Abstract
Surgical site infections are common, so effective antibiotic concentrations at the sites of infection, i.e., in the interstitial fluid (ISF), are required. The aim of this study was to evaluate contemporary perioperative prophylactic dosing of cefazolin by determining plasma and subcutaneous ISF concentrations in patients undergoing elective/semielective abdominal aortic aneurysm (AAA) open repair surgery. This was a prospective pharmacokinetic study in a tertiary referral hospital. Cefazolin (2 g) was administered as a 3-min slow bolus 30 min prior to incision in 12 enrolled patients undergoing elective/semielective AAA open repair surgery. Serial blood, urine, and ISF (via microdialysis) samples were collected and analyzed using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. Cardiac output was determined using pulse waveform contours with Vigileo. The recruited patients had a median (interquartile range) age of 70 (66 to 76) years and weight of 88 (81 to 95) kg. The median (interquartile range) terminal volume of distribution was 0.14 (0.11 to 0.15) liter/kg, total clearance was 0.05 (0.03 to 0.06) liter/h, and minimum observed unbound concentration was 5.7 (5.4 to 8.1) mg/liter. The penetration of unbound drug from plasma to ISF was 85% (78% to 106%). We found correlations present, albeit weak, between cefazolin clearance and cardiac output (r2 = 0.11) and urinary creatinine clearance (r2 = 0.12). In conclusion, we found that a single 2-g dose of cefazolin administered 30 min before incision provides plasma and ISF concentrations in excess of the likely MICs for susceptible pathogens in patients undergoing AAA open repair surgery.
INTRODUCTION
Clean and clean-contaminated surgeries are reported to have infection rates of 1% and 11%, respectively (1). This is the second most common type of adverse event occurring in hospitalized patients (6). The impact of surgical site infection (SSI) is dependent upon the site and severity of the disease and the development of further complications. These complications include bronchopneumonia (8%), renal insufficiency (5%), and wound infection (3%) as well as myocardial infarction (3%), stroke (2%), and pulmonary embolism (1%) (14). In addition to increased morbidity, there are clinical, logistic, and financial implications, such as antibiotic selection pressure with evolution of resistance patterns and bed management issues. Prevention of SSI may therefore represent significant advantages to the patient and the health care system.
Surgical prophylaxis mandates a comprehensive understanding of pharmacokinetic properties of the selected agent such that operative perturbations in physiology do not render the antibiotic redundant. There are a number of confounding issues which should be considered with abdominal aortic aneurysm (AAA) open repair surgery. First, significant hemorrhage may necessitate volume expansion, transfusion, and vasopressor and/or inotropic support, thereby increasing the clearance of hydrophilic compounds, including cephalosporin antibiotics (16). Second, the population is aged (mostly 65 to 75 years old), with obesity, diabetes, and hypertension, and concomitant nephropathy and vasculopathy (3, 11, 14) are not uncommon. The ultimate effect is determined by the balance of a complex interrelationship of altered physiology and pharmacokinetics, which has been described in part (16, 17). Finally, the surgery entails surgically clamping major vessels and the temporary reduction of renal blood flow and perfusion of other tissues.
The Australian Therapeutic Guidelines recommends the cephalosporin cefazolin for vascular surgery antibiotic prophylaxis (2). It provides excellent Gram-positive and modest Gram-negative coverage, but consistent with its class, it requires a maximal duration of exposure of the unbound or free fraction at a level higher than the MIC (fT>MIC) for the suspected pathogens. This is otherwise known as time-dependent killing.
Tissue concentrations of antibiotics are best determined by a technique known as microdialysis (4, 7, 16). The relationship between suboptimal perioperative antibiotic concentration and surgical site infections has been established for aminoglycosides (21). At this time, there are no studies that describe tissue concentrations of antibiotics during AAA open repair surgery or the effect of patient physiology on pharmacokinetics in this setting.
The main aim of this study was to describe the pharmacokinetics of cefazolin administered intravenously to patients undergoing elective and semielective AAA open repair surgery, including the kinetics of the drug in interstitial fluid (ISF). In addition, we aimed to measure the effects of cardiac output and urinary creatinine clearance (CLCR) on cefazolin clearance in these patients.
MATERIALS AND METHODS
This prospective observational pharmacokinetic study was conducted exactly in accordance with a recently published protocol paper (9). It was performed in the operating theaters and intensive care unit of a 918-bed teaching hospital in Australia. The study was approved by the Human Research and Ethics Committee of the Royal Brisbane and Women's Hospital (2007/187) and the Medical Research Ethics Committee of The University of Queensland (2008002032).
