Abstract
Treating biofilm infections on implanted medical devices is formidable, even with extensive antibiotic therapy. The aim of this study was to investigate whether ultrasound (US)-targeted microbubble (MB) destruction (UTMD) could enhance vancomycin activity against Staphylococcus epidermidis RP62A biofilms. Twelve-hour biofilms were treated with vancomycin combined with UTMD. The vancomycin and MB (SonoVue) were used at concentrations of 100 μg/ml and 30% (vol/vol), respectively, in studies in vitro. After US exposure (0.08 MHz, 1.0 W/cm2, 50% duty cycle, and 10-min duration), the biofilms were cultured at 37°C for another 12 h. The results showed that many micropores were found in biofilms treated with vancomycin combined with UTMD. Biofilm densities (A570 values) and the viable counts of S. epidermidis recovered from the biofilm were significantly decreased compared with those of any other groups. Furthermore, the highest percentage of dead cells was found, using confocal laser scanning microscopy, in the biofilm treated with vancomycin combined with UTMD. The viable counts of bacteria in biofilms in an in vivo rabbit model also confirmed the enhanced effect of vancomycin combined with UTMD. UTMD may have great potential for improving antibiotic activity against biofilm infections.
INTRODUCTION
Bacteria that adhere to implanted medical devices, such as vascular catheters, prosthetic joints, and artificial heart valves, can cause persistent infections because bacteria sequestered in biofilms show increased tolerance of normal antibiotic therapies (6). The mechanism of this resistance is very complicated. Current hypotheses on the subject include the heterogeneity of biofilm-encased bacteria and the failure of an antibiotic to penetrate the full depth of the biofilm (10). So far, the most effective treatment against biofilm infections is to remove the implant, treat the patient with antibiotics at safe levels, and later insert a new implant, which is a costly and difficult procedure (6, 7). Therefore, it is necessary to explore more effective methods of dealing with infections caused by biofilms on implanted devices.
Studies have shown that low-frequency ultrasound (US) combined with antibiotics can significantly enhance the bactericidal activity of antibiotics in both planktonic and biofilm forms (2, 4, 12). The mechanism may be that US increases cell membrane permeability (27), enhances microconvection from ultrasonic heating, and stimulates active or passive uptake of the antibiotics, as US can give rise to high-pressure, high-shear stress, or cavitation, causing cell membrane and biofilm disruption (23, 31).
Recently, microbubble (MB)-based US contrast agents have been used widely in clinical sonography. MB agents can provide nuclei for inertial cavitation and lower the threshold for US-induced cavitation. Destruction of MBs by US increases the membrane permeability of cells by shear stress, rising temperature, and activation of reactive oxygen species. Therefore, such MBs have also evolved as tools to deliver drugs or genetic materials (9). Many studies have shown that US-targeted MB destruction (UTMD) could improve transfection efficiency significantly (13, 30), and this technology may also be used to enhance the efficacy of thrombolysis therapy (20, 33). However, to our knowledge, the ability of UTMD to enhance antibiotic transport and improve antibiotic activity against biofilms is still unknown. Therefore, we designed this study to investigate the effect of UTMD combined with vancomycin on Staphylococcus epidermidis biofilms.
MATERIALS AND METHODS
Bacterial strains, media, and antibiotics.
Staphylococcus epidermidis RP62A (ATCC 35984) was selected for this study because this strain can produce a thick biofilm after 24-hour incubation (21, 34). Tryptic soy broth (TSB; Oxoid, Basingstoke, United Kingdom) medium containing 0.25% glucose was used for biofilm formation. Vancomycin (Sigma, St. Louis, MO) was reconstituted in distilled water and filter sterilized.
MB and US.
SonoVue MB contrast agent (Bracco, Milan, Italy) was reconstituted in 5 ml normal saline according to the manufacturer's protocol. The SonoVue reconstitution resulted in a preparation that contained 2 × 108 to 5 × 108 MBs/ml. The MB shell is composed of phospholipids and encapsulates sulfur hexafluoride gas. The diameter of MBs is typically 1 to 8 μm (mean, 2.5 μm) (29). In our study the concentration of the MB solution was defined as 100%, and the MB solution was further diluted in the TSB medium (1:3.3) in the UTMD experiments; therefore, the concentration of MB in medium was defined as 30% (vol/vol).
The ultrasonic apparatus (US-100; ITO Co., Tokyo, Japan) was hospital-use, physiotherapeutic equipment with a 30-mm-diameter ultrasonic transducer (US frequency, 0.08 MHz). The acoustic intensity of the output was set from 0.1 W/cm2 to 3.0 W/cm2, and the duty cycle was changed from 5% to 100%.
Protocol of biofilm treatment combining vancomycin with UTMD in vitro.
