Abstract
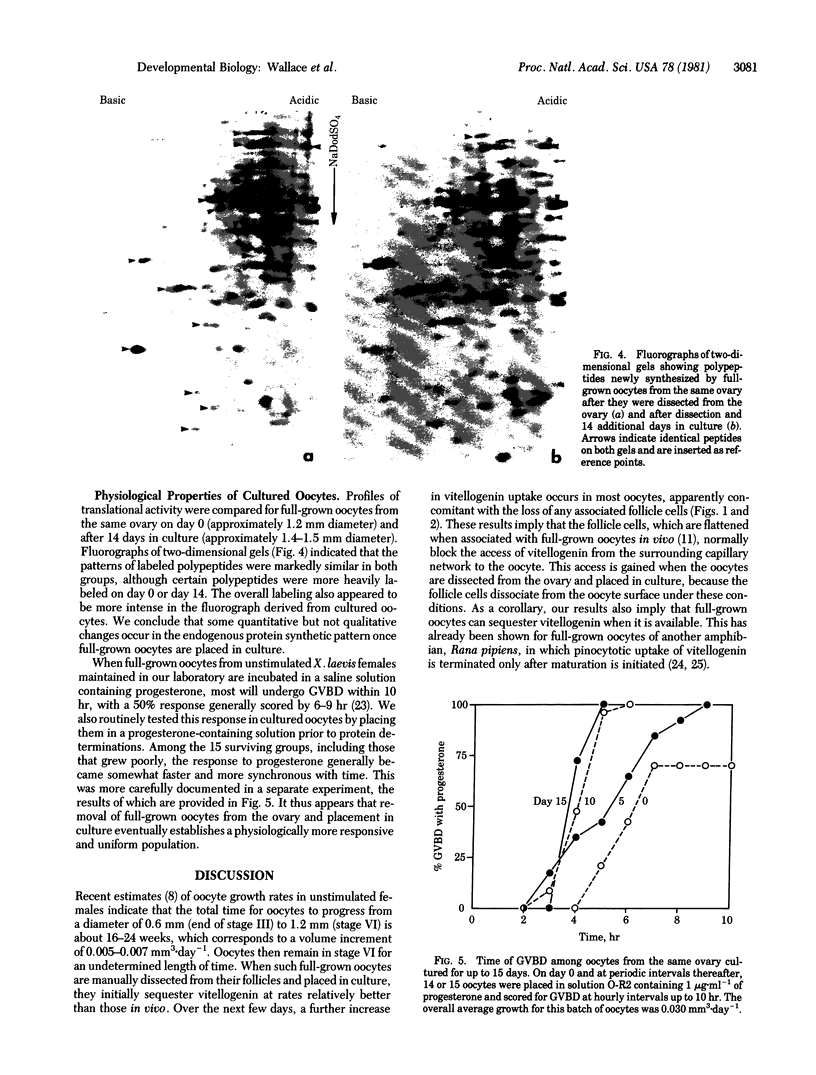
When most full-grown, follicle cell-invested oocytes from Xenopus laevis are placed in an appropriate culture medium, they resume growth and remain physiologically healthy for at least 2-3 weeks. Rates of growth by full-grown oocytes in vitro generally approximate and can even exceed the most rapid growth rate achieved by vitellogenic oocytes in vivo. Resumption of oocyte growth can be correlated with the loss of investing follicle cells, which under normal conditions appear to interfere with vitellogenin and nutrient access to the oocyte. The final size reached by the oocyte within the ovary is thus not an intrinsic property of the oocyte but is extrinsically imposed by the somatic environment.
