Abstract
An azithromycin extended-release (ER) oral suspension was developed to improve the gastrointestinal tolerability profile without substantially compromising systemic exposure. A single dose of 30 mg/kg azithromycin immediate-release (IR) oral suspension has been used in children to treat acute otitis media (AOM). This study was conducted to compare the pharmacokinetics of a 60-mg/kg azithromycin ER single dose with a 30-mg/kg azithromycin IR single dose in children with AOM aged 6 months to 6 years (n = 19 per treatment). Serum samples were collected at 1, 2, 3, 4, 8, 24, 48, and 72 h after dosing. The area under the curve from time zero to 72 h postdosing (AUC0-72) was calculated based on a noncompartmental method. One-way analysis of variance (ANOVA) was used to compare exposure parameters (e.g., AUC0-72 and peak concentration) as well as concentrations at each time point. The adjusted geometric mean ratio of the ER/IR AUC0-72 was 157.98% (90% confidence interval [CI], 98.87%, 252.44%), which met the predefined criterion of the lower boundary of the 90% CI of ≥80%. As expected, due to the slower-release profile of the ER formulation, the concentrations of the ER formulation during the first 3 h were lower than those of the IR formulation. After 3 h postdosing, the lower boundaries of the 90% CI for the ER/IR concentration ratios were greater than 100%. These results indicated that a 60-mg/kg single dose of ER azithromycin provides similar or greater systemic exposure in children than the 30-mg/kg single dose of IR azithromycin.
INTRODUCTION
A single-dose regimen of an azithromycin extended-release (ER) oral suspension (Zmax) has been developed to deliver systemic exposure that is comparable to the cumulative exposure observed with the currently approved multiple-oral-dose regimens of the immediate-release (IR) formulation (5, 14). The ER formulation releases the drug more slowly than conventional IR formulations and in the lower gastrointestinal (GI) tract, thereby reducing GI side effects such as nausea and vomiting. Since the ER formulation partially bypasses the absorption window (upper GI tract), the oral bioavailability of ER azithromycin was compromised to a certain extent. Therefore, a higher numeric dose was selected for the ER formulation to ensure that sufficient systemic exposure to azithromycin could be achieved. The 2-g azithromycin ER single-dose regimen has been approved worldwide for the treatment of acute bacterial sinusitis and community-acquired pneumonia (CAP) in adults.
The pharmacokinetics of ER azithromycin have been characterized for pediatric patients aged 3 months to 16 years following a single dose of ER azithromycin of 60 mg/kg (maximum of 2 g) (14). Although there was large intersubject variability in systemic exposure (area under the concentration-time curve [AUC] and maximum concentration of drug in serum [Cmax]) across the age groups studied, individual azithromycin AUC and Cmax values in pediatric subjects were comparable to or higher than those in adults following a 2-g single dose of azithromycin in the ER formulation.
Acute otitis media (AOM) is an important health problem in children. The currently approved azithromycin IR oral suspension for AOM is a 30-mg/kg total dose given as a single dose or given over 3 or 5 days (7, 11, 13, 15). It has been demonstrated that the 30-mg/kg azithromycin IR single-dose regimen was as effective as the 10-day regimen of high-dose amoxicillin-clavulanate (90/6.4 mg/kg/day, given in divided doses every 12 h [q12h]) for the treatment of AOM in children, whereas rates of adverse events (AEs) were lower and compliance was improved with the single-dose regimen (2). Nonetheless, the azithromycin pharmacokinetics for the 30-mg/kg IR single dose have not been well characterized in children previously.
To assess if the 60-mg/kg ER formulation in a single dose is as effective as the approved 30-mg/kg IR formulation in a single dose for the treatment of AOM in children, this study was conducted to characterize and compare the pharmacokinetic profiles of these two regimens in children with AOM to evaluate if systemic exposure to azithromycin from a single dose of the 60-mg/kg ER formulation is similar to or greater than that of the 30-mg/kg IR formulation in a single dose. Additionally, the safety and clinical response of azithromycin were evaluated in children with AOM following a single dose of either the 60-mg/kg ER or the 30-mg/kg IR formulation.
