Abstract
Tedizolid (TR-700, formerly torezolid) is the active component of the new oxazolidinone prodrug tedizolid phosphate (TR-701). We had previously demonstrated that tedizolid possessed potent antistaphylococcal activity superior to that of linezolid in a neutropenic mouse thigh infection model (A. Louie, W. Liu, R. Kulawy, and G. L. Drusano, Antimicrob. Agents Chemother. 55:3453-3460, 2011). In the current investigation, we used a mouse thigh infection model to delineate the effect of an interaction of TR-700 and granulocytes on staphylococcal cell killing. We compared the antistaphylococcal killing effect of doses of TR-701 equivalent to human exposures ranging from 200 to 3,200 mg/day in both granulocytopenic and normal mice. The mice were evaluated at 24, 48, and 72 h after therapy initiation. In granulocytopenic mice, a clear exposure response in which, depending on the time point of evaluation, stasis was achieved at “human-equivalent” doses of slightly below 2,300 mg/day (at 24 h) to slightly below 2,000 mg/day (at 72 h) was observed. In immune-normal animals, stasis was achieved at human-equivalent doses of slightly greater than 100 mg/day or less. The variance in bacterial cell killing results was attributable to the presence of granulocytes (without drug), the direct effect of TR-700 on Staphylococcus aureus, and the effect of the drug on Staphylococcus aureus mediated through granulocytes. The majority of the bacterial cell killing in normal animals was attributable to the effect of TR-700 mediated through granulocytes. Additional studies need to be undertaken to elucidate the mechanism underlying this observation.
INTRODUCTION
One of the most important determinations necessary when developing a new antimicrobial agent is that of how large of a dose is required to generate a good clinical and microbiological outcome in a large population of severely infected patients. For acute bacterial skin and skin structure infections (ABSSSIs), formerly classified as complicated skin and skin structure infections (cSSSI), the mouse thigh infection model is a standard for the preclinical identification of optimal doses and schedules. This model has demonstrated excellent clinical correlation over time (1).
Tedizolid (TR-700, formerly torezolid) is a new oxazolidinone antibiotic that has potent activity against Gram-positive bacteria, including methicillin-susceptible Staphylococcus aureus and -resistant S. aureus (MRSA). In order to improve the solubility and bioavailability of this drug, TR-700 was linked to a phosphate by an ester bond. The resulting prodrug, tedizolid phosphate (TR-701) lacks antimicrobial activity. In humans and other mammals, the monoester phosphate is very rapidly hydrolyzed, producing the active moiety TR-700.
Previously we conducted dose range and dose fractionation studies for TR-701 in a neutropenic mouse thigh model of S. aureus infection and demonstrated that the area under the concentration-time curve over 24 h in the steady state divided by the MIC (AUC/MIC ratio) is the pharmacodynamic index most closely linked to the bacterial cell killing rate (9). Linezolid served as a positive control in this evaluation. We showed that, even at matched values for the AUC/MIC ratio for linezolid and TR-700, the latter significantly outperformed linezolid. Lemaire et al. demonstrated that TR-700 penetrated into macrophages significantly better than linezolid and also translated this penetration into a better rate of intracellular killing of S. aureus (8).
Granulocytes are a critical element in the body's response to ABSSSIs. Given the findings of Lemaire et al. (8), we hypothesized that treatment with TR-700 would result in a significant increase in activity in the presence of granulocytes relative to the period when the animals were neutropenic. Andes and Craig (2) demonstrated that granulocytes decreased the AUC/MIC target value by 2- to 4-fold when a quinolone was used but that little change was seen for a strain of Klebsiella pneumoniae when ceftobiprole was used for cohorts of normal versus neutropenic mice (4). Given the known penetration of macrolides and quinolones into macrophages and white cells, we expected a larger boost in microbiological activity when tedizolid, which penetrates macrophages well (8), was employed in the presence of granulocytes in a mouse thigh model of S. aureus infection.
MATERIALS AND METHODS
Bacterial strains.
An MRSA ATCC 33591 isolate was obtained from the American Type Culture Collection (Manassas, VA). This isolate was stored at −80°C in 10% glycerol. For each experiment, the bacterial strain was cultured overnight on blood agar plates to confirm the purity and viability of the microbe. Then, a few colonies taken from the overnight agar culture were grown at 35°C to the late log phase in cation-adjusted Mueller-Hinton II broth in a water-shaker bath. The bacterial suspension was diluted to the desired concentration and was used immediately. The bacterial density in the suspension was confirmed by quantitative culture.
