Abstract
Pyrazinoic acid, the active form of the first-line antituberculosis drug pyrazinamide, decreased the proton motive force and respiratory ATP synthesis rates in subcellular mycobacterial membrane assays. Pyrazinoic acid also significantly lowered cellular ATP levels in Mycobacterium bovis BCG. These results indicate that the predominant mechanism of killing by this drug may operate by depletion of cellular ATP reserves.
TEXT
Shortening tuberculosis treatment duration is a key objective in order to reduce noncompliance and to combat recently emerging multidrug-resistant strains of Mycobacterium tuberculosis (6, 19, 36). Pyrazinamide (PZA), an important first-line drug employed in tuberculosis chemotherapy, played a key role in shortening the duration of tuberculosis treatment from 9 months to 6 months (22). PZA is a sterilizing drug that efficiently kills populations of Mycobacterium tuberculosis residing in acidic environments, as found during active inflammation (1, 7, 10, 11, 20, 21, 28, 32). Despite the importance of PZA, no cellular target proteins have been clearly identified (4, 23, 30, 41), and its mechanism of action is probably the least understood among all first- and second-line antituberculosis drugs. A better understanding of PZA action may help in development of new drugs to further shorten tuberculosis treatment.
PZA constitutes a prodrug that is hydrolyzed in the mycobacterial cell by pyrazinamidase to yield the active entity pyrazinoic acid (POA) (15, 16). According to the hypothesis put forward by Zhang and colleagues, POA, a weak acid (pKa, 2.9), acts as an uncoupling agent by breaking down the bacterial membrane potential (39, 40). POA in its unprotonated form can leave the cell by means of an unknown efflux system (37, 40), take up a proton in the acidic environment, and enter the mycobacterial cell again in its protonated, less polar form (39). The resultant decrease in proton motive force then blocks, among other processes, uptake of metabolites required for growth (40). However, it is not known whether the decreased membrane potential observed for PZA in whole mycobacterial cells (40) is due to the postulated uncoupling effect or indirectly caused by interference of PZA or POA with other cellular targets. Moreover, the impact of POA on respiratory ATP synthesis and on cellular ATP levels has not been investigated. In the present study, we used subcellular and cellular assays to address these open issues. We used M. bovis BCG, which is resistant to PZA due to mutations in pyrazinamidase (16, 29, 30) but is fully susceptible to POA (15, 31, 37), as a model system.
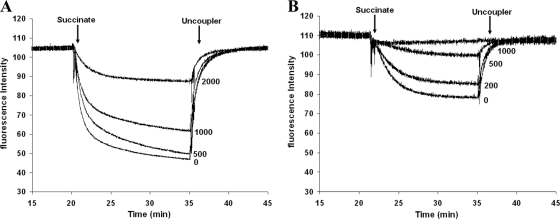
POA directly interferes with the proton motive force. We isolated membrane vesicles from M. bovis BCG as previously reported (9). In this subcellular system, the cytosolic fraction is removed, allowing a more specific investigation of drug action directed to membrane components (8, 9). First we determined whether pyrazinoic acid (POA) directly interferes with the proton motive force. The proton motive force was monitored with the ACMA (9-amino-6-chloro-2-methoxyacridine) quenching method as described previously (9). Addition of succinate caused fluorescence quenching, which was eased upon addition of an uncoupler (SF6847), proving that the quenching was caused by a proton motive force across the membrane (Fig. 1). In the presence of POA, the proton motive force decreased in a dose-dependent manner (Fig. 1). This effect was more pronounced at pH 5.5 than at pH 6.5 (Fig. 1). The POA concentrations needed to significantly decrease the proton motive force were comparable to values reported earlier for M. tuberculosis at the whole-cell level (40). This result shows that POA directly interferes with membrane energetics.
Fig. 1.
Pyrazinoic acid decreases the proton motive force in a subcellular assay. The proton motive force in membrane vesicles from M. bovis BCG was monitored in the presence of pyrazinoic acid at the indicated concentrations (measured in micrograms per milliliter) by quenching of ACMA as a fluorescence indicator at pH 6.5 (A) and pH 5.5 (B). Succinate and the uncoupler SF6847 were added to establish and collapse the proton motive force at the time points indicated by the arrows. Each experiment was carried out in triplicate; representative results are shown.