MATERIALS AND METHODS
Study design.
This was an open-label, randomized, single-dose, parallel-group pharmacokinetic study of 38 children with AOM. Subjects were screened within 48 h of dosing. Subjects who satisfied all inclusion/exclusion criteria were randomized in a 1:1 ratio to receive a single oral dose of azithromycin in either the 30-mg/kg IR formulation or the 60-mg/kg ER formulation. Subjects were confined to the clinical research unit until the 8-h postdose pharmacokinetic sample was collected on day 1 and returned on days 2 to 4 for pharmacokinetic blood sampling. The clinical response was assessed by the investigator at the test-of-cure (TOC) visit (7 to 10 days after dosing). Exclusive of the screening period, the total time of participation in the study for each subject was approximately 10 days.
The study was conducted in compliance with the Declaration of Helsinki and with International Conference on Harmonization Good Clinical Practice guidelines. The study protocol and informed consent documentation were reviewed and approved by the Independent Ethics Committees at the investigational center participating in the study. Written informed consent was obtained prior to the subject entering the study.
Patients.
Male or female children aged 6 months to 6 years, inclusive, with clinical signs and/or symptoms of AOM in at least one ear were included in the study. The clinical signs and/or symptoms of AOM were defined as follows: (i) purulent otorrhea of a duration of ≤24 h or (ii) at least 2 otoscopic signs of middle ear effusion (i.e., decreased or absent tympanic membrane mobility by pneumatic otoscopy, yellow or white discoloration of the tympanic membrane, and opacification of the tympanic membrane [other than scarring]) and (iii) at least 1 indicator of acute inflammation to support the diagnosis of AOM (i.e., ear pain, including unaccustomed tugging or rubbing; marked redness of the tympanic membrane; and a distinct fullness or bulging of the tympanic membrane). Subjects were excluded if they had known or suspected hypersensitivity or intolerance to azithromycin or other macrolides or to any penicillin, beta-lactam antibiotic, or beta-lactamase inhibitor. Subjects were excluded if they were unable to take oral medications or any condition possibly affecting drug absorption. Subjects were excluded if they had used prescription or nonprescription drugs and dietary supplements or consumed grapefruit (including grapefruit-containing products) within 7 days or 5 half-lives (whichever was longer) prior to the study dosing. As an exception, analgesics such as ibuprofen and acetaminophen could have been used. Other antibiotics without drug-drug interactions with azithromycin were also allowed, such as amoxicillin and cephalosporins. Subjects were excluded if they had any medical condition that could have interfered with the evaluation of the study drug and/or would have made the subject unsuitable for enrollment (e.g., tympanostomy tubes in place, otitis externa, evidence of chronic middle ear disease, or perforations of the tympanic membrane in the affected ear for >24 h prior to study entry). Subjects were also excluded if they had any other condition which, in the opinion of the investigator, made the subject unsuitable for enrollment.
Study treatment.
Each subject received his or her single oral dose of 60 mg/kg in the ER formulation or 30 mg/kg in the IR formulation on an empty stomach (1 h before or 2 h after a meal). The concentration for the azithromycin ER suspension was 27 mg/ml, and the concentration for the azithromycin IR suspension was 20 mg/ml. Subjects were observed for 1 h after study drug administration. Any subject who vomited within 1 h of administration was to receive alternative therapy. The study drug was not to be readministered to any subject who vomited.
Pharmacokinetic sampling and analysis.
Blood samples (approximately 0.75 ml per sample to provide a minimum of 0.3 ml of serum) were to be collected at 1, 2, 3, 4, 8, 24, 48, and 72 h postdose for pharmacokinetic analysis. The serum samples were stored frozen at −20°C or lower prior to analysis.