Antibiotics.
Tedizolid phosphate (TR-701) (molecular weight [MW], 494.28) and tedizolid (TR-700) (MW, 370.17) were supplied by Trius Therapeutics, Inc. (San Diego, CA). The structures of TR-701 and TR-700 are shown in Fig. 1. For each in vivo experiment, TR-701 (lot no. 287/07-TR3) was dissolved in sterile water and used immediately. For the susceptibility studies, TR-700 (lot no. 1040-69-2) was dissolved in dimethyl sulfoxide (DMSO) and then further diluted with medium to the desired concentrations.
Fig. 1.
Structures of tedizolid phosphate and tedizolid. (A) Tedizolid phosphate (TR-701 [prodrug]). (B) Tedizolid (TR-700).
Susceptibility studies.
The MICs of TR-700 for S. aureus ATCC 33591 were determined using cation-adjusted Mueller-Hinton II broth (BBL, Sparks, MD) and the macrobroth serial dilution method specified by CLSI (3). Subsequently, microdilution broth susceptibility studies were conducted to determine the effect of 80% complement-inactivated mouse and human sera on the activity of TR-700 (as performed previously [9]). The susceptibility studies were conducted at least twice for each bacterial strain.
Mice.
Female outbred Swiss Webster mice (Taconic Farms, Taconic, NY) (22 to 25 g) were employed. For the studies where granulocytopenia was required, transient neutropenia was induced by treating each mouse with cyclophosphamide (Sigma-Aldrich Inc., St. Louis, MO) (150 mg/kg of body weight) via the intraperitoneal (i.p.) route 4 days prior to bacterial inoculation and with cyclophosphamide at 100 mg/kg via the i.p. route 1 day prior to bacterial challenge. This regimen resulted in neutropenia in mice (neutrophil count < 100 cells/ml) for at least 5 days from the time the second dose of cyclophosphamide was administered (data not shown). All experimental methods using mice described in the proposal were approved by our IACUC.
Pharmacokinetic studies for TR-701 and TR-700.
The pharmacokinetics of TR-701 and TR-700 in mice have been studied by us previously (9). The concentrations in plasma for both TR-701 and TR-700 were determined by a validated liquid-chromatography, dual-mass-spectrometry (LC/MS/MS) technique. All data were fitted simultaneously in a large-population model employing NPAG software (7). Adaptive γ determinations were not employed, because only one sample was obtained from each mouse. Previously published (9) mean parameter values were employed for all the experiments described here to estimate the exposure to tedizolid (both TR-701 and TR-700).
TR-701 dose range experiment.
It should be noted that all TR-700 exposures were provided as tedizolid phosphate human dose equivalents. The total drug AUC from 0 to 24 h (AUC0-24) corresponding to exposure of a 200-mg dose of TR-701/day in humans was 20.1 mg of TR-700·h/liter (multiple-dose phase 1 study results supplied by Trius Therapeutics, Inc.). Normal and neutropenic mice differed slightly by weight. To attain the “human-equivalent” 24-h AUC value, normal and neutropenic mice were intraperitoneally administered 8.42 mg/kg of body weight, i.e., circa 0.202 mg for normal mice and 0.194 mg for neutropenic mice. All human-equivalent doses were scaled to this dose.
In the dose-range studies, MRSA ATCC 33591 at the mid-log-phase growth stage was inoculated into the posterior thigh muscles of normal and neutropenic mice at 107 CFU/thigh and 5 × 105 CFU/thigh, respectively. The bacterial inocula were confirmed by quantitative culture analyses. Two mice were immediately sacrificed, and homogenates of the posterior thigh muscle tissue were washed and then quantitatively cultured to determine the bacterial density in the thigh muscles at baseline. Two hours later, tissues collected from three additional mice were similarly evaluated to confirm that the bacteria in the thigh muscles were in log-phase growth prior to initiation of therapy. Treatment was then initiated, and mice were given TR-701 at doses that would produce AUC0-24 values of total drug that ranged from a 200 to 3,200 mg (200, 400, 600, 800, 1,200, 1,600, and 3,200 mg)/day human equivalent as a single dose via the i.p. route. The remaining infected mice were divided into 48 groups, each consisting of 4 mice for dosing groups and no-treatment controls for granuolocytopenic and granulocyte-replete groups, with sacrifice times of 24, 48, and 72 h. Groups of mice from the no-treatment control group and the groups corresponding to each of the doses of TR-701 were sacrificed at 24, 48, and 72 h after treatment initiation, and the posterior thigh muscles were collected aseptically. Homogenates of the infected thigh tissues were washed twice with sterile saline to prevent drug carryover and were then quantitatively cultured onto blood agar plates. After 24 h of incubation at 35°C, the colonies were enumerated.