Patient selection and data collection.
Patients scheduled to undergo elective and semielective AAA open repair surgery with cefazolin as surgical prophylaxis were eligible for enrolment. Informed consent was obtained from the patient or the patient's legally authorized representative.
Cefazolin administration.
All participants received 2 g of cefazolin (Cephazolin; DBL, Sydney, Australia) in 10 ml 0.9% sodium chloride as a 3-min slow-bolus intravenous injection 30 min prior to the first incision of surgery.
Sample collection.
Pharmacokinetic sampling occurred in the immediate perioperative period. Blood was sampled at 0 min (immediately prior to antibiotic administration) and then at 3, 10, 30, 60, 90, 180, 300, and 480 min or until at least the conclusion of surgery. Specimens were centrifuged at 3,000 rpm for 10 min and then frozen at −80°C for subsequent analysis. Urine was collected at 2-hourly intervals during the immediate perioperative period and concluded with an 8-h postoperative urinary creatinine clearance sample. Urine was stored at −80°C until assay.
In vivo microdialysis.
Microdialysis was the technique chosen to measure the free (or unbound) antibiotic concentration in subcutaneous tissue. Given that the free antibiotic concentration determines the antibacterial effect (15), this information is particularly useful. The principles and details of microdialysis have been described previously (8). Briefly, microdialysis is based on the sampling of analytes from the extracellular space by diffusion across a semipermeable membrane. In vivo, this process is accomplished by constantly perfusing the microdialysis probe with a physiological solution at a low flow rate. Once the probe is implanted in tissue, analytes diffuse across the membrane from the extracellular fluid into the perfusate and may be sampled and analyzed. In this study, a microdialysis probe (CMA 60; Microdialysis AB, Stockholm, Sweden) with a molecular mass cutoff of 20 kDa, an outer diameter of 0.6 mm, and a membrane length of 30 mm was placed aseptically in the subcutaneous tissue of the upper arm of each patient. The probe was perfused with cephalothin (10 mg/liter; internal standard) in 0.9% sodium chloride at a flow rate of 1.6 μl/min (7). After administration of cefazolin, microdialysis samples were collected at 30-min intervals until the conclusion of blood sampling. Samples were stored at −80°C for assay. The recovery of cefazolin in the microdialysate solution was interpolated from the loss of internal standard (cephalothin) across the microdialysis membrane into tissue (20), as follows: % cefazolin recovery = 100 × (Cin − mean Cout/Cin), where Cin is cephalothin at 10 mg/liter (perfusate) and Cout is the measured cephalothin concentration in the microdialysate.
Creatinine clearance measurement.
Samples for determination of urinary creatinine clearance were collected over an 8-hour period via an indwelling urinary catheter. This was performed to determine correlations between renal function and cefazolin clearance. CLCR was calculated using the formula CLCR (ml/min) = (urine creatinine concentration [μmol/liter] × volume [ml])/(plasma creatinine concentration [μmol/liter] × 480 [min]).
Cardiac output measurement.
Cardiac output was measured using pulse waveform contour analysis with FloTrac/Vigileo (Edwards Lifesciences, Irvine, CA).
Sample analysis.
Blood samples were centrifuged at 3,000 rpm for 10 min, and plasmas were stored at −80°C until assay. The plasma, urine, and microdialysis samples were analyzed utilizing validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) methodology at the Therapeutics Research Unit, The University of Queensland.
Plasma samples were prepared by addition of an internal standard (150 μl of 10 μg/ml cloxacillin in acetonitrile) to 50 μl of plasma. After centrifugation, 2 μl of supernatant was injected into the LC-MS/MS instrument to measure total cefazolin levels. The unbound plasma levels were determined by ultrafiltration of 200 μl of plasma (Amicon Ultra [0.5 ml, 10 kDa]) at room temperature to produce <100 μl of filtrate, of which 50 μl was then assayed as described for total plasma.
Chromatographic analysis used a Phenomenex Luna C18(2) column (50 mm × 2 mm × 5 μm) with mobile phases A (0.1% formic acid) and B (95:5:0.1 acetonitrile-water-formic acid). The mobile phase gradient increased (at 300 μl/min) from 0% to 90% phase B over 2 min; 90% phase B was maintained for 1.5 min before reverting to 0% phase B for a total run time of 5 min.
