit | KPC-2 | + | + | + | pPA-8 | 45 | + | + | A1 | ST308 |
| PA-9 | Cali | 2007 | Peritoneal fluid | General ward | KPC-2 | + | + | + | − | − | − | − | D | ST235 |
| PA-10 | Pereira | 2010 | Urine | Intensive care unit | KPC-2 | + | + | + | − | − | − | − | E | ST235 |
Transformants into E. coli (EC) DH10B.
Transformants into P. aeruginosa (PA) KG2505.
General ward: patients hospitalized for surgical or medical disease.
Fig. 1.
Dendrogram of pulsed-field gel electrophoresis data (SpeI digests) showing genetic relatedness between the 10 KPC-producing reference isolates. M, molecular weight.
Antibiotic susceptibility was determined by the disk diffusion method, and MICs of carbapenems were determined using Etest strips (bioMérieux, Marcy l'Etoile, France) and interpreted according to Clinical and Laboratory Standards Institute guidelines (2). The 10 isolates were resistant to all penicillins and expanded-spectrum cephalosporins tested and showed carbapenem MIC values above 32 mg/ml (Table 1). The use of cloxacillin (200 mg/liter)-containing Mueller-Hinton (MH) agar plates did not alter the antibiogram results. All isolates remained susceptible to colistin. Eight isolates (PA-1 and PA-4 to -10) were also fully resistant to all tested aminoglycosides (amikacin, gentamicin, netilmicin, and tobramycin) and to ciprofloxacin; one isolate (PA-2) was susceptible to all tested aminoglycosides and resistant to ciprofloxacin; and a single isolate (PA-3) was susceptible to ciprofloxacin, amikacin, and fosfomycin, according to guidelines from CA-SFM (http://www.sfm-microbiologie.org).
PCR and sequencing (Table 2) (11) revealed that all isolates possessed blaKPC-2 genes and naturally and chromosomally encoded blaAmpC and blaOXA-50 genes, but blaSHV, blaTEM, blaCTX-M, blaVIM, blaIMP, and blaOXA-9 genes could not be evidenced.
Table 2.
Primers used in this study
| Name of primer | Fig. 2 lane no. | Sequence (5′-3′) | Reference |
|---|---|---|---|
| KPC-A | 1 | CTGTCTTGTCTCTCATGGCC | 11 |
| KPC-B | 2 | CCTCGCTGTGCTTGTCATCC | 11 |
| 3098U | 3 | TGACCCTGAGCGGCGAAAGC | 11 |
| 4914L | 4 | GAAGATGCCAAGGTCAATGC | 11 |
| TnpA-178U | 5 | CTTGCAGATCGGCTACTTCA | This study |
| TnpA-1198U | 6 | CGCCATCTTCGTCCACTGTA | This study |
| TnpA-2537L | 7 | GCCCTGCGTCATTTCCTTCA | This study |
| ISKpn7-24U | 8 | CCCACCCCAGCGAAGTAAAAC | This study |
| ISKpn7-1916L | 9 | GACCCACTTTACCCCTGAAT | This study |
| ISKpn6-82U | 10 | CGTCGGCGCCAAGGATACCA | This study |
| ISKpn6-1429L | 11 | ATCTGCTGCCCCCTTCTCTG | This study |
| 141R-6 | 12 | TCACCGGCCCTCACCTTTGG | 11 |
| EcoRI-out | 13 | CACCCGACCTGGACGAACTA | 11 |
| Delta SHVa | AAGATCCACTATCGCCAGCAG | 4 | |
| Delta SHVb | ATTCAGTTCCGTTTCCCAGCGG | 4 | |
| Pre-TEM1 | GTATCCGCTCATGAGACAATA | 4 | |
| Pre-TEM2 | TCTAAAGTATATATGAGTAAACTTGGTCTG | 4 | |
| CTX-MA | CGCTTTGCGATGTGCAG | 4 | |
| CTX-MB | ACCGCGATATCGTTGGT | 4 | |
| VIM-B | ATGGTGTTTGGTCGCATATC | 11 | |
| VIM-F | TGGGCCATTCAGCCAGATC | 1 | |
| IMP-2004A | ACAYGGYTTGGTDGTTCTTG | 1 | |
| IMP-2004B | GGTTTAAYAAAACAACCACC | 1 | |
| OXA-9A | TTCGTTTCCGCCACTCTCCC | 4 | |
| OXA-9B | ACGAGAATATCCTCTCGTGC | 4 |
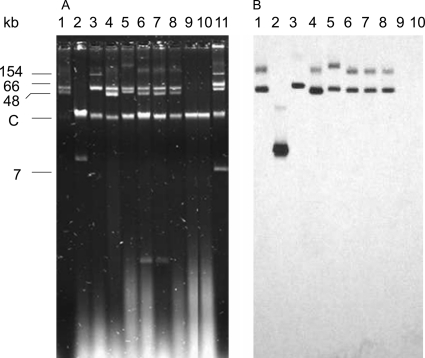