POA decreases rates of respiratory ATP synthesis. Next, we investigated the extent to which POA, by decreasing the proton motive force, interferes with respiratory ATP production. Rates of ATP synthesis by the mycobacterial membranes were determined as described previously (8, 9). As depicted in Fig. 2, POA inhibited ATP synthesis in a dose-dependent manner. The affinity for POA significantly decreased at higher pH, with a 50% inhibitory concentration (IC50) value of 200 μg/ml at pH 5.5 compared with 850 μg/ml at pH 6.5 (Fig. 2, closed bars). This result is consistent with the enhanced killing previously observed at acidic pH for PZA in vivo and for POA in vitro (38, 40). As shown by the results of a control experiment, only a minor effect was observed for PZA, with IC50s exceeding 2,000 μg/ml at both pH values tested (Fig. 2, open bars). These results strongly suggest that POA is the active entity that diminishes rates of ATP synthesis. By interfering both with uptake of metabolites as reported earlier (40) and with respiratory ATP synthesis as shown here, POA thus exerts at least a dual action, which potentially renders it an exceptionally powerful drug. This unusual property of POA may hold for bacteria under conditions of a low energy supply in particular (12, 34) and may in part explain why pyrazinamide appears to constitute an essential component of antituberculosis drug regimens.
Fig. 2.
Pyrazinoic acid inhibits ATP synthesis. ATP synthesis activity by membrane vesicles was determined using the glucose-6-phosphate method for quantification of the ATP produced (9). The reaction was carried out in the presence of the indicated concentrations (Con) of pyrazinoic acid (closed bars) or pyrazinamide (open bars) at pH 6.5 (A) or pH 5.5 (B). Each graph shows the mean values and standard deviations of the results of three independent experiments.
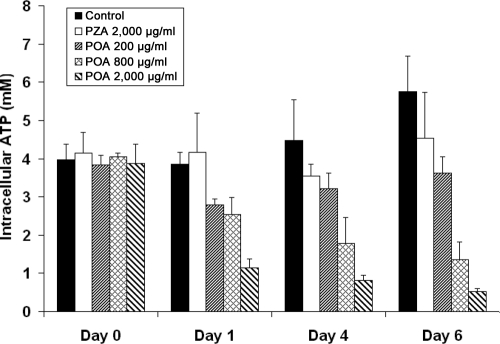
POA decreases cellular ATP levels. We investigated the impact of ATP synthesis inhibition by POA on cellular ATP levels. Addition of POA to M. bovis BCG grown in liquid culture significantly decreased cellular ATP levels in both a time-dependent and a dose-dependent manner (Fig. 3). The concentrations of POA needed to reduce cellular ATP levels correlate well with those required for ATP synthesis inhibition; e.g., 800 μg/ml POA (the IC50 in the ATP synthesis assay) decreased cellular ATP levels by ∼40% after 1 day and >60% after 6 days. In control experiments, PZA did not significantly change ATP levels. These results suggest that POA interference with respiratory ATP synthesis has a significant impact on cellular ATP levels and may be the cause of bacterial killing.
Fig. 3.
Pyrazinoic acid decreases cellular ATP levels. M. bovis BCG was grown in liquid culture (pH = 6.5) as described by Haagsma et al. (9). Cellular ATP levels in the presence of pyrazinoic acid or pyrazinamide were determined at the indicated time points using the luciferase bioluminescent method (17), based on cell volumes determined as described by Rao et al. (25). Bars represent the mean values and standard deviations of the results of three independent experiments.
PZA and POA share several interesting characteristics with a new series of ATP synthesis inhibitors, the diarylquinolines (2, 5, 9a), as follows. Both drugs show delayed action in vitro and in vivo, with time-dependent killing observed only from days 3 and 4 on (27, 40). Moreover, both drugs display a particularly strong effect on mycobacteria under (semi-) dormant conditions (10, 18, 26, 33, 38). Finally, in similarity to our data reported here for POA, TMC207 reduces cellular ATP levels (17, 18, 26). Based on the similarities in activity signatures, we suggest that the two drugs share the same predominant mechanism of killing, i.e., depleting the cellular ATP pool. It needs to be determined to what extent alternative targets are involved and to what extent they contribute to the pronounced bactericidal action of pyrazinamide.
The chain of events leading from (too) low ATP levels to bacterial killing is presumably complex (14, 24), and the factors involved need to be elucidated. Drugs interfering with cellular energy pools appear to be very powerful, especially against dormant bacteria. Membrane function and respiratory ATP synthesis may constitute examples of a new generation of antibiotic targets for treatment of persistent infections (3, 13, 19, 40). The subcellular membrane assay for characterization of pyrazinoic acid described here can be applied for screening and characterization of this new generation of compounds targeting respiratory ATP production.
Acknowledgments
P.L. gratefully acknowledges financial support from the Chinese Scholarship Council. A.C.H. and D.B. gratefully acknowledge financial support from the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO-ECHO grant 700.55.017).