Application of a mathematical model.
All of the groups at all time points were simultaneously analyzed using a 17-parameter mathematical model as follows:
dX(1)/dT through dX(5)/dT are the rates of change of the amount of TR-701 with respect to time [dX(1)/dT and dX(2)/dT], the rates of change of TR-700 with respect to time [dX(3)/dT and dX(4)/dT], and the number of CFU in the murine thigh with respect to time. X(1) is the absorption compartment, X(2) is the central compartment for TR-701, X(3) is the central compartment for TR-700, X(4) is the peripheral compartment for TR-700, and X(5) is the compartment for the infection. Ka represents the first-order absorption rate constant, Khydrolysis represents the first-order rate constant for the relationship between prodrug TR-701 and the microbiologically active TR-700 moiety. CL701 and CL700 represent the drug clearance rates for TR-701 and TR-700, respectively, and K34 and K43 represent the first-order intercompartmental transfer rate constants for TR-700. These values were fixed at the values identified in our previous pharmacokinetic analysis of TR-701 and 700 in mice (9).
The fifth differential equation represents the bacterial cell killing rate. It includes terms for cell growth and cell death. Cell death was categorized in two ways in this model: death attributable directly to drug and death attributable to granulocytes. Bayesian estimates for the latter differentiated the cell kill rates seen with and without drug so that the impact of TR-700 working through the granulocytes could be estimated. As we did not measure the numbers of granulocytes present in the mice in the normal and granulocytopenic states, we introduced two sets of relationships into the equation, one for white blood cell (WBC) killing in normal mice and one for WBC killing in granulocytopenic mice. We previously demonstrated (6) that bacterial cell killing by granulocytes represents a saturable function and is well represented by a Michaelis-Menten model. Therefore, we used this form for describing WBC killing. Had we measured WBC numbers, we would have made the Vmax terms (WBCKillN and WBCKillNN) multiply the WBC number and could have collapsed the model.
In the fifth differential equation, Kg represents the first-order growth constant, Popmax represents the maximal size of the bacterial population in stationary phase, Kk-max represents the value of the first-order directly drug-induced kill rate constant, C50k represents the value of the drug concentration in the plasma at which the kill rate was half the maximal rate, and Hk represents the Hill's constant. WBCKillN and WBCKillNN represent the maximal kill rate terms attributable to granulocytes for the neutropenic and nonneutropenic groups, respectively. WBCKillN50 and WBCKillNN50 represent the numbers of bacterial cells at which the kill rate value represents half the saturation value. R (1) and R (2) represent the piecewise input functions limited to 0 or 1 such that a specific animal's data were correlated to the proper relationship (neutropenic versus nonneutropenic). R (3) represents a piecewise input function that placed the proper number of bacteria in the fifth compartment at baseline, as different inoculum sizes were used for granulocytopenic versus normal animals. The BigNPAG program (7) was also used for this analysis. Simulations based on the Bayesian posterior parameter estimates were performed with the ADAPT II package of D'Argenio and Schumitzky (5).
RESULTS
Bacterial cell killing rate.