By the Kieser extraction method (9), the six isolates from Medellin (PA-1 and PA-4 to -8) and Barranquilla (PA-3) showed several plasmids of different sizes (45 kb to ≥150 kb) (Fig. 2A). One isolate from Bogota (PA-2) showed only one plasmid (13 kb), and no plasmid could be evidenced for strains from Cali (PA-9) and Pereira (PA-10), suggesting a chromosomal location of the blaKPC-2 gene, as has already been described in P. aeruginosa isolates (17) (Fig. 2B). Only one plasmid hybridized with an internal probe for a blaKPC gene in each isolate, with sizes ranging from 13 to 60 kb (Fig. 2A; Table 1). Transformants into Escherichia coli DH10B could be obtained with all the natural plasmids except with the 60-kb pPA-3 plasmid. No transformants could be obtained with Kieser extracts from PA-9 and PA-10. No other antibiotic resistance marker was cotransferred. The natural plasmids pPA-1, pPA-2, and pPA-4 to -8 were also electroporated into the AmpC-deficient P. aeruginosa KG2505 strain (16) and conferred a high level of resistance to all ß-lactams tested, including carbapenems. Previous studies have reported that OprD porin was absent in numerous KPC-producing P. aeruginosa isolates (17, 18) and is associated both with overexpression of the mexAB-oprM efflux pump and with increases in carbapenem MICs. However, in the present study, electroporation of plasmids harboring the blaKPC-2 gene was sufficient to confer a high level of resistance to the naturally susceptible P. aeruginosa KG2505 isolate.
Fig. 2.
(A) Plasmid extractions from cultures of the different isolates by the Kieser method. (B) Southern hybridization carried out with an internal probe for the blaKPC-2 gene. Lane 1, P. aeruginosa PA-1; lane 2, P. aeruginosa PA-2; lane 3, P. aeruginosa PA-3; lane 4, P. aeruginosa PA-4; lane 5, P. aeruginosa PA-5; lane 6, P. aeruginosa PA-6; lane 7, P. aeruginosa PA-7; lane 8, P. aeruginosa PA-8; lane 9, P. aeruginosa PA-9; lane 10, P. aeruginosa PA-10; lane 11, E. coli 50192 harboring four (7-, 48-, 66-, and 154-kb) plasmids. C, chromosome.
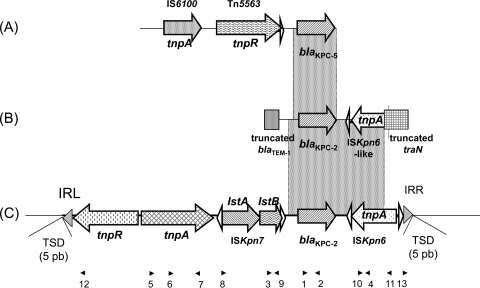
A PCR mapping approach revealed that the blaKPC-2 gene was associated with Tn4401b isoform in PA-1 and PA-3 to -10 (Table 2, Fig. 3). The natural plasmids pPA-1 and pPA-4 to -8 were extracted from electroporants and directly sequenced using outward-directed primers located next to the inverted repeats (IRs). For these six plasmids, the flanking sequences of Tn4401b were identical; it was inserted into an open reading frame (ORF) of 297 bp initially described as being carried on plasmid pRSB105, which was extracted from uncultured bacteria of a sewage plant in Germany (15). Upon insertion, Tn4401b generated identical 5-bp GCGCT target site duplications. For plasmid pPA-2, PCR mapping gave negative results, suggesting a different genetic organization, which was confirmed by direct sequencing of the plasmid. Only a 2,072-bp region, including the blaKPC-2 gene and an ISKpn6-like ORF previously described in a study of K. pneumoniae isolates collected in China, was identical to Tn4401 (8). This ISKpn6-like element shared a 1,039-bp fragment with ISKpn6. The target site duplication and the left inverted repeat (IRL) as described in Tn4401 were present, but the ORF encoding the putative transposase (439 amino acids in ISKpn6) was truncated in its N terminus by a 117-bp fragment encoding the C-terminal region of a protein sharing 100% amino acid identity with the traN protein from plasmid pFBAOT6 previously described in a study of Aeromonas punctata (GenBank accession no. NC_006143) (14a). Upstream of the blaKPC-2 gene, only a 73-bp segment, which is interrupted by a truncated blaTEM gene, is identical to Tn4401.
Fig. 3.