We thank B. Appelmelk and W. Bitter (Medical Microbiology & Infection Control, VU University Medical Center) and M. Braster (Molecular Cell Physiology, VU University) for assistance with bacterial culture and luminescence measurements, respectively.
Footnotes
Published ahead of print on 29 August 2011.
The authors have paid a fee to allow immediate free access to this article.
ADDENDUM IN PROOF
While this paper was in press, W. Shi et al. (Science 333:1630–1632, 2011) reported the ribosomal protein RpsA as an additional target of pyrazinamide. The relative importance of the respective targets for bacterial killing needs to be investigated.
REFERENCES
- 1. Ahmad Z., et al. 2011. Dose-dependent activity of pyrazinamide in animal models of intracellular and extracellular tuberculosis infections. Antimicrob. Agents Chemother. 55:1527–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andries K., et al. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227 [DOI] [PubMed] [Google Scholar]
- 3. Bald D., Koul A. 2010. Respiratory ATP synthesis: the new generation of mycobacterial drug targets? FEMS Microbiol. Lett. 308:1–7 [DOI] [PubMed] [Google Scholar]
- 4. Boshoff H. I., Mizrahi V., Barry C. E., III 2002. Effects of pyrazinamide on fatty acid synthesis by whole mycobacterial cells and purified fatty acid synthase I. J. Bacteriol. 184:2167–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diacon A. H., et al. 2009. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N. Engl. J. Med. 360:2397–2405 [DOI] [PubMed] [Google Scholar]
- 6. Gandhi N. R., et al. 2010. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 375:1830–1843 [DOI] [PubMed] [Google Scholar]
- 7. Grosset J. 1978. The sterilizing value of rifampicin and pyrazinamide in experimental short-course chemotherapy. Bull. Int. Union Tuberc. 53:5–12 [PubMed] [Google Scholar]
- 8. Haagsma A. C., et al. 2009. Selectivity of TMC207 towards mycobacterial ATP synthase compared with that towards the eukaryotic homologue. Antimicrob. Agents Chemother. 53:1290–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haagsma A. C., Driessen N. N., Hahn M. M., Lill H., Bald D. 2010. ATP synthase in slow- and fast-growing mycobacteria is active in ATP synthesis and blocked in ATP hydrolysis direction. FEMS Microbiol. Lett. 313:68–74 [DOI] [PubMed] [Google Scholar]
- 9a. Haagsma A. C., et al. 2011. Probing the interaction of the diarylquinoline TMC207 with its target mycobacterial ATP synthase. PLOS One 6:e23575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heifets L., Lindholm-Levy P. 1992. Pyrazinamide sterilizing activity in vitro against semidormant Mycobacterium tuberculosis bacterial populations. Am. Rev. Respir. Dis. 145:1223–1225 [DOI] [PubMed] [Google Scholar]
- 11. Hu Y., Coates A. R., Mitchison D. A. 2006. Sterilising action of pyrazinamide in models of dormant and rifampicin-tolerant Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 10:317–322 [PubMed] [Google Scholar]
- 12. Huang Q., et al. 2007. Nutrient-starved incubation conditions enhance pyrazinamide activity against Mycobacterium tuberculosis. Chemotherapy 53:338–343 [DOI] [PubMed] [Google Scholar]
- 13. Hurdle J. G., O'Neill A. J., Chopra I., Lee R. E. 2011. Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 9:62–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kohanski M. A., Dwyer D. J., Collins J. J. 2010. How antibiotics kill bacteria: from targets to networks. Nat. Rev. Microbiol. 8:423–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Konno K., Feldmann F. M., McDermott W. 1967. Pyrazinamide susceptibility and amidase activity of tubercle bacilli. Am. Rev. Respir. Dis. 95:461–469 [DOI] [PubMed] [Google Scholar]
- 16. Konno K., Nagayama H., Oka S. 1959. Nicotinamidase in Mycobacteria: a method for distinguishing bovine type tubercle bacilli from other Mycobacteria. Nature 184:1743–1744 [DOI] [PubMed] [Google Scholar]
- 17. Koul A., et al. 2007. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat. Chem. Biol. 3:323–324 [DOI] [PubMed] [Google Scholar]
- 18. Koul A., et al. 2008. Diarylquinolines are bactericidal for dormant mycobacteria as a result of disturbed ATP homeostasis. J. Biol. Chem. 283:25273–25280 [DOI] [PubMed] [Google Scholar]
