Abstract
Moxidectin, registered worldwide as a veterinary antiparasitic agent, is currently under development for humans for the treatment of onchocerciasis in collaboration with the World Health Organization. The objective of this study was to assess the pharmacokinetics of moxidectin in healthy lactating women, including the excretion into breast milk. Twelve women, ages 23 to 38 years, weighing 54 to 79 kg, all more than 5 months postpartum, were enrolled, following their plan to wean their infants and provision of informed consent. A single 8-mg, open-label dose was administered orally after consumption of a standard breakfast. Complete milk collection was done for approximately 28 days, and plasma samples were collected for 90 days. Moxidectin concentrations were measured by high-performance liquid chromatography (HPLC) with fluorescence detection, with a validated range of 0.08 to 120 ng/ml. Noncompartmental pharmacokinetic methods were used to find the following results: peak concentration in plasma (Cmax), 87 ± 25 ng/ml; time to Cmax (tmax), 4.18 ± 1.59 h; terminal-phase elimination half-life (t1/2), 832 ± 321 h; total area under the concentration-time curve (AUC), 4,046 ± 1,796 ng·h/ml; apparent oral dose clearance (CL/F), 2.35 ± 1.07 l/h; ratio of CL/F to the terminal-phase disposition rate constant, λz (Vλz/F), 2,526 ± 772 liters; percentage of maternal dose excreted in milk, 0.701 ± 0.299%; absolute amount excreted in milk, 0.056 ± 0.024 mg; relative infant dose, 8.73 ± 3.17% of maternal dose assuming complete absorption; clearance in milk (CLmilk), 0.016 ± 0.009 liter/h. Nine of 12 subjects reported adverse events, all of which were considered treatment emergent but not drug related and were mostly reported during the long outpatient period 8 to 90 days after dose administration. The most frequently reported adverse events were headache and nausea (n = 4), oropharyngeal pain (n = 2), rhinitis, viral pharyngitis, and viral upper respiratory tract infection (n = 2).
INTRODUCTION
Moxidectin is a macrocyclic lactone drug derived from the actinomycete Streptomyces cyanogriseus and is currently being used as a veterinary product for the prevention of canine heartworm disease and for the treatment of internal and external parasites in cattle, sheep, goats, and horses. It is being developed by the World Health Organization (WHO) as a potential macrofilaricidal agent for mass drug administration for the elimination of onchocerciasis (river blindness) in humans caused by the parasitic worm Onchocerca volvulus. Ivermectin (Stromectol; Merck & Co., Inc.) is currently used in mass drug administration programs, but an alternative agent would potentially offer a choice in treatment. Mass drug administration is a process that would call for administration of single doses of medicine, under the supervision of a community health worker, to an entire community at one time.
Knowing the extent of excretion of moxidectin into breast milk and the pharmacokinetics of the drug in lactating women will help the community health worker make appropriate decisions about dosing, hence the need for this study.
Preclinical studies have shown that the absolute bioavailability of moxidectin is variable, ranging from 19% in rats to 90% in dogs (9). After oral administration, moxidectin is quickly absorbed, with the time to peak plasma concentration (tmax) being 3.7 ± 1.5 h in 27 fasting healthy volunteers receiving an 8-mg dose. Administration of moxidectin with food has been shown to increase the mean peak plasma concentration (Cmax) and total area under the concentration-time curve (AUC) by 34% and 39%, respectively (8).
The apparent volume of distribution (Vz/F) of moxidectin is large (2,000 to 3,500 liters), and moxidectin has a low clearance (CL) (2.37 to 3.50 liters/h) and undergoes very slow elimination, with the terminal-phase elimination half-life (t1/2) being 485 to 842 h (4).