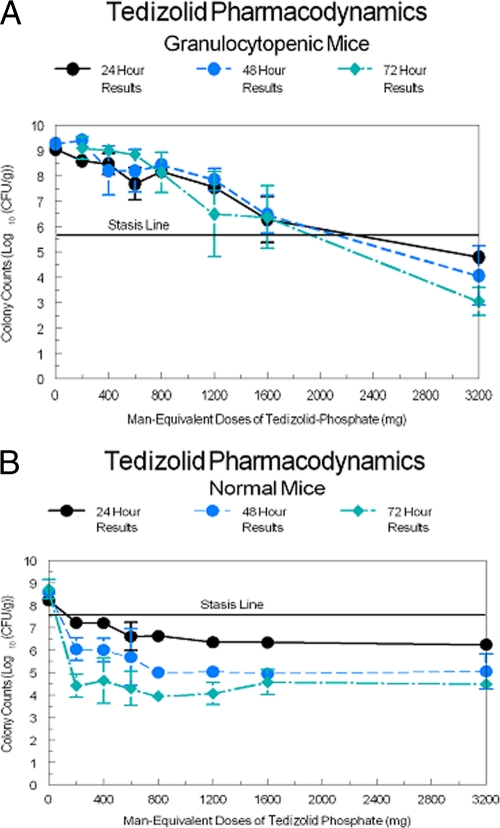
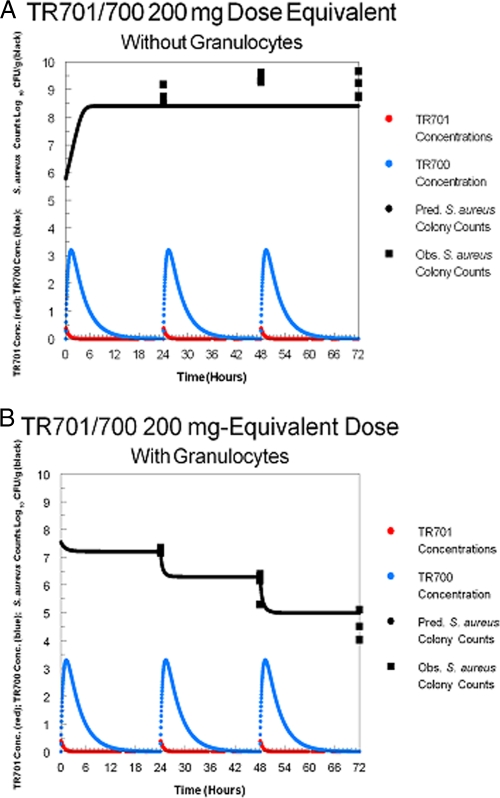
The TR-700 MIC for the challenge strain was 0.5 μg/ml. The bacterial growth/kill curves for the granulocytopenic animals are displayed in Fig. 2A and for the normal mice in Fig. 2B. The smallest dose administered produced a total drug AUC for TR-700 approximately equivalent to that produced by a 200-mg TR-701 dose (20.1 μg·h/ml). All other doses were scaled to this. Consequently, the AUC0-24/MIC ratio for a 200-mg-equivalent TR-701 dose was 20.1 μg·h/ml and the 3,200 mg-equivalent TR-701 dose produced an exposure of 643.3 μg·h/ml. It was apparent by inspection that the bacterial counts in the neutropenic animals did not achieve stasis by 72 h with TR-701 doses equivalent to human doses of 1,600 mg/day. At the 3,200 mg/day human-equivalent dose, a 2.75 log CFU/g killing rate was achieved at 72 h, and 1.73 and 1.0 log CFU/g killing rates were achieved at 48 and 24 h, respectively.
Fig. 2.
(A) Colony counts over 72 h of S. aureus in the thighs of granulocytopenic mice. (B) Colony counts over 72 h of S. aureus in the thighs of granulocyte-replete mice.
For the normal animals, Fig. 2B clearly demonstrates the major impact of the presence of granulocytes. There is a clear dose response at 24 h, with the 1,200 mg/day human-equivalent dosage providing the maximal effect. At 48 h, 800 mg/day human-equivalent doses produced the maximal effect, while the minimal dose (200 mg/day human equivalent) produced a killing rate reduced by 1.03 log CFU/g. At 72 h, the minimal dose (200 mg/day human equivalent) produced a near-maximal effect, with the 200 mg/day human-equivalent dose producing an effect not significantly different from the 3,200 mg/day human-equivalent dose according to the results of analysis of variance (ANOVA).
The pharmacokinetics of the drug were not different between groups (data not shown).
Model fit to the data.
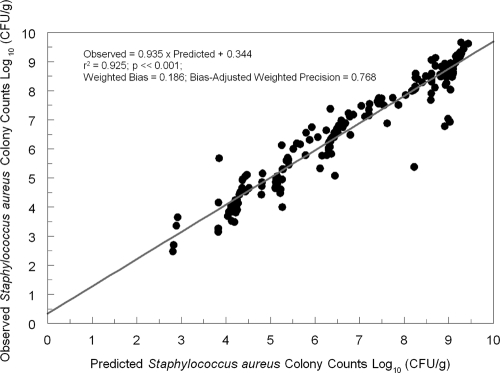
In Fig. 3, we show a plot comparing the predicted data to the observed data. The model fit the data well, with an r2 of 0.925 after the Bayesian step. The regression relationship was as follows: observed value = 0.935 × predicted value + 0.344. The point estimates of the parameter values are given in Table 1.
Fig. 3.
Predicted-observed regression of total colony counts of S. aureus in the thighs of tedizolid phosphate-treated mice (tedizolid-active moiety).
Table 1.