- 19. Koul A., Arnoult E., Lounis N., Guillemont J., Andries K. 2011. The challenge of new drug discovery for tuberculosis. Nature 469:483–490 [DOI] [PubMed] [Google Scholar]
- 20. McCune R. M., Jr., McDermott W., Tompsett R. 1956. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculosis infection to the latent state by administration of pyrazinamide and a companion drug. J. Exp. Med. 104:763–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McDermott W., Tompsett R. 1954. Activation of pyrazinamide and nicotinamide in acidic environments in vitro. Am. Rev. Tuberc. 70:748–754 [DOI] [PubMed] [Google Scholar]
- 22. Mitchison D. A. 1985. The action of antituberculosis drugs in short-course chemotherapy. Tubercle 66:219–225 [DOI] [PubMed] [Google Scholar]
- 23. Ngo S. C., Zimhony O., Chung W. J., Sayahi H., Jacobs W. R., Jr., Welch J. T. 2007. Inhibition of isolated Mycobacterium tuberculosis fatty acid synthase I by pyrazinamide analogs. Antimicrob. Agents Chemother. 51:2430–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pethe K., et al. 24 August 2010, posting date A chemical genetic screen in Mycobacterium tuberculosis identifies carbon-source-dependent growth inhibitors devoid of in vivo efficacy. Nat. Comm. 1:57 doi:10.1038/ncomms1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rao M., Streur T. L., Aldwell F. E., Cook G. M. 2001. Intracellular pH regulation by Mycobacterium smegmatis and Mycobacterium bovis BCG. Microbiology 147:1017–1024 [DOI] [PubMed] [Google Scholar]
- 26. Rao S. P., Alonso S., Rand L., Dick T., Pethe K. 2008. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 105:11945–11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rustomjee R., et al. 2008. Early bactericidal activity and pharmacokinetics of the diarylquinoline TMC207 in treatment of pulmonary tuberculosis. Antimicrob Agents Chemother. 52:2831–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salfinger M., Heifets L. B. 1988. Determination of pyrazinamide MICs for Mycobacterium tuberculosis at different pHs by the radiometric method. Antimicrob. Agents Chemother. 32:1002–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scorpio A., Zhang Y. 1996. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat. Med. 2:662–667 [DOI] [PubMed] [Google Scholar]
- 30. Scorpio A., et al. 1997. Characterization of pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 41:540–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Somoskovi A., Wade M. M., Sun Z., Zhang Y. 2004. Iron enhances the antituberculous activity of pyrazinamide. J. Antimicrob. Chemother. 53:192–196 [DOI] [PubMed] [Google Scholar]
- 32. Tarshis M. S., Weed W. A., Jr 1953. Lack of significant in vitro sensitivity of Mycobacterium tuberculosis to pyrazinamide on three different solid media. Am. Rev. Tuberc. 67:391–395 [DOI] [PubMed] [Google Scholar]
- 33. Tuberculosis (Edinburgh) 2008. Pyrazinamide. Tuberculosis (Edinburgh) 88:141–144 [DOI] [PubMed] [Google Scholar]
- 34. Wade M. M., Zhang Y. 2004. Anaerobic incubation conditions enhance pyrazinamide activity against Mycobacterium tuberculosis. J. Med. Microbiol. 53:769–773 [DOI] [PubMed] [Google Scholar]
- 35. Reference deleted.
- 36. World Health Organization 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB): global report on surveillance and response. World Health Organization, Geneva, Switzerland [Google Scholar]
- 37. Zhang Y., Scorpio A., Nikaido H., Sun Z. 1999. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Bacteriol. 181:2044–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Y., Permar S., Sun Z. 2002. Conditions that may affect the results of susceptibility testing of Mycobacterium tuberculosis to pyrazinamide. J. Med. Microbiol. 51:42–49 [DOI] [PubMed] [Google Scholar]
- 39. Zhang Y., Mitchison D. A. 2003. The curious characteristics of pyrazinamide: a review. Int. J. Tuberc. Lung Dis. 7:6–21 [PubMed] [Google Scholar]
- 40. Zhang Y., Wade M. M., Scorpio A., Zhang H., Sun Z. 2003. Mode of action of pyrazinamide: disruption of Mycobacterium tuberculosis membrane transport and energetics by pyrazinoic acid. J. Antimicrob. Chemother. 52:790–795 [DOI] [PubMed] [Google Scholar]
- 41. Zimhony O., Cox J. S., Welch J. T., Vilchèze C., Jacobs W. R., Jr 2000. Pyrazinamide inhibits the eukaryotic-like fatty acid synthetase I (FASI) of Mycobacterium tuberculosis. Nat. Med. 6:1043–1047 [DOI] [PubMed] [Google Scholar]