Bioanalytical Systems Ltd. (Kenilworth, Warwickshire, United Kingdom) analyzed serum samples for azithromycin concentrations using a validated high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method. The serum samples (50 μl) were extracted by using a liquid-liquid extraction procedure and employed azithromycin-D3 as the internal standard. The mass spectrometer was operated in the positive-ionization mode and monitored the transition ions m/z 749.5→591.1 and 752.6→594.1 for azithromycin and azithromycin-D3, respectively. The dynamic range for the assay was 10.0 to 500 ng/ml. The accuracy (percent difference from nominal) of the quality control samples used during sample analysis ranged from −1.6% to 3.5%, with a precision (as measured by the percent relative standard deviation) of ≤2.9%.
A noncompartmental pharmacokinetic analysis was performed by using an internally validated system, eNCA v2.2.1. The peak concentration (Cmax) and the time to Cmax (Tmax) were estimated directly from the concentration-time profiles. The area under the curve from time zero to 8 h postdosing (AUC0-8), AUC0-24, and AUC0-72 were estimated by using the linear-log trapezoidal approximation. Since no predose samples were obtained in order to spare the children an extra blood draw, predose concentrations were assigned a value of zero for AUC calculations. Samples above the limit of quantification were diluted appropriately within the range for assay. Samples below the lower limit of quantification were set to 0 ng/ml for analysis. Actual sample collection times were used for the pharmacokinetic analysis.
Safety assessment.
Adverse events (AEs) were monitored throughout the study. Safety laboratory tests were performed at screening (and day 2 for subjects who were discontinued from the study), and vital signs and physical examinations were performed at screening, prior to dosing on day 1, and at the TOC visit (on days 7 to 10).
Clinical response assessment.
At the TOC visit (between days 7 and 10), or when subjects discontinued the study prematurely (if applicable), the investigator assessed the subject's response to therapy as being cure, where clinical signs and symptoms related to the acute illness had resolved or clinical improvement was such that no additional therapy was necessary, or failure, with one or more of the following: (i) signs and symptoms related to the acute illness had persisted or worsened, and additional therapy was necessary, and (ii) new clinical signs and symptoms of acute illness had developed, and additional therapy was necessary.
Any worsening of existing signs and symptoms, or new signs and symptoms, was also documented as AEs.
Statistical analysis.
A sample size of 36 subjects (18 subjects per treatment group) was required to provide 90% power that the lower boundary of the 90% confidence interval (CI) for the ER/IR AUC0-72 ratio was ≥80%. This estimate was based on the assumption that the true ratio between the AUC0-72 for the ER formulation (60 mg/kg) and the AUC0-72 for the IR formulation (30 mg/kg) was 1.20 and also on the assumption of intersubject standard deviation of 0.4 for the natural log AUC0-72 based on historical data (5, 14).
One-way analysis of variance (ANOVA) was used to compare natural-log-transformed AUC0-8, AUC0-24, AUC0-72, and Cmax values as well as concentrations at each time point. The 30-mg/kg IR formulation was the reference treatment, and the 60-mg/kg ER formulation was the test treatment. The adjusted mean differences (test-reference) between treatments and 90% CIs for the differences were exponentiated to provide estimates of the ratio of adjusted geometric means (test-reference) and 90% CIs for the ratios.
The criterion for primary comparisons (AUC0-72) between treatments was predefined as maintaining at least a lower 90% CI boundary of 80% to demonstrate that the exposure of the ER formulation was similar to or greater than that of the IR formulation. Other secondary comparisons between treatments were also evaluated using the same criteria.
No formal inferential statistics were applied to the safety and clinical response data, and these data are listed for descriptive purpose.
RESULTS
Subject disposition and demography.