Microdialysate samples were assayed for cefazolin and cephalothin by LC-MS/MS. Five microliters of sample was injected directly using a similar chromatographic method to that for plasma (no internal standard; 10 mM ammonium acetate substituted for the formic acid in the mobile phase). Urine samples were also assayed for cefazolin by this method, after appropriate dilution with water.
The antibiotics were detected using an API2000 tandem mass spectrometer (AB Sciex). The mass spectrometer ion masses were 455 → 323 (cefazolin), 437 → 278 (cloxacillin), and 397 → 152 (cephalothin).
All assay methods were validated according to best practice guidelines, with precision and accuracy within 15% of the nominal value. The reliability of assay batches was monitored at three levels with quality control samples.
Pharmacokinetic analysis.
The pharmacokinetic values were calculated using noncompartmental methods. The area under the concentration-time curve from 0 h to the last measurement at the completion of surgery (AUC0-last) was calculated using the linear trapezoidal rule. The area under the moment curve from 0 to 8 h (AUMC0–8) was calculated using the linear trapezoidal rule. The apparent terminal elimination rate constant (λz) was determined from log-linear least-squares regression analysis of concentrations from the final 3 concentration-time values for each subject. The area under the concentration-time curve from 0 h to infinity (AUC0-∞) was calculated using the AUC0-last, the concentration at the last time point (Clast), and λz. The area under the moment curve from 0 h to ∞ (AUMC0-∞) was calculated using the AUMC0-last, Clast, and λz. The mean residence time (MRT) was calculated as AUMC0-∞/AUC0-∞. Total body clearance (CLtotal) was calculated as dose/AUC0-∞. The maximum concentration for the dosing period (Cmax) and the minimum concentration for the dosing period (Cmin) were observed values; the apparent volume of distribution during terminal phase (Vz) was calculated as CL/λz, and the half-life (t1/2) was calculated as ln(2)/λz.
Statistical analysis.
Summary data are presented as medians with interquartile ranges (IQR), and statistical significance was defined for P values of <0.05. Correlations between factors were determined using linear regression, with r2 values calculated to determine goodness of fit. Statistical analyses were performed using GraphPad Prism, version 4.03 (GraphPad, San Diego, CA), and Microsoft Excel (Microsoft Office 2007; Microsoft, Redmond, WA).
RESULTS
Clinical data.
Twelve patients were enrolled in the study, and there were no microdialysis catheter-related complications or associated morbidity. The clinical and demographic characteristics of the patients are described in Table 1. The median cross-clamp time of 54 min (IQR, 48 to 66 min) was not dependent upon the overall duration of surgery, which had an IQR of 2.6 to 3.2 h. Pharmacokinetic data are presented in Table 2. Based on the AUC ratios, unbound cefazolin concentrations in the plasma closely mirrored unbound concentrations found within the tissues. The median percent protein binding of cefazolin was 87% (interquartile range, 74 to 90%). The median plasma albumin concentration was 34 g/liter (interquartile range, 29 to 38 g/liter). The median volume of intravenous fluids administered perioperatively was 3,180 ml (interquartile range, 2,589 to 4,036 ml), which correlated with urinary creatinine clearance (linear regression r2 = 0.37).
Table 1.
Patient demographicsa
| Patient no. | Age (yr) | Wt (kg) | Ht (cm) | Serum creatinine concn (μmol/liter) | 8-h urinary CLCR (ml/min) | Cardiac output (liters/h) | Duration of surgery (h) | Duration of aortic clamping (h) |
|---|---|---|---|---|---|---|---|---|
| 1 | 81 | 88 | 178 | 137 | 38 | 4.8 | 4.6 | 2.5 |
| 2 | 65 | 87 | 176 | 82 | 91 | 5.6 | 2.5 | 0.8 |
| 3 | 77 | 108 | 183 | 86 | 93 | 5.4 | 3.0 | 1.0 |
| 4 | 69 | 81 | 173 | 114 | 61 | 4.6 | 2.0 | 0.5 |
| 5 | 59 | 128 | 181 | 83 | 125 | 5.6 | 3.9 | 1.0 |
| 6 | 66 | 80 | 180 | 68 | 129 | 5.9 | 2.6 | 0.8 |
| 7 | 80 | 92 | 180 | 102 | 37 | 1.8 | 0.8 | |
| 8 | 76 | 80 | 178 | 72 | 68 | 3.0 | 1.0 | |
| 9 | 62 | 80 | 180 | 90 | 77 | 6.4 | 3.6 | 0.5 |
| 10 | 76 | 87 | 181 | 91 | 236 | 5.0 | 3.1 | 1.3 |
| 11 | 70 | 93 | 170 | 91 | 149 | 5.4 | 3.1 | 2.1 |
| 12 | 66 | 100 | 175 | 75 | 67 | 5.9 | 2.6 | 0.7 |
| Median | 70 | 88 | 180 | 88 | 98 | 5.5 | 3.0 | 0.9 |
| 25th percentile | 66 | 81 | 176 | 80 | 76 | 5.1 | 2.6 | 0.8 |
| 75th percentile | 76 | 95 | 180 | 94 | 141 | 5.8 | 3.2 | 1.1 |
All patients were male.