A study in lactating dairy sheep showed that after administration of a single oral dose of 200 μg/kg of body weight of moxidectin, 2.1% ± 0.33% of the administered dose was excreted in milk for more than 35 days after dosing (7). The ratio of the total AUC curve for moxidectin in breast milk (AUCmilk) to the AUC was 14.3 ± 1.88, indicating that although the total amount of drug that entered the breast milk was small, moxidectin preferentially entered breast milk compared to results for plasma. Similar results were seen in a study in goats (1), where 5.7% ± 1.0% of an orally administered dose of 0.2 mg/kg of moxidectin was recovered from breast milk, which was collected for 40 days after dosing.
The amount of medication excreted into breast milk in lactating animals and women depends on the characteristics of the medication, including its lipophilicity, ionization, extent of protein binding in plasma, and although not extensively investigated, in transporters (6). Medications with high oil/water distribution coefficients, those that are not highly ionized at the pH of plasma or breast milk, or those which have a low fraction bound to plasma proteins are more likely to be excreted in breast milk than medications that have low oil/water coefficients or are extensively ionized or highly protein bound. Transfer of medications into breast milk is also governed by maternal characteristics, including the number of pregnancies, age, duration of lactation, volume of breast milk secreted, breast-feeding patterns, whether the breast milk is foremilk, the initial milk secreted during lactation, or hindmilk, the final milk secreted during lactation, and maternal size. Although maternal age is not usually investigated as a cofactor in pharmacokinetic (PK) studies of lactating women, there is evidence that breast milk concentrations of dioxins increase with maternal age, perhaps due to increase in the extent of body fat, where dioxins accumulate. Breast milk concentrations of dioxins decrease with increasing numbers of pregnancies (3). Breast milk composition changes from the first milk, colostrum, secreted immediately after birth for approximately 5 to 7 days, which is high in protein (10%) and low in fat (1%). After the first week, the protein levels in breast milk decrease to approximately 1%, and the fat levels increase to about 4% by the first month and then stay relatively constant. Within feeding sessions, breast milk composition may also vary, with foremilk containing less fat than the breast milk at the end of the session and fat content increasing from 1 to 2% to 4 to 6% (6, 11). The extent of protein binding of moxidectin has not been determined due to extensive, nonspecific binding of moxidectin to the apparatus. However, the oil/water distribution coefficient is 58,300, and the drug is largely un-ionized (10). These chemistry characteristics are consistent with the excretion of moxidectin into milk.
This study was designed to determine the PK of moxidectin in healthy lactating women, the extent to which moxidectin is excreted into breast milk as measured by CLmilk, the percentage of maternal dose excreted in breast milk, and the absolute and relative infant doses.
MATERIALS AND METHODS
This was a single-center, open-label study that was conducted at Veeda Clinical Research Ltd. (Plymouth, England). The study protocol, investigator's brochure, and informed-consent form were reviewed and approved by the Ethics Committee of Plymouth, United Kingdom, and the study was conducted in accordance with the International Conference on Harmonisation Guideline for Good Clinical Practice and the ethical principles that have their origins in the Declaration of Helsinki.
Subjects.
The inclusion criteria for the study were healthy women of ages 21 to 45 years, more than 12 weeks postpartum after an uncomplicated delivery, with established breast milk, who had decided to discontinue breast-feeding their infants prior to recruitment into the study and were not planning to breast-feed within 9 months of study drug administration, were willing and able to express breast milk fully, and who had a body mass index (BMI) of 18 to 35 kg/m2. The exclusion criteria were an unwillingness to use a medically acceptable form of contraception for the duration of the study and for 9 months following study completion, the presence of any surgical or medical condition that might interfere with the absorption, distribution, metabolism, or excretion of moxidectin, or evidence of postnatal depression determined using the Edinburgh Postnatal Depression Scale (5).
Twelve women enrolled in and completed the study. Their mean age ± standard deviation (SD) was 30.1 ± 4.8 years. Eleven were white, and one was Asian. Their mean weight and BMI ± SD were 64.0 ± 8.5 kg and 22.8 ± 3.6 kg/m2, respectively. The median (range) time postpartum was 29.6 (21.0 to 100) weeks.
Drug administration.
Four 2-mg moxidectin tablets were administered with 240 ml of room-temperature water at approximately 8 a.m. as open-label, single, oral doses within 5 min of consumption of a standard breakfast.