Parameter values for the model linking tedizolid exposure to killing of Staphylococcus aureus ATCC 33591
| Parameter | Unit of measurement | Mean | Median | SD |
|---|---|---|---|---|
| Kg | h−1 | 1.300 | 0.884 | 0.879 |
| Popmax | CFU/g | 1.36 × 109 | 1.72 × 109 | 6.48 × 108 |
| Kk-max | h−1 | 1.782 | 0.104 | 2.768 |
| C50k | mg/liter | 3.860 | 4.513 | 1.848 |
| Hk | Unitless | 8.056 | 7.895 | 0.431 |
| WBCKillN | (CFU/g)/h | 0.657 | 0.536 | 0.557 |
| WBCKillN50 | CFU/g | 3.47 × 106 | 2.41 × 106 | 3.02 × 106 |
| WBCKillNN | (CFU/g)/h | 2.638 | 0.995 | 3.797 |
| WBCKillNN50 | CFU/g | 1.58 × 106 | 1.80 × 104 | 3.22 × 106 |
Kg values represent the first-order growth rate constant; Popmax values represent the estimated maximal number of organisms in stationary phase; Kk-max values represent the first-order kill rate constant directly induced by tedizolid; C50k values represent the tedizolid concentration at which kill rate is half maximal; Hk values represent the Hill constant; WBCKillN values represent the maximal kill rate induced by granulocytes in the granulocytopenic cohort; WBCKillN50 values represent the organism load at which the system is half saturated; WBCKillNN values represent the maximal kill rate induced by granulocytes in the normal cohort; WBCKillNN50 values represent the organism load at which the system is half saturated. In the Bayesian estimates, the WBCKillN and WBCKillNN give the estimated killing rates for the granulocytes alone (no-treatment control) or for the granulocyte killing effect plus the tedizolid killing effect (treated cohorts). The pharmacokinetic parameter values for TR-701 and TR-700 were fixed at values identified previously (2). Ka = 66.4; Khydrol = 1.48; CL701 = 0.0102; V701 = 0.384; CL700 = 0.0101; V700 = 0.0164; K34 = 12.7; K43 = 12.8.
Simulations from the Bayesian estimates of the parameter values.
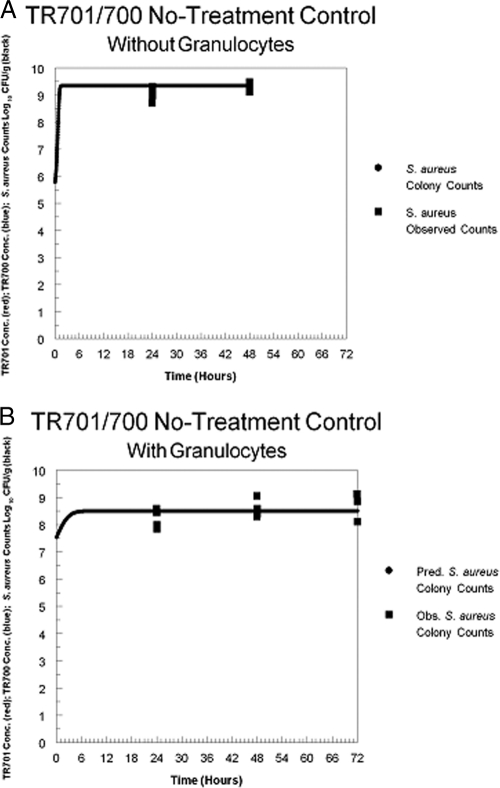
Figure 4 A shows the results of simulation seen with the no-treatment control for the granulocytopenic cohort. There was rapid growth by the staphylococci in the absence of granulocytes, resulting in attainment of the maximal number of organisms in the stationary phase, corresponding to the estimate of Popmax (approximately 9.1 logs). Figure 4B shows the results for the normal mouse cohort. Here, the inoculum started at a higher value (7.54 logs; lower inocula autocleared) and ended at a lower value (8.57 logs). This limitation in growth over the period of observation was directly attributable to the effect of the presence of the granulocytes.
Fig. 4.
(A) Simulation of Staphylococcus aureus growth in the thighs of no-treatment control mice that were granulocytopenic. The solid line represents predicted colony counts. Squares represent observed colony counts. (B) Simulation of Staphylococcus aureus growth in the thighs of no-treatment granulocyte-replete control mice. The solid line represents predicted colony counts. Squares represent observed colony counts.