Keywords: follicle cells, vitellogenin, nutrient availability, oocyte maturation
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- Canfield R. E., Morgan F. J., Kammerman S., Bell J. J., Agosto G. M. Studies of human chorionic gonadotropin. Recent Prog Horm Res. 1971;27:121–164. doi: 10.1016/b978-0-12-571127-2.50028-6. [DOI] [PubMed] [Google Scholar]
- De Robertis E. M., Partington G. A., Longthorne R. F., Gurdon J. B. Somatic nuclei in amphibian oocytes: evidence for selective gene expression. J Embryol Exp Morphol. 1977 Aug;40:199–214. [PubMed] [Google Scholar]
- Dolecki G. J., Smith L. D. Poly(A)+ RNA metabolism during oogenesis in Xenopus laevis. Dev Biol. 1979 Mar;69(1):217–236. doi: 10.1016/0012-1606(79)90287-2. [DOI] [PubMed] [Google Scholar]
- Dumont J. N., Brummett A. R. Oogenesis in Xenopus laevis (Daudin). V. Relationships between developing oocytes and their investing follicular tissues. J Morphol. 1978 Jan;155(1):73–97. doi: 10.1002/jmor.1051550106. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Changes in somatic cell nuclei inserted into growing and maturing amphibian oocytes. J Embryol Exp Morphol. 1968 Nov;20(3):401–414. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Masui Y., Clarke H. J. Oocyte maturation. Int Rev Cytol. 1979;57:185–282. doi: 10.1016/s0074-7696(08)61464-3. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Reynhout J. K., Taddei C., Smith L. D., LaMarca M. J. Response of large oocytes of Xenopus laevis to progesterone in vitro in relation to oocyte size and time after previous HCG-induced ovulation. Dev Biol. 1975 Jun;44(2):375–379. doi: 10.1016/0012-1606(75)90408-x. [DOI] [PubMed] [Google Scholar]
- Scheer U. Nuclear pore flow rate of ribosomal RNA and chain growth rate of its precursor during oogenesis of Xenopus laevis. Dev Biol. 1973 Jan;30(1):13–28. doi: 10.1016/0012-1606(73)90044-4. [DOI] [PubMed] [Google Scholar]
- Schuetz A. W., Hollinger T. G., Wallace R. A., Samson D. A. Inhibition of (3H)vitellogenin uptake by isolated amphibian oocytes injected with cytoplasm from progesterone-treated mature oocytes. Dev Biol. 1977 Jul 15;58(2):428–433. doi: 10.1016/0012-1606(77)90103-8. [DOI] [PubMed] [Google Scholar]
- Schuetz A. W. Role of hormones in oocyte maturation. Biol Reprod. 1974 Mar;10(2):150–178. doi: 10.1095/biolreprod10.2.150. [DOI] [PubMed] [Google Scholar]
- Schuetz A. W., Wallace R. A., Dumont J. N. Steroid inhibition of protein incorporation by isolated amphibian oocytes. J Cell Biol. 1974 Apr;61(1):26–34. doi: 10.1083/jcb.61.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitto A., Jr, wallace R. A. Maturation of Xenopus oocytes. I. Facilitation by ouabain. Exp Cell Res. 1976 Jan;97:56–62. doi: 10.1016/0014-4827(76)90654-6. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Ho T., Salter D. W., Jared D. W. Protein incorporation by isolated amphibian oocytes. IV. The role of follicle cells and calcium during protein uptake. Exp Cell Res. 1973 Dec;82(2):287–295. doi: 10.1016/0014-4827(73)90343-1. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Hollinger T. G. Turnover of endogenous, microinjected, and sequestered protein in Xenopus oocytes. Exp Cell Res. 1979 Mar 15;119(2):277–287. doi: 10.1016/0014-4827(79)90355-0. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W. Studies on amphibian yolk. VII. Serum phosphoprotein synthesis by vitellogenic females and estrogen-treated males of Xenopus laevis. Can J Biochem. 1968 Aug;46(8):953–959. doi: 10.1139/o68-142. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Misulovin Z. Long-term growth and differentiation of Xenopus oocytes in a defined medium. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5534–5538. doi: 10.1073/pnas.75.11.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. A., Misulovin Z., Wiley H. S. Growth of anuran oocytes in serum-supplemented medium. Reprod Nutr Dev. 1980;20(3A):699–708. doi: 10.1051/rnd:19800412. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Nickol J. M., Ho T., Jared D. W. Studies on amphibian yolk. X. The relative roles of autosynthetic and heterosynthetic processes during yolk protein assembly by isolated oocytes. Dev Biol. 1972 Nov;29(3):255–272. doi: 10.1016/0012-1606(72)90066-8. [DOI] [PubMed] [Google Scholar]
- Wiley H. S., Opresko L., Wallace R. A. New methods for the purification of vertebrate vitellogenin. Anal Biochem. 1979 Aug;97(1):145–152. doi: 10.1016/0003-2697(79)90338-5. [DOI] [PubMed] [Google Scholar]