Thirty-eight children with AOM were enrolled at a single study center in Costa Rica (19 in each treatment group), and 36 of them completed the study. One subject in each treatment group discontinued the study: in the IR group, one subject was inadvertently given a low dose due to a miscalculation based on weight, while in the ER group, one subject vomited while receiving the study drug. The two subjects who were discontinued from the study had safety laboratory tests performed on day 2, but their data were excluded from pharmacokinetic analyses.
All subjects were Hispanic. As shown in Table 1, demographic data were similar between the two treatment groups, although the mean age was slightly higher in the IR group (34.5 months) than in the ER group (24.3 months).
Table 1.
Baseline demographic characteristics
| Parameter | Value for group |
|
|---|---|---|
| 60 mg/kg ER azithromycin (n = 19) | 30 mg/kg IR azithromycin (n = 19) | |
| No. of males/no. of females | 11/8 | 12/7 |
| Age (mo) | ||
| Mean (SD) | 24.3 (20.6) | 34.5 (21.1) |
| Range | 6–76 | 9–78 |
| Wt (kg) | ||
| Mean (SD) | 13.1 (6.1) | 14.0 (5.1) |
| Range | 6.7–31.2 | 7.0–28.3 |
| Body mass index (kg/m2) | ||
| Mean (SD) | 17.8 (3.0) | 16.7 (2.0) |
| Range | 9.2–22.4 | 12.6–20.7 |
Concomitant treatments.
Seven subjects in the 30-mg/kg IR azithromycin group and four subjects in the 60-mg/kg ER azithromycin group received concomitant mediations during the study, and the most commonly taken concomitant treatment was paracetamol (acetaminophen). Two subjects in the IR group and one subject in the ER group received concomitant antibiotic therapy (i.e., ceftriaxone).
Comparison of azithromycin pharmacokinetics between the ER and IR formulations.
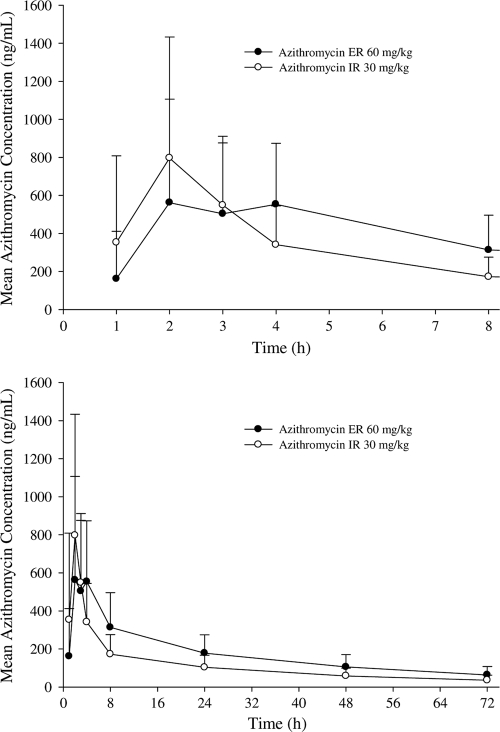
Mean azithromycin serum concentration-time profiles for the 60-mg/kg ER and 30-mg/kg IR single doses are presented in Fig. 1, and the corresponding pharmacokinetic parameters are summarized in Table 2. As expected, the IR single-dose regimen had a higher peak concentration and a shorter Tmax than those of the ER single-dose regimen since the ER formulation was designed to lower the absorption rate. As shown in Table 2, for the AUC0-72, the ER/IR ratio of the adjusted geometric means was 157.98%, with a 90% CI of 98.87% to 252.44%. The lower boundary of the 90% CI was greater than the predefined criterion of ≥80%. In addition, the ER/IR ratio for the adjusted means of the AUC0-8 was 120.09%, with a 90% CI of 74.92% to 192.51%, and for the AUC0-24, the ER/IR ratio for the adjusted means was 145.76%, with a 90% CI of 93.74 to 226.64% (Table 2). For the Cmax, the ER/IR ratio for the adjusted means was 91.63%, with a 90% CI of 56.21% to 149.38%.