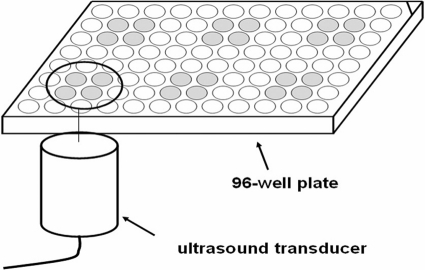
Biofilms were cultured and detected by using a semiquantitative plate assay (5). A polystyrene microtiter 96-well plate was divided into six areas, and biofilm was cultivated in the central four wells of each area, as illustrated in Fig. 1. The effective area of the US transducer is just larger than the area of the four wells. The ultrasonic transducer was placed 2 mm below each of the four wells, and US was transmitted through the bottom of the 96-well plate via a coupling gel, as reported previously (16). In the in vitro assay, vancomycin was used at a concentration of 100 μg/ml (a high concentration, given that the minimal bactericidal concentration of S. epidermidis planktonic cells is 8 μg/ml [24]). During experiments in vitro, the acoustic intensity was set at 1.0 W/cm2, with a 50% duty cycle. Sixty microliters of SonoVue was added to each well before US exposure. The MB concentration was 30% as we defined previously.
Fig. 1.
Schematic diagram of biofilm treatment. Ultrasound is transmitted though the bottom of the wells via coupling gels.
Overnight cultures of S. epidermidis strains grown in TSB medium were diluted 1:200, inoculated into wells of polystyrene microtiter plates (200 μl per well), and incubated for 12 h at 37°C. Biofilms formed in the six areas were treated under six different conditions: (i) not treated (control group), (ii) vancomycin, (iii) US only, (iv) vancomycin plus US, (v) MB + US, and (vi) vancomycin + MB + US.
Viable count of S. epidermidis.
Numbers of CFU were used to determine the viable bacteria in biofilms and supernatants. Twelve hours after treatment under the above six conditions, the biofilms were carefully scraped from the bottom of the 96-well plate, digested with 0.5% trypsin, and then dispersed thoroughly in TSB by vortexing. The samples were serially diluted in TSB, and 100 μl of each dilution was plated onto TSB agar plates and incubated at 37°C for 24 h. The numbers of CFU were determined and expressed relative to the area of the well (CFU/cm2) for biofilm bacteria and expressed relative to the amount of TSB for planktonic bacteria (CFU/ml). The experiments were repeated three times.
Changes in biofilm density and morphology.
After US treatment, the biofilms in the 96-well plate were washed gently three times with 200 μl phosphate-buffered saline (PBS) and stained with 2% crystal violet for 5 min. Then, the plate was rinsed under running tap water and air dried. The biofilm density was evaluated by measuring the absorbance at 570 nm using a spectrophotometer (DTX880; Beckman Coulter, CA). Each assay was repeated three times.
After spectrophotometer readings, the biofilms were observed by macroscopy and by optical microscopy (YS100; Nikon, Tokyo, Japan).
Observation of biofilms by CLSM.
To observe the biofilms by confocal laser scanning microscopy (CLSM), the S. epidermidis cells were cultivated in FluoroDish (FD35-100; WPI, United States). Briefly, overnight cultures of S. epidermidis grown in TSB medium were diluted 1:200, inoculated into the dishes (2 ml per dish), and then incubated at 37°C for 12 h. After the incubation, the biofilms were treated under the six different conditions described above. The sterile transducer was placed 10 mm above the biofilm, and US was transmitted through the TSB medium to the biofilms. SonoVue microbubbles (900 μl) were added into the corresponding dishes. The acoustic intensity and duty cycle were set at 1.0 W/cm2 and 50%. Then, the biofilms were incubated at 37°C for another 12 h. The dishes were washed carefully with PBS and stained with a LIVE/DEAD kit (containing SYTO9 and propidium iodide [PI]; Invitrogen Molecular Probes, United States) according to the manufacturer's instructions. SYTO9 stains viable bacteria green, while PI stains dead bacteria red. The biofilms of S. epidermidis were observed under a TCS SP5 CLSM (Leica, Germany) using a 63×, 1.3 oil water immersion lens. Images were generated by the Imaris software package (Bitplane, Zurich, Switzerland).
Protocol for in vivo studies.
All animals were cared for according to the approved guidelines of the Institute of Animal Care and Use Committee, and the protocol was approved by the committee. Forty-eight hours prior to surgery, sterile polyethylene dishes (8-mm diameter, 1-mm thickness, with a 20-mm chimb) were placed in separate sterile glass petri dishes containing 20 ml TSB and 100 μl overnight cultures of S. epidermidis. The dishes were inoculated at 37°C for 24 h. This procedure was repeated again for formation of a biofilm on the polyethylene dishes. Twelve New Zealand White female rabbits (2.0 to 2.5 kg) were randomly divided into six groups and anesthetized with ketamine (35 mg/kg of body weight) and diazepam (5 mg/kg), which was administered intramuscularly into the caudal thigh muscles as a single dose. After the back of the rabbit was denuded of fur, biofilm-infected polyethylene dishes were implanted subcutaneously bilateral to the spine, as reported previously (2, 25, 26), with three dishes on each side (Fig. 2).