Schematic representations of genetic structures surrounding the blaKPC gene in different P. aeruginosa isolates. (A) P. aeruginosa PR280 harboring blaKPC-5 from Puerto Rico (19); upstream of the blaKPC-5 gene, a portion of the transposable element Tn5563, previously described in plasmid pRA2 from P. alcaligenes, was found. (B) Novel structure characterized in PA-2 from Colombia. (C) Structure of Tn4401b. The dotted rectangles indicate the common regions between the different structures. Horizontal arrows indicate genes and their corresponding transcription orientations. Gray triangles represent the left inverted repeat (IRL) and right inverted repeat (IRR) of Tn4401. Small and empty triangles represent the inverted repeats of ISKpn6 and ISKpn7. Target site duplications (TSDs) are indicated above the sequence. Small black triangles above the sequence represent the primers listed in Table 2.
Unlike Enterobacteriaceae, P. aeruginosa isolates expressing KPC carbapenemase seem to be geographically limited. The presence of blaKPC in P. aeruginosa isolates is worrying in a species that is known to be prone to becoming carbapenem resistant by multiple mechanisms. Our analysis of several KPC-producing P. aeruginosa isolates from different Colombian hospitals revealed the spread of different clones that harbor different plasmids and different genetic structures, including the Tn4401b isoform associated with the blaKPC-2 gene. The emergence of blaKPC-2 in different species of Enterobactriaceae and the spread of this gene to P. aeruginosa isolates in different countries emphasizes the potential for dissemination worldwide. Since phenotypic detection is still difficult, it remains to be determined to what extent KPC-producing P. aeruginosa have spread worldwide, particularly in South America.
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been assigned to the GenBank nucleotide database (accession number JN545009).
Acknowledgments
The work was funded by INSERM, France, by a grant from the Ministère de l'Education Nationale et de la Recherche (UPRES-EA3539), Université Paris XI, France, by the Assistance Publique-Hôpitaux de Paris, France, by the European Community (6th PCRD [LSHMCT-2003-503-335], TROCAR contract HEALTH-F3-2008-223031), and by the Chicago Infectious Diseases Research Institute.
This publication made use of the Pseudomonas aeruginosa MLST website (http://pubmlst.org/paeruginosa/) developed by Keith Jolley and sited at the University of Oxford (8a). The development of the site has been funded by the Wellcome Trust.
We thank the institutions that are part of the Colombian Nosocomial Resistance Study Group, whose members are as follows: Henry Mendoza, Flor Angela Cubides, Martha Patricia Melendez, Luz Angelica Quintero, and Ilba Liliana Galeano at Hospital Central de la Policía; Beatriz Porras, Guillermo Ortiz, and Luz Mila Lopez at Hospital Santa Clara; Henry Oliveros, Maria Nilse Gonzalez, Angela Pescador, Monica Ballesteros, Sandra Valderrama, Alirio Rodriguez, and Jairo Perez at Hospital Militar Central; Carlos Ignacio Gomez, Jaime Lopez, Jorge Donado, Monica Cuartas, Ana Lucia Correa, and Lina Marcela Castañeda at Hospital Pablo Tobon Uribe; Julian Betancourth, Juan David Villa, Jorge Nagles, Nancy Estella Gonzalez, Magda Orjuela, Ana Cristina Quiroga, Rodrigo Agudelo, and Carolina Rios at Clínica de las Américas; Martha Vallejo, Luz Marina Melguizo, Dora Rivas, and Sergio Velez at Hospital General de Medellín; Ernesto Martinez, Jose Millan Oñate, Christian Pallares, Luz Marina Gallardo, Alba Lucia Bohorques, and Nancy Villamarin at Hospital Universitario del Valle; Fernando Rosso, Juan Diego Velez, Jose Garcia, Monica Recalde, Alejandra Toala, and John Jairo Echeverry at Clínica Fundación Valle del Lili; Beatriz Lopez and Claudia Barcenas at La Foscal; Karol Monsalve, Luis Angel Villar, and Mayerly Anaya at Fundación Cardiovascular; Agustin Vega, Miriam Fanny Amaya, Matha Jacome, Rocio Abaunza, and Martin Mejía at Hospital Universitario de Santander; Angela Mendoza at Hospital Universitario San Jorge; Carmen Elisa Llano, Myriam Gomez, and Rodolfo Cabrales at Clínica General del Norte, and Amparo Ovalle, Claudia Echeverry, M. del Rosario Aldana, Pablo Lopez, and Luis Gonzalez at Hospital Federico Lleras Acosta.
The formation of the network of institutions of the Colombian Nosocomial Resistance Study Group was made possible thanks in part to the support of: Merck Sharp & Dohme; Janssen-Cilag SA; Pfizer SA; AstraZeneca Colombia SA; Merck Colombia; and Novartis.