In Fig. 5A and B, the effects of the smallest dose of TR-701 are shown for both the normal and granulocytopenic cohorts. There is a clear difference, with the normal cohort exhibiting a decline in the number of organisms throughout the period, whereas the granulocytopenic cohort exhibited a clear net growth of 1 log CFU/g over the observation period. The difference was due to the granulocytes. The ability of the low dose of TR-700 to induce a multilog decline in the bacterial numbers for immunocompetent mice showed that the effect of the drug was mediated through the granulocytes, since the low dose of the drug in neutropenic mice resulted in net growth in bacterial numbers.
Fig. 5.
(A) Simulation of Staphylococcus aureus growth in the thighs of granulocytopenic mice that were treated with an exposure to tedizolid equivalent to 200 mg of tedizolid phosphate in humans (AUC0-24 = 20.1 mg·h/liter). The solid line represents predicted colony counts. Squares represent observed colony counts. (B) Simulation of Staphylococcus aureus killing in the thighs of granulocyte-replete mice that were treated with an exposure to tedizolid equivalent to 200 mg of tedizolid phosphate in humans (AUC0-24 = 20.1 mg·h/liter). The solid line represents predicted colony counts. Squares represent observed colony counts.
DISCUSSION
In both normal and granulocytopenic animals, treatment with TR-701 resulted in a good dose-response curve. The difference between the two groups represented an improvement in the exposure response as a function of the presence of granulocytes. Examination of Fig. 2A and B shows that, for the granulocytopenic animals, stasis was achieved with a human-equivalent dose exposure of slightly less than the 2,000, 2,100, and 2,300 mg administered daily for the 72-, 48-, and 24-h endpoints, respectively. In the normal animals, stasis was achieved at human-equivalent exposures of slightly more than 100 mg/day at the 24-h endpoint and less than 100 mg/day at the 48-h and 72-h endpoints. The maximal killing rate was achieved at 72 h, with the lowest dose (200 mg/day) providing an effect similar to that seen with the highest dose (3,200 mg/day); those regimens were associated with bacterial burdens in thigh muscles of 4.42 ± 0.433 log CFU/g and 4.49 + 0.264 log CFU/g, respectively, which represent bacterial killing levels that were 3.12 and 3.05 log CFU/g greater than the stasis response. Thus, after 72 h of therapy with TR-701, the lowest and the highest doses produced equivalent microbiological effects. On day 1, both achieved stasis. This suggests that human patients with ABSSSIs who are treated with 200 mg/day of TR-701 should have a successful treatment outcome, since it has been shown that the stasis response is predictive of clinical success in immunocompetent human patients with ABSSSIs (1). A successful treatment outcome has recently been validated in a human clinical phase 2 study of cSSSI in which a clinical cure rate of 98.2% was found for clinically evaluable patients treated with 200 mg of TR-701 (10).
The results showing the differences between the effects of treatment with TR-700 in the presence and absence of granulocytes are unprecedented. For β-lactams and quinolones, differences in efficacy of 2- to 4-fold are seen (2, 4). The stasis endpoint values corresponding to the effects of TR-700 treatment differed on the order of 20-fold. When looking at the maximal killing effect, it is best to compare the differences in numbers of log CFU per gram of organisms between the 200 and 3,200 mg/day human-equivalent doses at the 24-, 48-, and 72-h endpoints. For the granulocytopenic mice, the differences were 3.8 log CFU/g at 24 h, 5.34 log CFU/g at 48 h, and 6.05 log CFU/g at 72 h. In nongranulocyotopenic mice, the differences were muted, at 0.98 log CFU/g at 24 h, 0.99 log CFU/g at 48 h, and −0.07 log CFU/g at 72 h. Thus, there was a major, multilog difference between the values corresponding to the effects mediated by the smallest versus largest doses in neutropenic mice at all time points evaluated whereas in the nongranulocyotopenic mice the difference was on the order of 1.0 log CFU/g at 24 and 48 h and was nonexistent at 72 h.