Fig. 2.
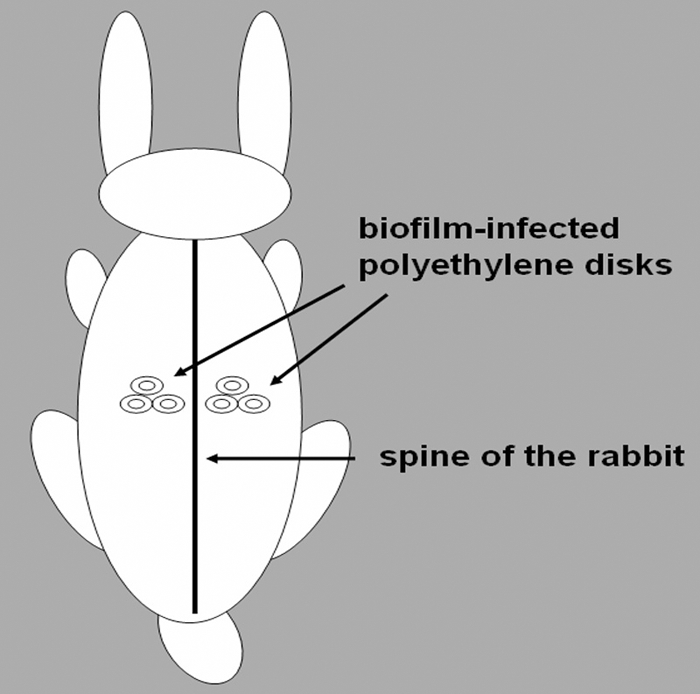
Schematic diagram of biofilm infection in a rabbit. Biofilm-infected polyethylene dishes were implanted subcutaneously bilateral to the spine, with three dishes on each side.
The rabbits in six groups were treated as follows: (i) untreated control, (ii) vancomycin, (iii) US only, (iv) vancomycin + US, (v) MB + US, and (vi) vancomycin + MB + US. Vancomycin (50 mg/kg) was administered through a marginal vein in the appropriate rabbits' ears immediately after surgery and 3 times a day thereafter. Twenty-four hours after surgery, US exposure or UTMD was performed for 20 min immediately following the administration of vancomycin, 3 times a day. The SonoVue MB (200 μl) was injected subcutaneously into the area of the dishes at 5-min intervals for a total 800 μl per treatment. The acoustic intensity was set at 500 mW/cm2, and the duty cycle was 50%. The ultrasonic transducer was placed on the skin over one of the implanted biofilm-infected dishes using an acoustically conductive gel adhesive. At 4 days after the surgery, the nine treatments were completed. Twelve hours after the last treatment, the rabbits were euthanized and the implants were taken out with sterile forceps. Biofilms were scraped from the dishes, and the viable bacteria were determined by CFU counting as previously described. The hearts, livers, and kidneys of rabbits were collected for independent histopathological examinations.
Blood samples were collected from the marginal ear veins before and after treatment to determine if bacteria had escaped from the biofilms into the bloodstreams. The first sample was taken before surgery, the second sample was made just prior to treatment with vancomycin or vancomycin plus US or UTMD, and the third sample was done prior to euthanasia and 12 h after the final vancomycin dose. Blood samples were diluted and plated onto TSB agar plates via the membrane filtration method.
Statistical analysis.
All data were expressed as the mean ± standard deviation. Differences in A570 values and log10 CFU counts among groups were assessed with one-way analysis of variance (ANOVA) tests. SPSS version 11.5 (SPSS, Inc., Chicago, IL) was used for statistical analyses. A P value of <0.05 was considered statistically significant.
RESULTS
Influence of vancomycin combined with UTMD on biofilm morphology.
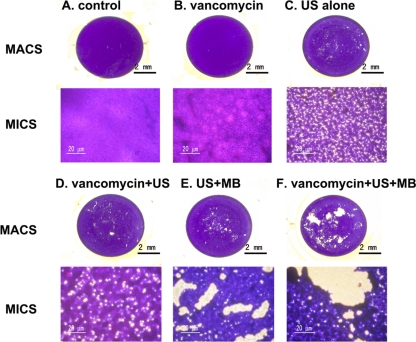
To investigate whether UTMD could influence the modality of S. epidermidis biofilms, the biofilms were examined by macroscopy and microscopy after treatment (Fig. 3). A thick and compact biofilm was observed with both the control group and the vancomycin group. Interestingly, many micropores were found in both biofilms of the US-only-treated group and vancomycin + US-treated group, and even bigger micropores were observed with the biofilms of both the US + MB-treated group and vancomycin + US + MB-treated group.