Fig. 1.
Mean (+SD) serum azithromycin concentration-time profiles for children with AOM following a single oral dose of 60 mg/kg azithromycin in the ER formulation or 30 mg/kg azithromycin in the IR formulation (top panel, 8-h profile; bottom panel, 72-h profile).
Table 2.
Statistical summary of pharmacokinetic parameters of azithromycin in children with AOM following a single oral dose of the 60 mg/kg ER or 30 mg/kg IR azithromycin formulationa
| Pharmacokinetic parameter | Geometric mean value (CV%) for group |
ER/IR geometric mean ratio (%) (90% CI) | |
|---|---|---|---|
| 60 mg/kg ER azithromycin (n = 18) | 30 mg/kg IR azithromycin (n = 18) | ||
| AUC0–8 (ng·h/ml) | 2,576 (53) | 2,145 (60) | 120.09 (74.92, 192.51) |
| AUC0–24 (ng·h/ml) | 5,765 (49) | 3,955 (58) | 145.76 (93.74, 226.64) |
| AUC0–72 (ng·h/ml) | 9,848 (52) | 6,234 (60) | 157.98 (98.87, 252.44) |
| Cmax (ng/ml) | 611 (62) | 667 (67) | 91.63 (56.21, 149.38) |
| Tmax (h)b | 3.0 (2.0–8.0) | 2.0 (1.0–4.1) | |
CV%, percent coefficient of variation; ER, extended release; IR, immediate release; AUC, area under the curve; Cmax, maximum concentration; Tmax, time to reach maximum concentration.
Median (range) for Tmax.
Results of the comparisons of the concentration data between treatments at each serial time point are summarized in Table 3. The lower boundaries of the 90% CIs for ER/IR concentration ratios at the first 3 time points (C1, C2, and C3) fell below 80%. The lower boundaries of the 90% CIs for ER/IR concentration ratios at all remaining time points (C4, C8, C24, C48, and C72) were greater than 100%.
Table 3.
Statistical summary of azithromycin concentration comparisons at each time point in children with AOM following a single oral dose of the 60-mg/kg ER or 30-mg/kg IR azithromycin formulation
| Concna (ng/ml) | Adjusted geometric mean |
ER/IR geometric mean ratio (%) | 90% CI for ER/IR ratio | |
|---|---|---|---|---|
| 60 mg/kg ER (n = 18) | 30 mg/kg IR (n = 18) | |||
| C1 | 100 | 153 | 65.44 | 23.56, 181.77 |
| C2 | 293 | 580 | 50.57 | 25.24, 101.30 |
| C3 | 382 | 382 | 100.07 | 57.91, 172.92 |
| C4 | 442 | 268 | 164.93 | 103.78, 262.12 |
| C8 | 245 | 140 | 174.41 | 110.07, 276.36 |
| C24 | 142 | 82 | 173.01 | 111.45, 268.55 |
| C48 | 95 | 50 | 189.35 | 129.76, 276.30 |
| C72 | 56 | 31 | 183.14 | 124.61, 269.14 |
Concentration at specific time (hour).
Safety assessment.
There were no serious AEs. All AEs were mild or moderate in severity, and all resolved by the end of the study. In the IR group, 5 out of 19 subjects reported 5 treatment-emergent AEs: treatment failure (2 subjects), anorexia (1), diarrhea (1), and vomiting (1). Among them, treatment failure and anorexia were assessed as being related to treatment by the investigator. In the ER group, 4 out of 19 subjects reported 4 treatment-emergent AEs: nausea (1) and vomiting (3). Among them, one vomiting event was assessed as being related to treatment by the investigator.