The only difference between the groups was the lack of granulocytopenia in one group versus its presence in the other. While we are cognizant of the importance of granulocytes in defense against many bacterial infections, this circumstance is unique. The true importance of the presence of granulocytes alone (i.e., without drug) is found in the contrast in bacterial growth rates in neutropenic versus nonneutropenic mice in the absence of drug therapy (Fig. 4A and B). Here, there was a net growth of 3.26 log CFU/g in the first 24 h in the neutropenic group. At 48 h, this difference had increased to 3.48 log CFU/g, and at 72 h, all control mice had died. In the nongranulocyotopenic mice, there was a net growth of 0.68 log CFU/g at 24 h, increasing at 48 and 72 h to 1.05 and 1.18 log CFU/g, respectively. All control mice survived to 72 h. Granulocytopenia allowed a rapid, multilog amplification in CFU per gram, resulting in animal death at 72 h, whereas the granulocyte-replete animals exhibited amplification limited to slightly greater than 1 log CFU/g. This is important, but it is clear that in the presence of granulocytes alone (i.e., without drug), net growth was observed. Use of the smallest dose of TR-701 is instructive (Fig. 2A and B). From the baseline, the granulocytopenic group treated with the lowest human equivalent dose of 200 mg/day exhibited net growth of 2.81, 3.62, and 3.31 log CFU/g at 24, 48, and 72 h, respectively. Normal animals receiving a 200 mg/day human-equivalent dose of TR-701 exhibited net killing levels (relative to the normal, no-treatment control level) of 0.998, 2.545, and 4.3 log CFU/g at 24, 48, and 72 h, respectively. One way of measuring the effect of the drug mediated by the presence of granulocytes is to contrast these figures, as the direct drug effect was seen in the neutropenic animals receiving the drug (note that the net amplification from baseline was less than in the no-treatment controls that were granulocytopenic and, importantly, that animals survived to 72 h). The cumulative effects of the presence of granulocytes plus the direct drug effect plus the drug effect mediated through the granulocytes is seen in the normal cohort receiving drug. Taking the differences in the net growth/net killing values shows that the level seen with the normal cohort was down by 3.12 log CFU/g at 24 h, 5.12 log CFU/g at 48 h, and 6.43 log CFU/g at 72 h relative to that seen with the neutropenic cohort receiving a 200 mg/day human-equivalent dose of TR-701. The granulocytopenic cohort showed amplification from the baseline, whereas the normal cohort exhibited net killing from the baseline, and the difference in the two results compared to the baseline give the preceding numbers contrasting the two groups. The vast bulk of this difference was due to the effect of drug mediated through the presence of the granulocytes.
This difference can be seen in Table 2. First, the direct effect of TR-700 can be estimated as the difference in organism amplification/kill ratios between the no-treatment control and the granulocytopenic cohorts subjected to the 200 mg/day TR-701 human-equivalent treatment. At 24 and 48 h, these differences resulted in 0.45 log CFU/g less amplification in the TR-701-treated group. At 48 h, the TR-701-treated group actually exhibited greater amplification (0.14 log CFU/g). This difference could be evaluated at 24 and 48 h, but not at 72 h, as all no-treatment controls had succumbed by this time. For the 72-h data, we simply fixed the value to zero. The direct effect of granulocytes together with the effect of TR-700 treatment mediated through the granulocytes is estimated as the difference between the counts seen with the normal cohort treated with a 200 mg/day human-equivalent dose of TR-701 and those seen when the direct effect of TR-700 is subtracted. For 24, 48, and 72 h, the effects attributable to those two factors were seen as reductions of 2.67, 5.26, and 6.43 log CFU/g. Finally, the effect of the drug mediated through the presence of granulocytes was calculated as the difference between the total effect (determined for the normal cohort treated with 200 mg/day of TR-701) and the effect mediated by the granulocytes without therapy. For the 24-, 48-, and 72-h time points, the estimates of the effect due to TR-700 acting through granulocytes were seen as killing levels of 1.16, 3.53, and 4.70 log CFU/g, respectively. These differences are well reflected in the modeling.
Table 2.
Determination of the effect of tedizolid mediated through the presence of granulocytes
| Parameter and time (h) | Value (log CFU/g) |
|---|---|
| Direct effect of tedizolid (net growth in no-treatment cohort [log CFU/g] − net growth in 200 mg human-equivalent dose cohort [log CFU/g] [both cohorts granulocytopenic]) | |
| 24 (3.26 − 2.81) | 0.45 |
| 48 (3.48 − 3.62) | −0.14 |
| Effect of tedizolid mediated through granulocytes + direct WBC effect (total effect [granulocytopenic cohort {log CFU/g} − normal treated cohort {log CFU/g}] − direct effect of tedizolid [log CFU/g]) | |
| 24 (−3.12 + 0.45) | −2.67 |
| 48 (−5.12 − 0.14) | −5.26 |
| 72a (−6.43 − 0.00) | −6.43 |
| Estimate of tedizolid effect mediated through granulocytes ([effect of tedizolid mediated through granulocytes{log CFU/g} + direct WBC effect {log CFU/g}] − WBC effect [log CFU/g])b | |
| 24 (−2.67 + 1.51) | −1.16 |
| 48 (−5.26 + 1.73) | −3.53 |
| 72 (−6.43 + 1.73) | −4.70 |
The actual direct drug effect cannot be calculated for the 72-h time point, because all the animals in the granulocytopenic no-treatment control cohort had expired before that time. As the actual value for 48 h was a negative value, the direct effect was fixed to zero for this time point.