Table 2.
Pharmacokinetic parameters of cefazolin in plasma (total and unbound) and ISF of subcutaneous adipose tissue
| Pharmacokinetic parametera | Median value (IQR) |
||
|---|---|---|---|
| Total plasma | Unbound plasma | Unbound ISF | |
| Cmax (mg/liter) | 404 (289–458) | 25.7 (21.6–27.6) | 23.7 (17.2–27.1) |
| Tmax (h) | 0.08 (0.08–0.09) | 0.08 (0.08–0.09) | 2.0 (1.5–3.0) |
| Cmin (mg/liter) | 35.7 (30.4–54.3) | 5.7 (5.4–8.1) | 6.7 (6.0–6.9) |
| t1/2 (h) | 2.7 (2.4–4.1) | ||
| AUMC0-last (mg-h/liter) | 53,486 (39,116–72,613) | 11,444 (6,650–16,165) | 8,675 (7,530–11,942) |
| AUMC0-∞ (mg-h/liter) | 178,899 (103,300–392,810) | 535,767 (304,118–641,091) | 1,484,890 (485,872–2,255,884) |
| AUC0-last (mg-h/liter) | 452 (420–542) | 77.6 (59.0–82.4) | 65.6 (51.7–83.5) |
| AUC0-∞ (mg-h/liter) | 670 (518–1,113) | 382 (295–420) | 2,044 (994–3,857) |
| MRT (h) | 259 (210–347) | 1,402 (1,030–1,525) | 147 (125–178) |
| kel (h) | 0.26 (0.17–0.28) | 0.04 (0.04–0.06) | 0.22 (0.18–0.32) |
| CLtotal (liters/h) | 3.01 (1.73–3.94) | ||
| Vz (liters) | 11.6 (10.2–14.1) | 119.3 (115.2–123.3) | |
| Vz (liters/kg) | 0.14 (0.11–0.15) | 1.31 (1.25–1.40) | |
| fAUCISF tissue /AUCplasmab | 0.15 (0.12–0.19) | ||
| fAUCISF tissue /fAUCplasmab | 0.85 (0.78–1.06) | ||
Cmax, observed maximum concentration; Tmax, time that Cmax was observed; Cmin, observed minimum concentration; t1/2, elimination half-life; AUMC0-last, area under the moment curve from 0 h to the last measurement at the completion of surgery; AUMC0-∞, area under the moment curve from 0 h to infinity; AUC0-last, area under the concentration-time curve from 0 h to the last measurement at the completion of surgery; AUC0-∞, area under the concentration-time curve from 0 h to infinity; MRT, mean residence time; kel, elimination rate constant; CLtotal, total clearance; Vz, apparent volume of distribution during terminal phase; fAUCISF tissue, unbound AUC in interstitial fluid of subcutaneous tissue; AUCplasma, AUC in plasma, obtained using total concentrations; fAUCplasma, AUC in plasma, obtained using unbound concentrations.
Calculated using AUC0-last.