The most commonly reported AE was vomiting (4 events). The vomiting event in the ER group, which was assessed as being related to treatment, led to discontinuation from the study. This event started approximately 5 min after dosing and resolved approximately 19 h after dosing, which was assessed as mild in severity. The other two vomiting events in subjects from the ER group occurred at a later time point, with a very short duration: 9 and 35 h after dosing, respectively. The vomiting in the subject from the IR group also occurred at a later time point, with a very short duration: 16 h after dosing. All three vomiting events were attributed to the disease under study. It is thought that nausea and vomiting that occur shortly after the oral dosing of macrolides, including azithromycin, are primarily local in origin and possibly due to the drug's action on the motilin receptors in the upper GI tract (12, 16).
There were no clinically significant laboratory tests or vital sign results other than the signs and symptoms of AOM.
Assessment of clinical response.
Sixteen (88.9%) subjects in the IR group and 18 (100%) subjects in the ER group had a clinical response assessed as cure. Two (11.1%) subjects in the IR group had a clinical response assessed as failure.
DISCUSSION
For azithromycin, the AUC0-24/MIC ratio is considered the pharmacokinetic-pharmacodynamic parameter that best predicts efficacy (1, 6). It has been demonstrated that higher AUC values, achieved by “front-loading” (i.e., giving the entire course of therapy as one dose), could result in improved bacteriologic efficacy based on preclinical infection models (9). It should be noted that the single-dose regimen maximizes patient compliance, therefore eliminating noncompliance as a reason for treatment failure. Also, because of the benefit conferred by delivering the entire dose up front—at a time when the bacterial burden is the greatest—single-dose therapy has the potential to minimize the emergence and spread of bacterial resistance in the community. To assess the utility of the azithromycin ER formulation for AOM, this study compared the systemic exposure of the 60-mg/kg single dose of the ER formulation to that of the approved 30-mg/kg single dose of the IR formulation in children with AOM.
In this study, the pharmacokinetic parameter AUC0-72 was the primary endpoint for comparisons between ER and IR formulations, since azithromycin has a long elimination half-life (approximately 60 h) (10). Per FDA guidance on bioavailability and bioequivalence studies for orally administered drug products, it is acceptable for drugs with a long elimination half-life that demonstrate low intrasubject variability in distribution and clearance to use an AUC truncated at 72 h (AUC0-72) in place of AUC0-∞ (8). The 72-h sample collection is considered adequate to ensure the completion of the gastrointestinal transit (approximately 2 to 3 days) of the drug product and absorption of the drug substance. The criterion for the AUC0-72 between treatments was predefined as maintaining at least a lower 90% CI boundary of 80% to demonstrate that the exposure from the ER formulation is similar to or greater than that from the IR formulation, which was consistent with the industry-accepted lower boundary of the bioequivalence range (80 to 125%). The ER/IR AUC0-72 ratio for the adjusted means (90% CI) was 157.98% (98.87%, 252.44%), which met the predefined criterion; thus, the exposure from a 60-mg/kg ER dose was considered similar to or greater than that from a 30-mg/kg IR dose.
Although the concentrations of the ER formulation during the first 3 h were lower than those of the IR formulation, the exposures over the first 8 h (AUC0-8) were comparable between these two treatments (Table 2). By 24 h after dosing, the exposure (AUC0-24) from ER treatment was similar to or higher than that from IR treatment (Table 2). This indicated that a slight delay in drug release for the ER formulation has a minimal impact on the total azithromycin exposure during the early state of treatment.
The azithromycin pharmacokinetic profiles for 7 subjects had a double peak: 3 subjects from the ER group and 4 from the IR group. The reason for the double peaks is unknown. This was also observed in other azithromycin pharmacokinetic studies (data available upon request).
Azithromycin was safe and well tolerated following the single-dose administration of either formulation (60-mg/kg ER or 30-mg/kg IR formulation) in children with AOM. Due to the small sample size, comparisons cannot be made between the ER and IR formulations regarding safety.