It is not a fair appraisal of direct granulocyte effect to examine amplification in the granulocytopenic no-treatment control cohort versus the normal no-treatment control cohort, because there were different starting inocula, but the upper boundary is fixed in both instances because of the stationary-phase growth status. We estimated the direct effect by assuming that the granulocytopenic no-treatment control cohort amplification started at 7.54 log CFU/g, which is the same as the value seen with the normal no-treatment control cohort, and that the direct granulocyte effect would be the difference in levels of organism amplification between the granulocytopenic and normal no-treatment control cohorts. Again, for the 72-h time point, we use the value from 48 h because all animals in the granulocytopenic cohort had expired.
The mechanism behind this finding is unclear. Lemaire et al. (8) demonstrated that TR-700 penetrates into macrophages better than linezolid in cell culture and, once there, kills intracellular staphylococci significantly better. The difference in our study is that we evaluated the interaction of TR-700 and granulocytes in an in vivo system. It would not be surprising to find that, as with macrophages, granulocyte penetration by TR-700 is better than that seen with linezolid and, most importantly, that killing of staphylococci within granulocytes is also improved.
It is clear that TR-701 causes a multilog decline in S. aureus CFU per gram in the mouse thigh model and that it differs from many other antibiotics in that a considerable portion of the multilog decline is attributable to the effect of the drug mediated through the activity of effector cells, especially granulocytes. This work was performed with a single isolate, and more work needs to be performed with multiple isolates. Also, additional basic research needs to be done in this area to place these observations into perspective.
ACKNOWLEDGMENTS
This project was sponsored by a grant provided by Trius Therapeutics, San Diego, CA.
Footnotes
Published ahead of print on 12 September 2011.
REFERENCES
- 1. Ambrose P. G., et al. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79–86 [DOI] [PubMed] [Google Scholar]
- 2. Andes D., Craig W. A. 2003. Pharmacodynamics of the new Des-F(6)-quinolone garenoxacin in a murine thigh infection model. Antimicrob. Agents Chemother. 47:3935–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clinical Laboratory Standards Institute 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standards—seventh edition. CLSI publication M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 4. Craig W. A., Andes D. R. 2008. In vivo pharmacodynamics of ceftobiprole against multiple bacterial pathogens in murine thigh and lung infection models. Antimicrob. Agents Chemother. 52:3492–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. D'Argenio D. Z., Schumitzky A. 1997. ADAPT II: a program for simulation, identification, and optimal experimental design, user manual. Biomedical Simulations Resource, University of Southern California, Los Angeles, Los Angeles, CA: http://bmsr.usc.edu/ [Google Scholar]
- 6. Drusano G. L., Fregeau C., Liu W., Brown D. L., Louie A. 2010. Impact of burden on granulocyte clearance of bacteria in a mouse thigh infection model. Antimicrob. Agents Chemother. 54:4368–4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leary R, Jelliffe R., Schumitzky A., Van Guilder M. 2001. An adaptive grid non-parametric approach to pharmacokinetic and dynamic (PK/PD) models, p. 389–394. In Proceedings of the 14th IEEE Symposium on Computer-Based Medical Systems IEEE Computer Society, Bethesda, MD [Google Scholar]
- 8. Lemaire S., Van Bambeke F., Appelbaum P., Tulkens P. 2009. Cellular pharmacokinetics and intracellular activity of tedizolid (TR-700): studies with human macrophage (THP-1) and endothelial (HUVEC) cell lines. J. Antimicrob. Chemother. 64:1035–1043 [DOI] [PubMed] [Google Scholar]
- 9. Louie A., Liu W., Kulawy R., Drusano G. L. 2011. In vivo pharmacodynamics of torezolid phosphate (TR-701), a new oxazolidinone antibiotic, against methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains in a mouse thigh infection model. Antimicrob. Agents Chemother. 55:3453–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prokocimer P., et al. 2011. Phase 2, randomized, double-blind, dose-ranging study evaluating the safety, tolerability, population pharmacokinetics, and efficacy of oral torezolid phosphate in patients with complicated skin and skin structure infections. Antimicrob. Agents Chemother. 55:583–592 [DOI] [PMC free article] [PubMed] [Google Scholar]