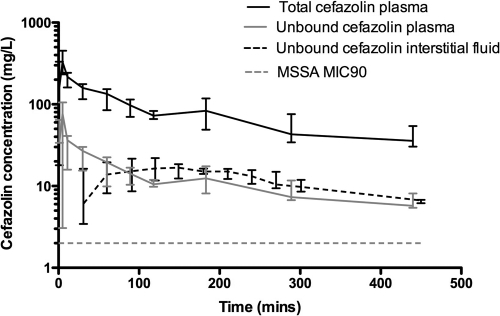
The median perioperative concentration-time profile for cefazolin fractions during abdominal aortic aneurysm repair surgery when cefazolin was administered 30 min prior to incision is shown in Fig. 1. The data show that the MIC for methicillin-sensitive Staphylococcus aureus (MSSA) at our institution, 2 mg/liter, was achieved within the ISF at 30 min, and the peak of 23.7 mg/liter was relatively delayed, at 2 h. Furthermore, unbound cefazolin concentrations exceed the MIC in both blood and ISF for the duration of the operation.
Fig. 1.
Median perioperative concentration-time profile for cefazolin during abdominal aortic aneurysm repair surgery. Total cefazolin plasma concentrations (solid black line), unbound cefazolin plasma concentrations (solid gray line), and unbound interstitial space fluid concentrations (dashed black line) are shown for cefazolin administered 30 min prior to incision. The dotted gray line represents the MIC90 (2 mg/liter) for methicillin-susceptible Staphylococcus aureus.
The median 8-h creatinine clearance of 98 ml/min (IQR, 76 to 141 ml/min) is within normal limits and was not dependent upon the clamp time.
The relationships between systemic cefazolin clearance and cardiac output and creatinine clearance are shown in Fig. 2 and 3. These data show that there is higher cefazolin clearance with higher cardiac output (linear regression r2 = 0.11) and higher urinary creatinine clearance (linear regression r2 = 0.12).
Fig. 2.
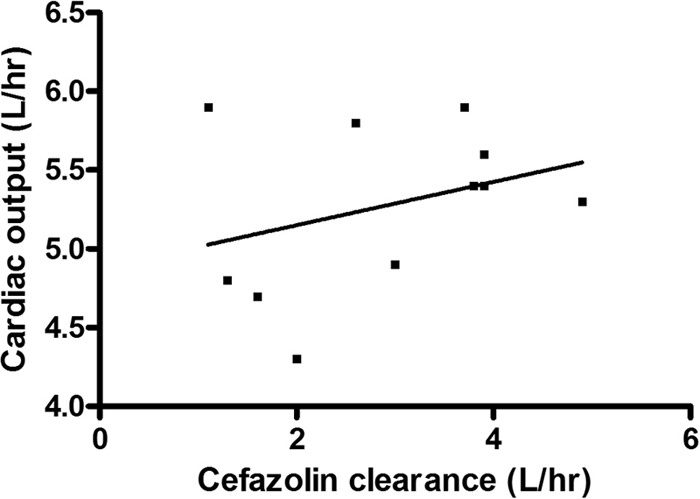
Relationship between cardiac output and systemic cefazolin clearance. The unbroken line represents the linear line of regression (r2 = 0.11).
Fig. 3.
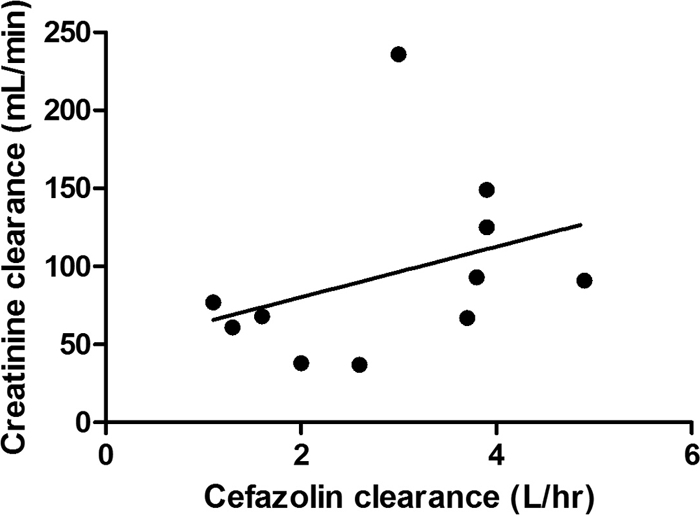
Relationship between urinary creatinine clearance and systemic cefazolin clearance. The unbroken line represents the linear line of regression (r2 = 0.12).
DISCUSSION
This is the first study to use microdialysis to investigate ISF concentrations of cefazolin while simultaneously measuring plasma pharmacokinetics following a single bolus of 2 g as prophylaxis during AAA open repair surgery.