Safety evaluations.
Safety was evaluated through reported adverse events (AEs), scheduled physical examination findings performed before moxidectin administration and then 14, 30, 60, and 90 days afterward and at the final study evaluation, frequent vital sign and 12-lead electrocardiogram (ECG) measurements, and clinical laboratory test results. Individual data for vital sign measurements, ECG findings, and laboratory test results were analyzed to detect values that met the predetermined criteria for potential clinical importance. Because of the nature of this study, no formal statistical analysis was planned. Evaluation of the data consisted primarily of summary displays (i.e., descriptive statistics and graphs).
Sample collection.
Blood samples of 4 ml (each) were collected in sodium heparin-containing collection tubes before and 2, 4, 6, 8, 12, 24, 48, 72, and 96 h after moxidectin administration and then on days 6, 8, 14, 30, 60, and 90. Plasma was separated by centrifugation, transferred to watertight, labeled polypropylene tubes, stored at −70°C until shipment, and then stored at −80°C until analysis.
Subjects entering this study were asked to express breast milk regularly, which was then collected at predetermined times and saved for the analysis of the quantity of study drug that was excreted. The type of breast pump preferred was determined on an individual basis. Those subjects with their own pumps and sterilizers were permitted to continue using these. Subjects without their own pumps were provided with a breast milk pump by the unit. Subjects were asked to express milk from both breasts completely at each time point and within a 20-min period. The collected milk was added to a bulk bottle that was labeled for that time period. The breast pump, used collection bottles, and any associated equipment were cleaned and resterilized as necessary, following manufacturers' guidelines, and were made ready for the next use. All subjects were expected to complete at least 8 days of milk collection and were asked to continue to day 14. Following successful completion of 14 days of milk expression, the women were further encouraged to continue through to day 30.
During the outpatient period (days 9 to 14 inclusive, followed by optional days 15 to 30), the following activities were continued at home: expressing breast milk using a breast pump every 6 to 8 h, with the next 24-h collection commencing at 8 p.m., which was saved into the next labeled container; recording of the start and stop times of expression on a diary card provided; at each pumping session, milk from both breasts was collected into a labeled container (prelabeled and provided to the woman during the inpatient stay), which was subsequently refrigerated until the following morning. The subject was asked to be available at daily intervals for the study site personnel to collect the milk, which was picked up by a member of the clinical team with the diary card for that day. This was then combined at the clinical site, the volumes for each collection were measured, and two representative samples (2 × 5 ml) were saved and stored in watertight, labeled polypropylene tubes at −70°C until shipment and at −80°C until analysis.
Assay procedure.
Moxidectin concentrations were determined in plasma and breast milk using modifications of a high-performance liquid chromatography method with fluorescence detection that was validated over the range of 0.08 to 120 ng/ml, using a 500-μl sample (2). The minimal quantifiable concentrations of moxidectin in plasma and in breast milk were approximately 0.08 ng/ml.
The interday accuracy (mean bias) and precision (coefficient of variation [CV%]) for moxidectin concentrations in plasma were less than 6.4% and 13.1%, respectively. The mean recovery of moxidectin from plasma samples measured at the four quality control levels was 84.6%. No potential interference was detected from six different lots of plasma.
The interday accuracy (mean bias) and precision (CV%) for moxidectin concentrations in milk were less than −7.7% and 6.9%, respectively. The mean recovery of moxidectin from milk samples measured at the four quality control levels was 82.5%.
Moxidectin was observed to be stable in samples on the bench top for 96 h, through three freeze-thaw cycles, for 2 weeks at +4°C and at −80°C for up to 6 months.
PK evaluation.
Blood samples for PK analyses were collected at the requested times, but the following windows for sample collection were allowed: ±2 min for the samples to be collected 1 h before moxidectin administration; ±5 min for the hour one through four samples; ±10 min for the hour six through 12 samples; and ±30 min for the hour 24 through day eight samples. Thereafter, on day 14, when the sample was collected ±24 h, the samples could have been collected ±1 to 2 days through day 30 and ±1 week up to 90 days and on the final study visit. The actual time of collection was noted and was used in the calculations.