The observed clinical cure rates for the 2 treatments appeared to be similar, as all 18 subjects with AOM who completed the study had a clinical response assessed as cure in the ER group, compared to 16 out of 18 subjects who completed the study in the IR group. Note that this study was not designed to demonstrate clinical noninferiority between these two treatments, and other antibiotic therapy was permitted during the study if clinically indicated.
Previously, the efficacy and safety of the 60-mg/kg azithromycin ER single-dose regimen in children with AOM was evaluated in a randomized, double-blinded, double-dummy study in comparison with a 10-day regimen of high-dose amoxicillin-clavulanate (90/6.4 mg/kg/day, given in divided doses q12h), particularly in children with or at risk for recurrent middle ear infection (4). In the bacteriologic eligible population (clinically eligible subjects with a key AOM pathogen isolated at baseline), the cure rates for the azithromycin ER arm (n = 258) and the amoxicillin-clavulanate arm (n = 239) were 80.2% and 84.5%, respectively; the age-adjusted difference was −3.9%, (95% CI, −10.4%, 2.6%). Unfortunately, the lower boundary of the 95% CI (−10.4%) marginally missed the study-defined noninferiority criterion of −10%. Vomiting on day 1 had a greater impact on the efficacy rate in the bacteriologic eligible population in the azithromycin ER arm than on that in the amoxicillin-clavulanate arm. Specifically, 4 subjects in the azithromycin ER arm vomited within 30 min of dosing on day 1 and were withdrawn from the study, in comparison with 2 subjects in the amoxicillin-clavulanate arm, and these subjects were assessed as clinical failures at the TOC visit. In the bacteriologic-per-protocol population (subjects eligible for study of bacteriologic efficacy with a TOC visit), the cure rates for the azithromycin ER arm (n = 239) and the amoxicillin-clavulanate arm (n = 217) were 85.8% and 89.9%, respectively; the age-adjusted difference was −3.4% (95% CI, −9.1%, 2.4%). The most common treatment-related AEs for the ER group were vomiting (10.7%), diarrhea and loose stools (9.3% each), and rash (5.1%). The most common treatment-related AEs for the amoxicillin-clavulanate group were diarrhea (17.7%), loose stools (12.8%), vomiting (8.2%), rash (7.7%), and dermatitis (5.1%). The AE profile of ER azithromycin was favorable compared with that of amoxicillin-clavulanate, particularly with respect to diarrhea. Although subjects given ER azithromycin had a higher incidence of immediate vomiting after dosing, the incidence of longer-term vomiting was higher for subjects given amoxicillin-clavulanate.
Subsequently, more effort was made to address the tolerability issue (early vomiting) with ER azithromycin. It was demonstrated previously that this could be effectively managed by use of a more dilute (less viscous) concentration (27 mg/ml versus the original 60-mg/ml suspension) and a standardized dosing technique (3). The more dilute suspension (27 mg/ml) of the ER azithromycin formulation was used in this study.
In summary, this study demonstrated that the 60-mg/kg ER azithromycin formulation given as a single dose provides similar or greater systemic exposure in children with AOM than that of the 30-mg/kg single dose of IR azithromycin.
ACKNOWLEDGMENTS
We acknowledge the study team for their contribution to the study, especially the study managers Laura Sears and Clarissa McDonough from Pfizer and Carolina Soley and Cecilia Loaiza from the Instituto de Atención Pediátrica.
This study was supported by Pfizer Inc. Ping Liu, Annie F. Fang, Robert R. LaBadie, and Penelope H. Crownover are Pfizer employees and stockholders. Adriano G. Arguedas was the principal investigator for this study. He has received research grants and honoraria for conferences and participation in advisory boards from Pfizer, Novartis, GlaxoSmithKline, Bristol Myers, Abbott, Sanofi Pasteur, Aventis, Replidyne, Wyeth, and Electrosonics.
Footnotes
Published ahead of print on 22 August 2011.