We have demonstrated that a 2-g dose administered 30 min prior to surgical incision will ensure penetration of the ISF of subcutaneous tissue to levels in excess of the 2-mg/liter MIC90 for MSSA (10). In addition to this, our data show that the levels are maintained for over 7 h, which is well in excess of the median duration of the surgery. This nearly complete rapid penetration of ISF by unbound cefazolin is surprising given the peripheral vascular disease (PVD) common to these patients. However, we are unable to compare the ISF time to maximum (Tmax) and concentration maximum (Cmax) of our population with those of a non-PVD population of patients. The sustained ISF level may reflect the reduced distribution from the tissues back to plasma, which is not unexpected due to the high concentrations in plasma resulting from high protein binding as well as the reduced vascular perfusion in the described AAA population.
The surgical conduct of AAA repair induces some interesting physiological compensatory measures which impact pharmacokinetics. ISF antibiotic levels were detected prior to the incision but may not have peaked prior to and/or at the time of securing of the aneurysm. This is significant, as this phase of the procedure can be associated with major hemorrhage, which may then require large volumes of intravenous fluids, with the former resulting in loss of antibiotic from the circulation and the latter resulting in dilution of an already reduced concentration. The application of cross clamps impairs supply to the dependent tissues, and perfusion is reliant on collateral blood flow.
The impact of application of the infrarenal cross clamp on renal function may fluctuate with the resultant change in cardiac output. Gross systemic hypertension or hypotension can occur, and at a regional level, the clamp can reflect higher pressures to the kidneys. The subsequent impact of cross-clamp application on renal function as defined by the 8-h creatinine clearance is quite unpredictable. However, it is clear that both increased cardiac output and increased creatinine clearance are associated with increased cefazolin clearance. We note that the correlation between creatinine clearance and cefazolin clearance is weak and postulate that this is due to the narrow range of creatinine clearances observed in this study. Notably, the results of our study show a greater-than-expected cefazolin clearance in the elderly population and are similar to those for younger morbidly obese patients, who would be expected to have a significantly higher clearance than the patients described in the present study (19). The reason for this is unclear, although it could reflect the high volume of concurrent fluid administration (median of >2.5 liters per patient), which was also found to be correlated with increasing creatinine clearance in these patients.
Our data are unique in this respect, as this study represents the first investigation to correlate drug clearance with cardiac output and creatinine clearance in the perioperative period. Although similar findings have been reported for critically ill septic patients, the correlation with cardiac output suggests an important consideration in dosing renally eliminated antimicrobials in such a setting (12, 13). In this respect, augmented renal perfusion secondary to hyperdynamic circulation may promote increased drug elimination (through greater solute delivery and augmented glomerular filtration) and may help to explain the increased clearances observed (18). In our cohort, drug concentrations in ISF and plasma were sufficient throughout the operative period, although similar pharmacokinetic studies should be planned for settings where both cardiac output and creatinine clearance are typically elevated, such as emergent surgery and trauma (5).
Limitations of the study include the small sample size and the fact that the sample did not include the morbidly and supermorbidly obese, but given the not insignificant difficulties associated with this patient group, it is worthy of a separate study (19).
Conclusions.
A single 2-g dose of cefazolin administered 30 min prior to surgical incision provides plasma and ISF concentrations in excess of the likely MICs for susceptible pathogens in patients undergoing AAA open repair surgery. Further studies like this are required for other surgeries to confirm the appropriateness of standard approaches to prophylactic antimicrobial dosing in the perioperative setting.
ACKNOWLEDGMENTS
We thank the nursing and medical staff of the operating theaters and intensive care units at the Royal Brisbane and Women's Hospital for their assistance with this study.
Funding for this project was received from the Royal Brisbane and Women's Hospital Research Foundation and the Australian and New Zealand College of Anaesthetists (ANZCA 09/032). We acknowledge funding of the Burns Trauma and Critical Care Research Centre by the National Health and Medical Research Council of Australia (project grant 519702). Jason A. Roberts was funded by a fellowship from the Australian National Health and Medical Research Council of Australia (Australian-based health professional research fellowship 569917).
Footnotes
Published ahead of print on 22 August 2011.