Individual parameters were derived for each subject from the plasma and breast milk concentration versus time using conventional noncompartmental methods, using the WinNonlin software program, version 5.1.1 (Pharsight). The Cmax and tmax of moxidectin were determined directly from the observed plasma concentration data. The terminal-phase disposition rate constant (λz) was estimated by a log-linear regression of the terminal monoexponential portion of the observed plasma concentrations. The t1/2 was calculated as 0.693/λz. The area under the concentration-time curve at time T (AUCT), truncated at the last measurable concentration at time T (CT), was calculated using the trapezoidal rule during the ascending portion of the curve and the log-trapezoidal rule during the descending portion of the curve. The AUC was then estimated as follows: AUC = AUCT + CT/λz. The apparent oral dose clearance (CL/F) was calculated as a ratio of moxidectin dose to AUC. Vλz/F was estimated as the ratio of CL/F to λz.
The midpoint time for milk expression was used as the milk collection time. The AUC was calculated for milk in the same way as for plasma, and the ratio of AUC for moxidectin in breast milk and AUC for moxidectin in plasma was determined. The amount of moxidectin excreted in milk (Xu) was calculated as the sum of the product of individual milk volumes and concentrations. Moxidectin clearance in milk (CLmilk) was calculated as the ratio of Xu to total AUC. The absolute infant dose, which is equal to Xu, was determined assuming 100% bioavailability of the amount excreted. Each relative infant dose was determined as the ratio of the absolute infant dose (normalized to an infant weight of 5 kg) to the maternal dose of 8 mg moxidectin (normalized by each woman's weight), expressed as a percentage.
The PK parameters were summarized as means ± SD. The relationship between maternal weight, BMI, and the length of lactation and CLmilk and Xu were evaluated graphically. Post hoc single linear regression examining the relationship between weight and BMI and CLmilk and Xu was also performed. Women were carefully selected to participate in the study as per the inclusion criteria, particularly with regard to maternal age (21 to 45 years) and an established breast milk supply, ensuring that variability with regard to milk composition and maternal condition were minimized, and therefore the relationship between these characteristics and moxidectin CLmilk or Xu was not evaluated.
RESULTS
Safety evaluations.
All 12 subjects completed the study. No serious AEs were experienced during the study, and no subject withdrew from the study due to an AE. Nine of the subjects reported treatment-emergent AEs (TEAEs), which are defined as events not present prior to the initiation of the treatments or events already present that worsen in either intensity or frequency following exposure to the treatments. The definition is broad so that every clinically relevant change is noted even if it is not related to drug treatment. The most frequently reported TEAEs during the study (≥2 subjects) were mostly experienced during the long outpatient period (>8 days and <90 days). These TEAEs include headache and nausea (4 subjects each), oropharyngeal pain (2 subjects), and rhinitis, viral pharyngitis, and viral upper respiratory tract infection (2 subjects each and all of which were experienced in the outpatient period). All were of mild to moderate intensity, and none were considered to be related to moxidectin by the principal investigator. No clinically significant changes in laboratory test results, vital sign measurement, and ECG findings occurred during the study. The AEs reported by healthy lactating women during this study were similar to those reported previously in clinical pharmacology trials by healthy male volunteers (4, 8).
PK evaluations.
PK sample collection was completed for all 12 enrolled subjects. Complete milk collection was made over approximately 30 days (720 h) for all 12 subjects: from 0 to 504 h (1 subject) and 600 h (1 subject), 696 h (3 subjects), and 720 h (7 subjects) postdose.