Fig. 3.
Morphology of biofilms examined by macroscopy (MACS) and light microscopy (MICS) (original magnification, ×200). Overnight cultures of bacteria were diluted 1:200 and cultured in 96-well plates (200 μl/well) at 37°C for 12 h, and then the biofilms underwent the six different treatments outlined in the figure (A to F). The biofilm was then washed gently 3 times with PBS and stained with crystal violet. After treatment, the biofilms were stained with 2% crystal violet.
A quantitative analysis was performed using the Image J program (quantitative analysis software; http://rsbweb.nih.gov/ij) to compare the ratios of the areas of the micropores to the total areas in photos taken under light microscopy. Five sample points were selected for each group, and the ratios for (i) the control group, (ii) the vancomycin group, (iii) the US-only group, (iv) the vancomycin + US group, (v) the MB + US group, and (vi) the vancomycin + MB + US group were 0.04% ± 0.02%, 0.08% ± 0.03%, 12.04% ± 1.21%, 18.06% ± 1.72%, 24.81% ± 3.02%, and 29.02% ± 3.16%, respectively. The total micropore area in the vancomycin + US + MB group was significantly larger than those in the other groups (P < 0.05).
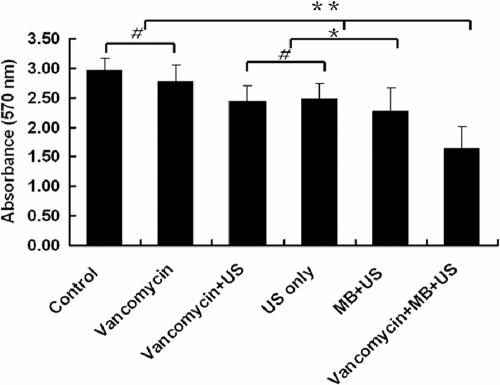
Effect of vancomycin combined with UTMD on biofilm density.
To test the effect of UTMD on biofilm density, the absorbance of biofilms stained with crystal violet was detected at 570 nm using a spectrophotometer. The A570 value in the control group, representing the biofilm density, was slightly higher than that of the vancomycin-treated group, but there was no significant difference between the two groups (P > 0.05). The biofilm density in the vancomycin + US-treated group, the US-only-treated group, the MB + US-treated group, and the vancomycin + MB + US-treated group was less than that of the control group and vancomycin-treated group (P < 0.01). The vancomycin + MB + US-treated group had the minimum lowest biofilm density (P < 0.01) (Fig. 4).
Fig. 4.
Semiquantitative analysis of biofilm density. The biofilms of S. epidermidis RP62A were detected by semiquantitative microtiter plate assay. Briefly, overnight cultures of bacteria were diluted 1:200 and cultured in a 96-well plate (200 μl/well) at 37°C for 12 h, and then the biofilms were treated with the six different conditions denoted in the figure. The biofilm was washed gently 3 times with PBS and stained with crystal violet, and the absorbance at 570 nm was measured by a spectrophotometer. Data are presented as means plus standard deviations (n = 12). The error bars represent 95% confidence intervals. Asterisks denote statistically significant differences: *, P < 0.05, and **, P < 0.01. # denotes no significant difference between groups, P > 0.05.
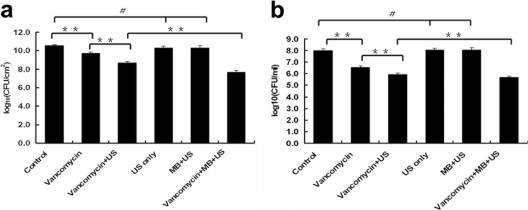
Bactericidal action of vancomycin combined with UTMD in vitro.
To assess the antibacterial activity of vancomycin combined with UTMD, viable Staphylococcus epidermidis from the biofilms and planktonic growth was recovered. The log10 number of CFU/cm2 of viable bacteria recovered from biofilms in the vancomycin-treated group (9.73 ± 0.12) was slightly lower than that of the control group (10.51 ± 0.14) (P < 0.01). The log10 numbers of viable CFU/cm2 from the biofilms of the US-only-treated group and the US + MB-treated group were 10.26 ± 0.16 and 10.19 ± 0.19, respectively, which is not significantly different from that of the control group. The log10 numbers of viable CFU/cm2 significantly decreased in the vancomycin + US-treated group (8.66 ± 0.16) (P < 0.01) and in the vancomycin + MB + US-treated group (7.17 ± 0.17) (P < 0.01) compared with those of other groups (Fig. 5a). The results of the log10 numbers of CFU/ml of viable cells obtained from the planktonic bacteria were similar to the results from the biofilms (Fig. 5b).