No financial or commercial interests were involved in the development of this study, except that J. P. Quinn is an employee of Pfizer Global Research and Development (New London, CT) and M. V. Villegas has received consulting fees and research grants from Merck Sharp & Dohme; Pfizer SA; Janssen-Cilag SA; Bayer SA; Novartis; Merck Colombia; and AstraZeneca Colombia SA.
Footnotes
Published ahead of print on 15 August 2011.
REFERENCES
- 1. Akpaka P. E., et al. 2009. Emergence of KPC-producing Pseudomonas aeruginosa in Trinidad and Tobago. J. Clin. Microbiol. 47:2670–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clinical Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement M100-S21. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 3. Curran B., Jonas D., Grundmann H., Pitt T., Dowson C. G. 2004. Development of a multilocus sequence typing scheme for the opportunistic pathogen Pseudomonas aeruginosa. J. Clin. Microbiol. 42:5644–5649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cuzon G., et al. 2010. Worldwide diversity of Klebsiella pneumoniae that produce ß-lactamase blaKPC-2 gene. Emerg. Infect. Dis. 16:1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Empel J., et al. 2007. Outbreak of Pseudomonas aeruginosa infections with PER-1 extended-spectrum ß-lactamase in Warsaw, Poland: further evidence for an international clonal complex. J. Clin. Microbiol. 45:2829–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ge C., et al. 2011. Identification of KPC-2-producing Pseudomonas aeruginosa isolates in China. J. Antimicrob. Chemother. 66:1184–1186 [DOI] [PubMed] [Google Scholar]
- 7. Giske C. G., et al. 2006. Establishing clonal relationships between VIM-1-like metallo-ß-lactamase-producing Pseudomonas aeruginosa strains from four European countries by multilocus sequence typing. J. Clin. Microbiol. 44:4309–4315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang Y., et al. 2010. Complete nucleotide sequence of Klebsiella pneumoniae multidrug resistance plasmid pKP048, carrying blaKPC-2, blaDHA-1, qnrB4, and armA. Antimicrob. Agents Chemother. 54:3967–3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a. Jolley K. A., Chan M. S., Maiden M. C. 2004. mlstdbNet—distributed multi-locus sequence typing (MLST) databases. BMC Bioinformatics 5:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kieser T. 1984. Factors affecting the isolation of cccDNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19–36 [DOI] [PubMed] [Google Scholar]
- 10. Livermore D. M. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis. 34:634–640 [DOI] [PubMed] [Google Scholar]
- 11. Naas T., et al. 2008. Genetic structures at the origin of acquisition of the beta-lactamase blaKPC gene. Antimicrob. Agents Chemother. 52:1257–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nordmann P., Cuzon G., Naas T. 2009. The real threat of KPC carbapenemase-producing bacteria. Lancet Infect. Dis. 9:228–236 [DOI] [PubMed] [Google Scholar]
- 13. Poirel L., Lagrutta E., Cleary T., Munoz-Price L. S., Nordmann P. 2010. Emergence of KPC-producing Pseudomonas aeruginosa, United States. Antimicrob. Agents Chemother. 54:3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Queenan A. M., Bush K. 2007. Carbapenemases: the versatile ß-lactamases. Clin. Microbiol. Rev. 20:440–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a. Rhodes G., et al. 2004. Complete nucleotide sequence of the conjugative tetracycline resistance plasmid pFBAOT6, a member of a group of IncU plasmids with global ubiquity. Appl. Environ. Microbiol. 70:7497–7510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schlüter A., et al. 2007. Erythromycin resistance-conferring plasmid pRSB105, isolated from a sewage treatment plant, harbors a new macrolide resistance determinant, an integron-containing Tn402-like element, and a large region of unknown function. Appl. Environ. Microbiol. 73:1952–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith A. W., Iglewski B. H. 1989. Transformation of Pseudomonas aeruginosa by electroporation. Nucleic Acids Res. 17:10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Villegas M. V., et al. 2007. First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing ß-lactamase. Antimicrob. Agents Chemother. 51:1553–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolter D. J., et al. 2009. Surveillance of carbapenem-resistant Pseudomonas aeruginosa isolates from Puerto Rican medical center hospitals: dissemination of KPC and IMP-18 ß-lactamases. Antimicrob. Agents Chemother. 53:1660–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolter D. J., et al. 2009. Phenotypic and enzymatic comparative analysis of the novel KPC variant KPC-5 and its evolutionary variants, KPC-2 and KPC-4. Antimicrob. Agents Chemother. 53:557–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yigit H., et al. 2001. Novel carbapenem-hydrolyzing ß-lactamase KPC-1 from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45:1151–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]