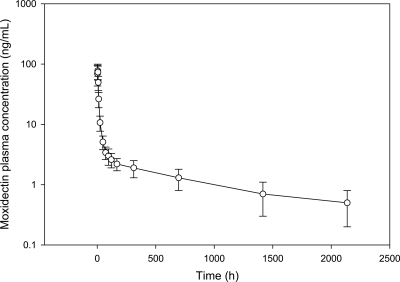
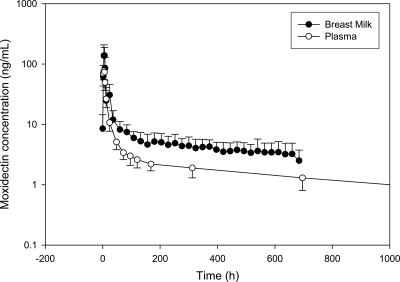
The mean (± standard deviation [SD]) plasma concentration-time profile after administration of a single oral 8-mg dose of moxidectin is presented in Fig. 1, and the mean breast milk and plasma concentration-time profiles after administration of a single oral 8-mg dose of moxidectin are simultaneously presented in Fig. 2.
Fig. 1.
Plasma moxidectin concentration-time profile for healthy lactating women after a single 8-mg dose (mean ± SD; n = 12).
Fig. 2.
Breast milk and plasma moxidectin concentration-time profiles in healthy lactating women after a single 8-mg dose (mean ± SD; n = 12).
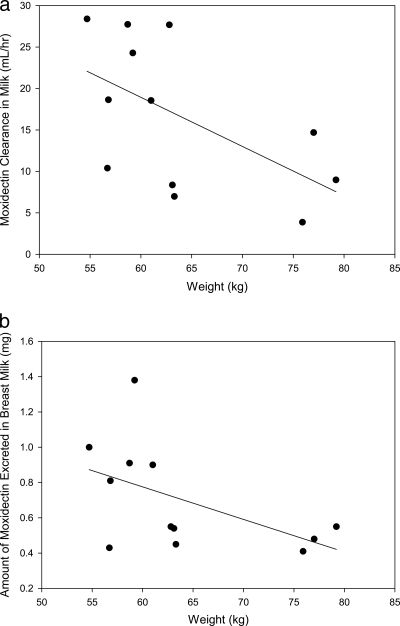
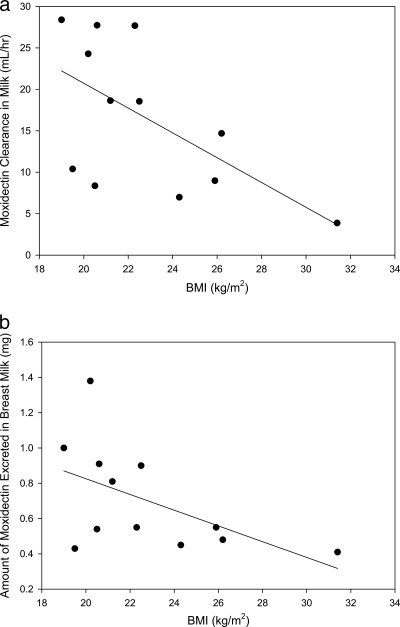
A summary of the PK analysis results is presented in Table 1. Graphic exploration of the relationship of subject weight and BMI with CLmilk and the amount of moxidectin excreted in milk are shown in Fig. 3a and b and 4a and b, respectively. Although the numbers are small, it appears that women who have a lower weight or smaller BMI have increased, although still small, amounts of moxidectin excreted in their breast milk and a higher CLmilk than women with higher weight or larger BMI. Post hoc linear regression analysis showed that weight or BMI did not explain variability in either CLmilk or Xu, with r2 values of 0.27 to 0.36. There appeared to be no relationship between the length of time postpartum that women were dosed and either the amount of moxidectin excreted in their milk or the CLmilk.
Table 1.