Fig. 5.
Comparison of viable bacteria recovered from biofilms and planktonic bacteria after treatments. Data are expressed as means plus standard deviations (n = 9). (a) Viable counts recovered from biofilms. (b) Viable counts from planktonic cells. The error bars represent 95% confidence intervals. Asterisks denote statistically significant differences; **, P < 0.01. # denotes no significant difference between groups, P > 0.05.
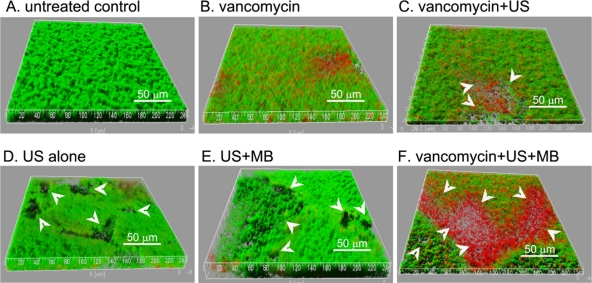
To confirm the activity of vancomycin combined with UTMD against bacteria in biofilms, the biofilms were examined by CLSM (Fig. 6). Packed and thick biofilms were observed with the control group and the vancomycin-treated group, and many dead bacteria could be found in the vancomycin-treated group. Similar to the results acquired by macroscopy and by light microscopy, many micropores were observed with the biofilms treated with US with or without vancomycin and/or MB. The biofilms treated with US + MB in the presence or absence of vancomycin exhibited larger micropores than those in other groups. Furthermore, many more dead bacteria were detected in biofilms treated with vancomycin + US or with vancomycin + MB + US, than in those of biofilms treated with vancomycin only, with US, or with US + MB.
Fig. 6.
(A to F) Three-dimensional structural images of biofilms. The biofilms of S. epidermidis RP62A in FluoroDishes were visualized by CLSM with the LIVE/DEAD viability stain (SYTO9/PI). Viable cells exhibit green fluorescence, whereas dead cells exhibit red fluorescence. Many micropores (white arrow) could be observed in the biofilms treated with US with or without MB. The images are three-dimensional reconstructions using Imaris software, based on CLSM data at approximately 0.5-μm-depth increments. The experiments were repeated twice with similar results.
To evaluate the ratios of viable cells to dead cells in biofilms after the different treatments, a fluorescence quantification based on the original CLSM data was conducted using the Image J program by measuring the green fluorescence (GF) and red fluorescence (RF) in the z-stack composite confocal photomicrographs. Those stacks of viable cells (GF), dead cells (RF), and both (GF + RF) were generated separately. The quantitative values (mean values and standard deviations of five sample points) were calculated (Table 1). Compared with those of the other groups, the total bacteria (RF + GF) and the percentage of viable cells (GF/total fluorescence [TF]) in the vancomycin + US + MB group were significantly decreased (P < 0.05).
Table 1.
Quantitative analysis of fluorescence in CLSM images of biofilms by use of the Image J programa
| Group | Fluorescence quantificationb |
Ratio |
|||
|---|---|---|---|---|---|
| GF | RF | TF | RF/GF | GF/TF | |
| Control | 1,492 ± 79 | 337 ± 23 | 1,829 ± 89 | 0.23 | 0.82 |
| Vancomycin | 1,254 ± 61 | 352 ± 26 | 1,606 ± 73 | 0.28 | 0.78 |
| US alone | 1,175 ± 53 | 184 ± 13 | 1,359 ± 64 | 0.16 | 0.86 |
| Vancomycin + US | 782 ± 32 | 463 ± 31 | 1,245 ± 56 | 0.59 | 0.63 |
| US + MB | 1,043 ± 47 | 186 ± 16 | 1,229 ± 51 | 0.18 | 0.85 |
| Vancomycin + US + MB | 522 ± 25 | 505 ± 33 | 1,027 ± 47 | 0.94* | 0.51* |
z-stack composite confocal photomicrographs were generated for fluorescence quantification based on the original CLSM data shown in Fig. 6, and those stacks of viable cells (GF), dead cells (RF), and all cells (TF = GF + RF, viable and dead) were generated separately. GF stands for the green fluorescence quantities of the viable cell stacks. RF stands for the red fluorescence quantities of the dead cell stacks. TF represents the fluorescence quantities of all the cell stacks. *, P < 0.05.
Values are expressed as means ± standard deviations of five sample points.
Bactericidal action of vancomycin combined with UTMD in vivo.