REFERENCES
- 1. Anonymous. 2004. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 through June 2004. Am. J. Infect. Control 32:470–485 [DOI] [PubMed] [Google Scholar]
- 2. Antibiotic Expert Group 2010. Therapeutic guidelines—antibiotic.Version 14. Therapeutic Guidelines Limited, Melbourne, Australia [Google Scholar]
- 3. Barba A., Estallo L., Rodriguez L., Baquer M., Vega de Ceniga M. 2005. Detection of abdominal aortic aneurysm in patients with peripheral artery disease. Eur. J. Vasc. Endovasc. Surg. 30:504–508 [DOI] [PubMed] [Google Scholar]
- 4. Barbour A., et al. 2009. Soft tissue penetration of cefuroxime determined by clinical microdialysis in morbidly obese patients undergoing abdominal surgery. Int. J. Antimicrob. Agents 34:231–235 [DOI] [PubMed] [Google Scholar]
- 5. Brown R., et al. 1980. Renal function in critically ill postoperative patients: sequential assessment of creatinine osmolar and free water clearance. Crit. Care Med. 8:68–72 [DOI] [PubMed] [Google Scholar]
- 6. Burke J. P. 2003. Infection control—a problem for patient safety. N. Engl. J. Med. 348:651–656 [DOI] [PubMed] [Google Scholar]
- 7. de Lange E. C. M., de Boer A. G., Breimer D. D. 2000. Methodological issues for microdialysis sampling for pharmacokinetic studies. Adv. Drug Deliv. Rev. 45:125–148 [DOI] [PubMed] [Google Scholar]
- 8. de la Pena A., Liu P., Derendorf H. 2000. Microdialysis in peripheral tissues. Adv. Drug Deliv. Rev. 45:189–216 [DOI] [PubMed] [Google Scholar]
- 9. Douglas A., et al. 2011. The pharmacokinetics of cefazolin in patients undergoing elective & semi-elective abdominal aortic aneurysm (AAA) open repair surgery. BMC Anesthesiol. 11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. European Committee on Antimicrobial Susceptibility Testing 2010. MIC distributions. www.eucast.org. Accessed 17 July 2010.
- 11. Janmahasatian S., et al. 2005. B: quantification of lean bodyweight. Clin. Pharmacokinet. 44:1051–1065 [DOI] [PubMed] [Google Scholar]
- 12. Lipman J., Wallis S. C., Rickard C. M., Fraenkel D. 2001. Low cefpirome levels during twice daily dosing in critically ill septic patients: pharmacokinetic modelling calls for more frequent dosing. Intensive Care Med. 27:363–370 [DOI] [PubMed] [Google Scholar]
- 13. Lipman J., Wallis S. C., Rickard C. 1999. Low plasma cefepime levels in critically ill septic patients: pharmacokinetic modeling indicates improved troughs with revised dosing. Antimicrob. Agents Chemother. 43:2559–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mohammdzade M. A., Akbar M. H., Mohammdzade A. 2007. Complications of elective abdominal aortic aneurysm surgery. Acta Med. Iran. 45:116–120 [Google Scholar]
- 15. Mouton J. W., et al. 2008. Tissue concentrations: do we ever learn? J. Antimicrob. Chemother. 61:235–237 [DOI] [PubMed] [Google Scholar]
- 16. Roberts J. A., Lipman J. 2009. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 37:840–851 [DOI] [PubMed] [Google Scholar]
- 17. Udy A., et al. 2010. Augmented creatinine clearance in traumatic brain injury. Anesth. Analg. 111:1505–1510 [DOI] [PubMed] [Google Scholar]
- 18. Udy A. A., Roberts J. A., Boots R. J., Paterson D. L., Lipman J. 2010. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin. Pharmacokinet. 49:1–16 [DOI] [PubMed] [Google Scholar]
- 19. van Kralingen S., et al. 2011. Pharmacokinetics and protein binding of cefazolin in morbidly obese patients. Eur. J. Clin. Pharmacol. 67:985–992 [DOI] [PubMed] [Google Scholar]
- 20. Wang Y., Wong S. L., Sawchuk R. J. 1993. Microdialysis calibration using retrodialysis and zero-net flux: application to a study of the distribution of zidovudine to rabbit cerebrospinal fluid and thalamus. Pharm. Res. 10:1411–1419 [DOI] [PubMed] [Google Scholar]
- 21. Zelenitsky S. A., Ariano R. E., Harding G. K., Silverman R. E. 2002. Antibiotic pharmacodynamics in surgical prophylaxis: an association between intraoperative antibiotic concentrations and efficacy. Antimicrob. Agents Chemother. 46:3026–3030 [DOI] [PMC free article] [PubMed] [Google Scholar]