Moxidectin plasma and breast milk PK results
| Parametera | Value (mean ± SD)b |
|---|---|
| Cmax (ng/ml) | 87 ± 25 |
| tmax (h) | 4.18 ± 1.59 |
| AUC (ng·h/ml) | 4,046 ± 1,796 |
| λz (h−1) | 0.00093 ± 0.00029 |
| t1/2 (h) | 832 ± 321 |
| CL/F (liters/h) | 2.349 ± 1.067 |
| Vλz/F (liters) | 2,526 ± 772 |
| % dose excreted in milk | 0.701 ± 0.299 |
| Amt excreted in milk (mg) | 0.056 ± 0.024 |
| CLmilk (liters/h) | 0.016 ± 0.009 |
| AUCmilk/AUC | 1.77 ± 0.66 |
| Absolute infant dose (mg) | 0.056 ± 0.024 |
| Relative infant dose (%) | 8.73 ± 3.17 |
AUC, total area under the concentration-time curve; AUCmilk, AUC for moxidectin in breast milk; Cmax, peak plasma concentration; CL/F, apparent oral dose clearance; CLmilk, clearance in milk; t½, terminal phase elimination half-life;, tmax, time to Cmax; Vz/F, apparent volume of distribution; λz, terminal-phase elimination rate constant.
n = 12.
Fig. 3.
(a) Subject weight versus moxidectin clearance in breast milk in healthy women after a single 8-mg dose given with food (n = 12; P = 0.055; y = −0.59x + 54.5; r2 = 0.32). (b) Subject weight versus amount of moxidectin excreted in breast milk in healthy women after a single 8-mg dose given with food (n = 12; P = 0.08; y = −0.018x + 1.88; r2 = 0.27).
Fig. 4.
(a) Subject BMI versus moxidectin clearance in breast milk in healthy lactating women after a single 8-mg dose given with food (n = 12; P = 0.038; y = −1.50x + 50.67; r2 = 0.36). (b) Subject BMI versus amount of moxidectin excreted in breast milk in healthy lactating women after receiving a single 8-mg dose with food (n = 12; P = 0.073; y = −0.045x + 1.72; r2 = 0.287).
DISCUSSION
This study was remarkable in that it represents one of the longest and most complete collections of breast milk in women who were preparing to wean their babies. The long half-life of moxidectin necessitated the development of straightforward study procedures that could be implemented by both the clinical staff and the patients.
The plasma PK parameters observed for the healthy lactating women enrolled in this study were similar to those observed for healthy young men who received moxidectin with food, where the following mean ± SD PK parameters were observed: Cmax was 79.1 ± 26.3 ng/ml, tmax was 5.3 ± 2.1 h, t1/2 was 700 ± 307 h, AUC was 4,885 ± 1,483 ng·h/ml, CL/F was 1.78 ± 0.54 liter/h, and Vλz/F was 1,708 ± 724 liters (8). Therefore, administration of similar doses would be expected to result in similar exposures in the two groups of subjects.
The milk excretion parameters observed for the women enrolled in this study were qualitatively similar to those observed in lactating sheep and goats. The women received doses similar to those for the animals, approximately 0.125 mg/kg, compared to 0.2 mg/kg for the animals. After 35 or 40 days of milk collection, 2 to 6% of the administered dose was recovered from the milk in the animals. The women in this study were weaning their babies, and so the volume of milk would be less than that collected from the animals, which combined with the shorter sampling time could account for the difference in amount of drug collected.
The mean ratio of drug in milk to that in plasma (M/P ratio) ± SD was 1.77 ± 0.66, confirming that moxidectin is more concentrated in breast milk than in plasma. The mean (± SD) absolute infant dose, assuming that the infants would consume the breast milk collected during the study, was 0.056 ± 0.024 mg, and the mean (± SD) relative infant dose compared to the maternal dose was 8.73% ± 3.17%. Some authors have suggested that a relative infant dose less than 10% would suggest little harm to a healthy full-term infant, assuming that the infant would have 33% of the maternal drug CL and similar bioavailability, which would in turn predict that the infant would have ∼30% of the maternal exposure (6). PK information from young children receiving moxidectin is necessary to increase understanding of the implications of the relative infant dose observed in this study.