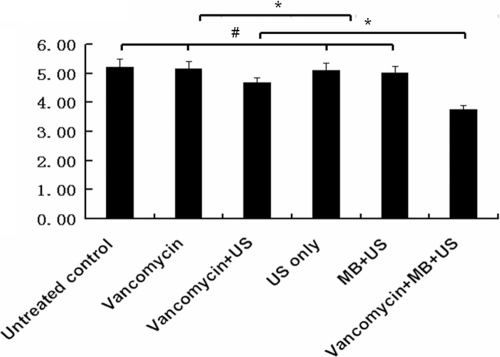
Visual observation showed that neither burns nor any type of skin damage was found after US or UTMD treatment. The viable counts of S. epidermidis CFU recovered from the implanted dishes in rabbits are shown in Fig. 7. The log10 numbers of viable CFU/cm2 from biofilms of (i) the untreated control group, (ii) the vancomycin group, (iii) the US-only group, (iv) the vancomycin + US group, (v) the MB + US group, and (vi) the vancomycin + MB + US group were 5.21 ± 0.28, 5.16 ± 0.25, 4.68 ± 0.17, 5.11 ± 0.26, 5.01 ± 0.23, and 3.76 ± 0.14, respectively. There was no significant difference among the untreated control group, the vancomycin group, the US-only group, and the MB + US group (P > 0.05). However, the log10 numbers of viable CFU/cm2 were significantly decreased in biofilms treated with vancomycin + US with or without MB (P < 0.05). The vancomycin + US + MB group contained the smallest log10 number of CFU/cm2 compared with the other groups (P < 0.05).
Fig. 7.
The log10 numbers of CFU/cm2 (y axis) of viable Staphylococcus epidermidis recovered from the implanted dishes in the in vivo rabbit model. Biofilm-infected polyethylene dishes were implanted in six groups of New Zealand White female rabbits. Twenty-four hours after surgery, the rabbits underwent the six treatments denoted in the figure, for 3 days. The data are expressed as means plus standard deviations (n = 12). The error bars represent 95% confidence intervals. Asterisks denote statistically significant difference, P < 0.05. # denotes no significant difference between groups, P > 0.05.
None of the blood samples, before or after treatment of any of the rabbits, showed any signs of S. epidermidis. The tissue around the implant dishes had large numbers of bacteria, but none of such organs as hearts, livers, or kidneys showed any colonization by S. epidermidis.
DISCUSSION
Biofilm infections are a common complication associated with implanted medical devices. Mature biofilms are demonstrably resistant to concentrations of antibiotics 500 to 5,000 times more than that necessary to kill planktonic cells of the same organism (15). Successful therapies for infections on implanted medical devices are very difficult and quite rare, even with extensive antibiotic treatment (8). To the best of our knowledge, this study is the first to investigate the effect of UTMD on enhancing vancomycin's antibacterial activity against biofilm cells both in vitro and in vivo. Compared with antibiotics combined with US alone, UTMD could further enhance the activity of antibiotics against bacterial biofilms. Our results indicated that the biofilms treated with vancomycin combined with UTMD contained the largest percentage of dead bacteria compared to the other groups. Furthermore, we found that the biofilm density was significantly lowered and larger micropores were observed after treatment with a combination of vancomycin and UTMD.
The mechanism of the enhancement of antibiotic action by US is complex. First, US can increase cell permeability (27) and stimulate transport of the antibiotics through the biofilm (4) due to cavitation, high pressure, and high shear stress. Second, a temperature increase and generation of intracellular reactive oxygen species following the application of US might permeabilize the cell membrane (14, 17). In this study, UTMD exhibited the ability to destroy the biofilm matrix and give rise to larger micropores in the biofilm than US alone, which improved antibiotic transport significantly. The reason for this may be that MB can act as cavitation nuclei and significantly lower the threshold energy needed for cavitation (13, 19). Many studies have shown that US can increase gene or drug delivery (16, 18) in eukaryotic cells, and the mechanism has been explained convincingly by the repairable sonoporation phenomenon, as visualized by scanning electron microscopy (32). However, bacteria are significantly smaller than eukaryotic cells and have a higher tensile-strength cell wall, and the occurrence of sonoporation through bacterial walls needs further research.
Qian et al. (22) reported that low-intensity US (10 mW/cm2, 2 h) could enhance the activity of antibiotics against biofilms without disrupting the biofilm or dispersing the bacteria. However, in the present study, many micropores were observed in the biofilms treated with US or US plus MB, which indicated that biofilms might be disrupted and portions of them detached. The findings might be attributed to the higher-intensity US (0.08 MHz, 1 W/cm2 with 50% duty cycle, but a duration of only 10 min) used here. These US parameters are within the range of clinical physical therapy (11, 28). The similar US parameters (0.07 MHz, 1.5 W/cm2, 45 min) were also utilized in another related study (4). Although no bacteria were found in blood samples from rabbits and no organs showed any colonization by S. epidermidis in the in vivo experiment, it is necessary to maintain an effective antibiotic dose to avoid a potential infection in other parts of the body resulting from dissemination of biofilm fragments, given that the technology might be applied in clinical practice in the future. Lowering the power of the US treatment might also be an alternative choice. Further research is necessary to resolve these problems.