REFERENCES
- 1. Andes D. 2001. Pharmacokinetic and pharmacodynamic properties of antimicrobials in the therapy of respiratory tract infections. Curr. Opin. Infect. Dis. 14:165–172 [DOI] [PubMed] [Google Scholar]
- 2. Arguedas A., et al. 2005. A randomized, multicenter, double blind, double dummy trial of single dose azithromycin versus high dose amoxicillin for treatment of uncomplicated acute otitis media. Pediatr. Infect. Dis. J. 24:153–161 [DOI] [PubMed] [Google Scholar]
- 3. Arguedas A., Loaiza C., Ferris J., Jorgensen D. 2007. Tolerability of extended-release azithromycin pediatric suspension (PEDS-AZM) vs. extended-release azithromycin adult suspension (ADULT-AZM) in children with acute otitis media (AOM), abstr. G-981. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL, 17 to 20 September 2007 [Google Scholar]
- 4. Arguedas A., Soley C., Jorgensen D. 2006. Single, high-dose azithromycin extended release (60 mg/kg) (AZ-ER) vs. 10 days high-dose amoxicillin clavulanate (AC) in children with acute otitis media (AOM) at high risk of persistent/recurrent otitis media (HR-P/ROM), abstr. G-347a. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 27 to 30 September 2006 [Google Scholar]
- 5. Chandra R., et al. 2007. Clinical pharmacokinetics and gastrointestinal tolerability of a novel extended-release microsphere formulation of azithromycin. Clin. Pharmacokinet. 46:247–259 [DOI] [PubMed] [Google Scholar]
- 6. Craig W. A. 2001. Does the dose matter? Clin. Infect. Dis. 33(Suppl. 3):S233–S237 [DOI] [PubMed] [Google Scholar]
- 7. Dunne M. W., et al. 2003. Efficacy of single-dose azithromycin in treatment of acute otitis media in children after a baseline tympanocentesis. Antimicrob. Agents Chemother. 47:2663–2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. FDA 2003. Bioavailability and bioequivalence studies for orally administered drug products—general considerations. FDA, Washington, DC [Google Scholar]
- 9. Girard D., Finegan S. M., Dunne M. W., Lame M. E. 2005. Enhanced efficacy of single-dose versus multi-dose azithromycin regimens in preclinical infection models. J. Antimicrob. Chemother. 56:365–371 [DOI] [PubMed] [Google Scholar]
- 10. Jacobs R. F., et al. 2005. Pharmacokinetics of intravenously administered azithromycin in pediatric patients. Pediatr. Infect. Dis. J. 24:34–39 [DOI] [PubMed] [Google Scholar]
- 11. Khurana C. M. 1996. A multicenter, randomized, open label comparison of azithromycin and amoxicillin/clavulanate in acute otitis media among children attending day care or school. Pediatr. Infect. Dis. J. 15:S24–S29 [DOI] [PubMed] [Google Scholar]
- 12. Periti P., Mazzei T., Mini E., Novelli A. 1993. Adverse effects of macrolide antibacterials. Drug Saf. 9:346–364 [DOI] [PubMed] [Google Scholar]
- 13. Pfizer Inc 2010. Zithromax: azithromycin immediate release United States package insert. Pfizer Inc., New York, NY [Google Scholar]
- 14. Pfizer Inc 2010. Zmax: azithromycin extended release United States package insert. Pfizer Inc., New York, NY [Google Scholar]
- 15. Soley C. A., Arguedas A. 2005. Single-dose azithromycin for the treatment of children with acute otitis media. Expert Rev. Anti Infect. Ther. 3:707–717 [DOI] [PubMed] [Google Scholar]
- 16. Weber F. H., Jr., Richards R. D., McCallum R. W. 1993. Erythromycin: a motilin agonist and gastrointestinal prokinetic agent. Am. J. Gastroenterol. 88:485–490 [PubMed] [Google Scholar]