All women who participated in the study had been lactating for at least 5 months. Therefore, the composition of the breast milk secreted would have been relatively consistent among the women and would not have been expected to account for the variability in either the CLmilk or the amount of moxidectin excreted in milk. Although the numbers are small, it appears that women with higher weight and BMI may have lower CLmilk than women with lower weight and lower BMI. A potential explanation for this observation would be that the lipophilicity of moxidectin would lead to an increased portion of the drug being distributed to adipose tissue and consequently being out of the blood and so not available to be excreted into breast milk. More work is needed to better understand this observation.
Moxidectin was safe and well tolerated by lactating women when administered as a single oral dose of 8 mg. The AEs reported by the study subjects were similar to those previously reported in trials of healthy male volunteers.
ACKNOWLEDGMENTS
This study was sponsored by Wyeth, which was acquired by Pfizer Inc. in October 2009. Veeda Clinical Research conducted the study, and the University of Iowa provided bioanalytical analysis, both of which were funded by Wyeth, now Pfizer Inc. Editorial support was provided by Raymond Magee at Complete Medical Communications and funded by Pfizer Inc.
We recognize the significant contributions of the Veeda Clinical Research staff members, the study subjects, and the bioanalytical laboratory staff at the College of Pharmacy, University of Iowa.
Virginia Parks and Stephan Chalon are former employees of Pfizer Inc. and are currently employed by Shire AG and the Roche Institute for Research and Translational Medicine, respectively.
Footnotes
Published ahead of print on 6 September 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Carceles C. M., et al. 2001. Milk kinetics of moxidectin and doramectin in goats. Res. Vet. Sci. 70:227–231 [DOI] [PubMed] [Google Scholar]
- 2. Chen Y. C., Hung Y. P., Fleckenstein L. 2002. Liquid chromatographic assay of moxidectin in human plasma for application to pharmacokinetic studies. J. Pharm. Biomed. Anal. 29:917–926 [DOI] [PubMed] [Google Scholar]
- 3. Clewell R. A., Gearhart J. M. 2002. Pharmacokinetics of toxic chemicals in breast milk: use of PBPK models to predict infant exposure. Environ. Health Perspect. 110:A333–A337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cotreau M. M., et al. 2003. The antiparasitic moxidectin: safety, tolerability, and pharmacokinetics in humans. J. Clin. Pharmacol. 43:1108–1115 [DOI] [PubMed] [Google Scholar]
- 5. Cox J. L., Holden J. M., Sagovsky R. 1987. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 150:782–786 [DOI] [PubMed] [Google Scholar]
- 6. Ilett K. F., Kristensen J. H. 2005. Drug use and breastfeeding. Expert Opin. Drug Saf. 4:745–768 [DOI] [PubMed] [Google Scholar]
- 7. Imperiale F., Lifschitz A., Sallovitz J., Virkel G., Lanusse C. 2004. Comparative depletion of ivermectin and moxidectin milk residues in dairy sheep after oral and subcutaneous administration. J. Dairy. Res. 71:427–433 [DOI] [PubMed] [Google Scholar]
- 8. Korth-Bradley J. M., et al. 2010. The effect of a high-fat breakfast on the pharmacokinetics of moxidectin in healthy male subjects. J. Clin. Pharmacol. 50:1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lallemand E., Lespine A., Alvinerie M., Bousquet-Melou A., Toutain P. L. 2007. Estimation of absolute oral bioavailability of moxidectin in dogs using a semi-simultaneous method: influence of lipid co-administration. J. Vet. Pharmacol. Ther. 30:375–380 [DOI] [PubMed] [Google Scholar]
- 10. Rock D. W., DeLay R. L., Gliddon M. J. 2002. Chemistry, pharmacology and safety: moxidectin, p. 77–96 In Vercruysse J., Rew R. S. (ed.), Macrocyclic lactones in antiparasitic therapy. CABI Publishing, New York, NY [Google Scholar]
- 11. U.S. Food and Drug Administration 2005. Guidance for industry: clinical lactation studies—study design, data analysis, and recommendations for labeling. U.S. Food and Drug Administration, Rockville, MD: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM072097.pdf [Google Scholar]