Multiple factors affect the activity of US-mediated antibiotics against bacteria in biofilms. First, the US parameters, including acoustic intensity, duty cycle, frequency, and duration, may be the main factors (23). Greater power and longer duration could promote antibiotic transport (4, 25). However, this may cause skin or tissue damage, although pulsed US could significantly reduce bacterial viability without tissue damage (25). It has been reported that the bactericidal effect of US combined with an antibiotic on biofilms decreased as the US frequency increased (23). Second, the species of bacteria and the age of the biofilm may also influence antibiotic action. Carmen et al. (3) have reported that the combination of gentamicin and ultrasonic irradiation for 24 h reduced the viability of Escherichia coli biofilms in vivo, and such treatment for 48 h reduced viable bacteria to nearly undetectable levels. However, when Pseudomonas aeruginosa biofilms were treated with US for 24 and 48 h, no appreciable reduction of viable bacteria was observed. The authors believed that the difference in the responses of these two organisms was due to the greater impermeability and stability of the outer membrane of Pseudomonas aeruginosa. In addition, it is noteworthy that the amount of antibiotic penetrating the biofilm significantly decreased with an increase in age of the biofilm (4). Older biofilms are frequently observed to be more resistant to antimicrobial agents (1). Therefore, it is very important to optimize the US exposure parameters and to treat biofilm infections as early as possible with antibiotics in combination with UTMD.
In conclusion, UTMD may enhance antibiotic activity against biofilms, providing a noninvasive and targeted approach to cure biofilm infections.
ACKNOWLEDGMENTS
This study was supported by the Program of the Ministry of Science and Technology of China (2009ZX09303-005 and 2008ZX10003-016), the National Natural Science Foundation of China (30800036), the Scientific Technology Development Foundation of Shanghai (08JC1401600 and 10410700600), the Shanghai Leading Academic Discipline Project (B112), and the High-Tech Research and Development Program of China (2006AA02A253).
Footnotes
Published ahead of print on 15 August 2011.
REFERENCES
- 1. Anwar H., Strap J. L., Costerton J. W. 1992. Establishment of aging biofilms: possible mechanism of bacterial resistance to antimicrobial therapy. Antimicrob. Agents Chemother. 36:1347–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carmen J. C., et al. 2004. Ultrasonically enhanced vancomycin activity against Staphylococcus epidermidis biofilms in vivo. J. Biomater. Appl. 18:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carmen J. C., et al. 2005. Treatment of biofilm infections on implants with low-frequency ultrasound and antibiotics. Am. J. Infect. Control 33:78–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carmen J. C., et al. 2004. Ultrasonic-enhanced gentamicin transport through colony biofilms of Pseudomonas aeruginosa and Escherichia coli. J. Infect. Chemother. 10:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Christensen G. D., et al. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Costerton J. W., Stewart P. S., Greenberg E. P. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 7. Costerton J. W., Stewart P. S. 2001. Battling biofilms. Sci. Am. 285:74–81 [DOI] [PubMed] [Google Scholar]
- 8. Darouiche R. O., et al. 1994. Vancomycin penetration into biofilm covering infected prostheses and effect on bacteria. J. Infect. Dis. 170:720–723 [DOI] [PubMed] [Google Scholar]
- 9. Dijkmans P. A., et al. 2004. Microbubbles and ultrasound: from diagnosis to therapy. Eur. J. Echocardiogr. 5:245–246 [DOI] [PubMed] [Google Scholar]
- 10. Donlan R. M. 2000. Role of biofilms in antimicrobial resistance. ASAIO J. 46:S47–S52 [DOI] [PubMed] [Google Scholar]
- 11. Ebenbichler G. R., et al. 1999. Ultrasound therapy for calcific tendinitis of the shoulder. N. Engl. J. Med. 340:1533–1538 [DOI] [PubMed] [Google Scholar]
- 12. Ensing G. T., Neut D., van Horn J. R., van der Mei H. C., Busscher H. J. 2006. The combination of ultrasound with antibiotics released from bone cement decreases the viability of planktonic and biofilm bacteria: an in vitro study with clinical strains. J. Antimicrob. Chemother. 58:1287–1290 [DOI] [PubMed] [Google Scholar]
- 13. Greenleaf W. J., Bolander M. E., Sarkar G., Goldring M. B., Greenleaf J. F. 1998. Artificial cavitation nuclei significantly enhance acoustically induced cell transfection. Ultrasound Med. Biol. 24:587–595 [DOI] [PubMed] [Google Scholar]
- 14. Juffermans L. J., Dijkmans P. A., Musters R. J., Visser C. A., Kamp O. 2006. Transient permeabilization of cell membranes by ultrasound-exposed microbubbles is related to formation of hydrogen peroxide. Am. J. Physiol. Heart Circ. Physiol. 291:H1595–H1601 [DOI] [PubMed] [Google Scholar]
- 15. Khoury A. E., Lam K., Ellis B., Costerton J. W. 1992. Prevention and control of bacterial infections associated with medical devices. ASAIO J. 38:M174–M178 [DOI] [PubMed] [Google Scholar]
- 16. Kim H. J., Greenleaf J. F., Kinnick R. R., Bronk J. T., Bolander M. E. 1996. Ultrasound-mediated transfection of mammalian cells. Hum. Gene Ther. 7:1339–1346 [DOI] [PubMed] [Google Scholar]
- 17. Kondo T., Misik V., Riesz P. 1998. Effect of gas-containing microspheres and echo contrast agents on free radical formation by ultrasound. Free Radic. Biol. Med. 25:605–612 [DOI] [PubMed] [Google Scholar]
- 18. Lawrie A., et al. 1999. Ultrasound enhances reporter gene expression after transfection of vascular cells in vitro. Circulation 99:2617–2620 [DOI] [PubMed] [Google Scholar]
- 19. Miller D. L., Thomas R. M. 1995. Ultrasound contrast agents nucleate inertial cavitation in vitro. Ultrasound Med. Biol. 21:1059–1065 [DOI] [PubMed] [Google Scholar]
- 20. Molina C. A., et al. 2006. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke 37:425–429 [DOI] [PubMed] [Google Scholar]
- 21. Patel J. D., Ebert M., Ward R., Anderson J. M. 2007. S. epidermidis biofilm formation: effects of biomaterial surface chemistry and serum proteins. J. Biomed. Mater. Res. A 80:742–751 [DOI] [PubMed] [Google Scholar]
- 22. Qian Z., Stoodley P., Pitt W. G. 1996. Effect of low-intensity ultrasound upon biofilm structure from confocal scanning laser microscopy observation. Biomaterials 17:1975–1980 [DOI] [PubMed] [Google Scholar]
- 23. Qian Z., Sagers R. D., Pitt W. G. 1999. Investigation of the mechanism of the bioacoustic effect. J. Biomed. Mater. Res. 44:198–205 [DOI] [PubMed] [Google Scholar]
- 24. Qin Z., et al. 2007. Formation and properties of in vitro biofilms of ica-negative Staphylococcus epidermidis clinical isolates. J. Med. Microbiol. 56:83–93 [DOI] [PubMed] [Google Scholar]
- 25. Rediske A. M., et al. 2000. Pulsed ultrasound enhances the killing of Escherichia coli biofilms by aminoglycoside antibiotics in vivo. Antimicrob. Agents Chemother. 44:771–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rediske A. M., et al. 1999. Ultrasonic enhancement of antibiotic action on Escherichia coli biofilms: an in vivo model. Antimicrob. Agents Chemother. 43:1211–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Runyan C. M., et al. 2006. Low-frequency ultrasound increases outer membrane permeability of Pseudomonas aeruginosa. J. Gen. Appl. Microbiol. 52:295–301 [DOI] [PubMed] [Google Scholar]
- 28. Santamato A., et al. 2009. Short-term effects of high-intensity laser therapy versus ultrasound therapy in the treatment of people with subacromial impingement syndrome: a randomized clinical trial. Phys. Ther. 89:643–652 [DOI] [PubMed] [Google Scholar]
- 29. Schneider M., et al. 1995. BR1: a new ultrasonographic contrast agent based on sulfur hexafluoride-filled microbubbles. Invest. Radiol. 30:451–457 [DOI] [PubMed] [Google Scholar]
- 30. Shohet R. V., et al. 2000. Echocardiographic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation 101:2554–2556 [DOI] [PubMed] [Google Scholar]
- 31. Sinisterra J. V. 1992. Application of ultrasound to biotechnology: an overview. Ultrasonics 30:180–185 [DOI] [PubMed] [Google Scholar]
- 32. Tachibana K., Uchida T., Ogawa K., Yamashita N., Tamura K. 1999. Induction of cell-membrane porosity by ultrasound. Lancet 353:1409. [DOI] [PubMed] [Google Scholar]
- 33. Tachibana K., Tachibana S. 1995. Albumin microbubble echo-contrast material as an enhancer for ultrasound accelerated thrombolysis. Circulation 92:1148–1150 [DOI] [PubMed] [Google Scholar]
- 34. Tojo M., Yamashita N., Goldmann D. A., Pier G. B. 1988. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J. Infect. Dis. 157:713–722 [DOI] [PubMed] [Google Scholar]