Abstract
CTX-M-15 now appears to be the dominant extended-spectrum β-lactamase worldwide, and a number of different factors may contribute to this success. These include associations between blaCTX-M-15 and particular plasmids (IncF) and/or strains, such as Escherichia coli ST131, as well as the genetic contexts in which this gene is found. We previously identified blaCTX-M-15 as the dominant ESBL gene in the western Sydney area, Australia, and found that it was carried mainly on IncF or IncI1 plasmids. Here, we have mapped the multiresistance regions of the 11 conjugative plasmids with one or more IncF replicons obtained from that survey and conducted a limited comparison of plasmid backbones. Two plasmids with only an IncFII replicon appear to be very similar to the published plasmids pC15-1a and pEK516. The remaining nine plasmids, with multiple IncF replicons, have multiresistance regions related to those of pC15-1a and pEK516, but eight contain additional modules previously found in resistance plasmids from different geographic locations that carry a variety of different resistance genes. Differences between the multiresistance regions are largely due to IS26-mediated deletions, insertions, and/or rearrangements, which can explain the observed variable associations between blaCTX-M-15 and certain other resistance genes. We found no evidence of independent movement of blaCTX-M-15 or of a large multiresistance region between different plasmid backbones. Instead, homologous recombination between common components, such as IS26 and Tn2, appeared to be more important in creating new multiresistance regions, and this may be coupled with recombination in plasmid backbones to reassort multiple IncF replicons as well as components of multiresistance regions.
INTRODUCTION
The CTX-M family of extended-spectrum β-lactamases (ESBLs) has recently become the dominant ESBL type in Enterobacteriaceae worldwide (5, 7). Among these, CTX-M-15, belonging to the CTX-M-1 group, appears to be the most widespread, particularly in Escherichia coli, and is associated with both hospital and community-acquired infections. The successful spread of a particular antibiotic resistance gene like blaCTX-M-15 may be influenced by a number of factors, including the mobile genetic element responsible for its capture/mobilization, the plasmids on which it is found, and clonal spread of strains. The international spread of blaCTX-M-15 is known to be partially linked to the E. coli O25 sequence type 131 (ST131) clonal group (10, 24, 32, 35), but blaCTX-M-15 is also found in other clonal groups, e.g., ST405 (10).
blaCTX-M-15 has always been found in association with the insertion sequence (IS) ISEcp1, which captures adjacent genes by using its left-hand inverted repeat (IRL) in conjunction with loosely related sequences downstream of each gene and also provides a promoter (19, 33, 34). Where enough flanking sequence is available, blaCTX-M-15 is generally found 48 bp beyond the right-hand inverted repeat (IRR) of ISEcp1 as part of a 2,971-bp transposition unit that also includes a partial open reading frame (orf477Δ) (6, 11, 13, 44). While an association with ISEcp1 may contribute to the success of blaCTX-M-15, many other genes associated with ISEcp1 are apparently much less widespread, suggesting that other factors are also important.
Surveys in several different countries (4, 10, 17, 20, 26, 50) have indicated that blaCTX-M-15 is often carried by IncF plasmids, many of which have multiple replicons and multiple antibiotic resistance genes. Unlike plasmids belonging to some other Inc groups, the backbones of IncF plasmids appear to be quite diverse, in terms of size, number of replicons present, and conjugation ability, due to extensive recombination events (28). The first IncF plasmid carrying blaCTX-M-15 to be completely sequenced was pC15-1a (GenBank accession no. AY458016), isolated in Canada (6). In pC15-1a, the ISEcp1-blaCTX-M-15-orf477Δ transposition unit is inserted in Tn2 (1), flanked by 5-bp direct repeats (DRs). Tn2 carries a tnpA (transposase) gene separated by a resolution (res) site from a tnpR (resolvase) gene and blaTEM-1b and is bounded by 38-bp inverted repeats (designated IRtnp and IRTEM here). In pC15-1a, the IRtnp end of Tn2 is truncated by IS26 and the Tn2-ISEcp1-blaCTX-M-15-orf477Δ-Tn2Δ structure is part of a larger (28.5 kb) multiresistance region (MRR) that also includes the aac(6′)-Ib-cr, aac(3)-IIe (29), blaOXA-30, and tetA(A) antibiotic resistance genes and several copies of IS26 (Fig. 1A).
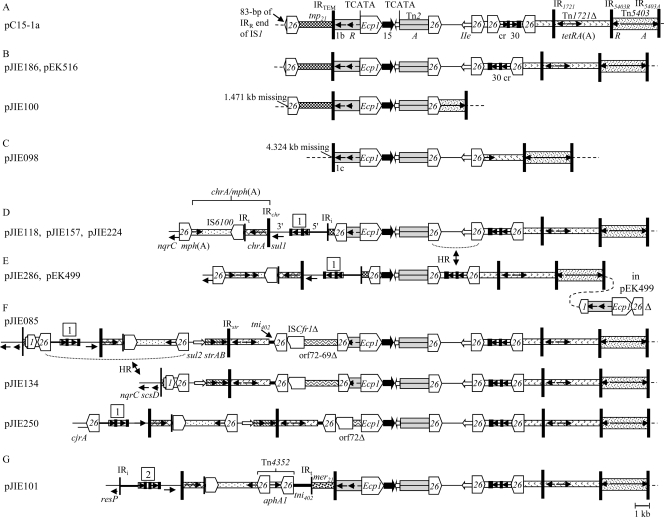
Fig. 1.
MRRs carrying blaCTX-M-15. Similar structures are grouped together. Different transposons and other modules have different shading and are generally labeled only once (tnp21 and mer21, transposition and mercury resistance regions of Tn21, respectively). ISs are labeled with their number/name, with the pointed end indicating IRR. Tall bars represent the 38-bp IR of transposons, as indicated. Positions/orientations of selected resistance and other genes are indicated by arrows, generally labeled only once. Abbreviations: A, tnpA; R, tnpR; 1b, blaTEM-1b, 1c, blaTEM-1c; 15, blaCTX-M-15; IIe, aac(3)-IIe; cr, aac(6′)-Ib-cr; 30, blaOXA-30. Class 1 integron components are indicated as follows: 5′, 5′-CS; 3′, 3′-CS; narrow boxes, gene cassettes; small black boxes, attC sites; 1, dfrA17-aadA5 cassette array; 2, dfrA12-gcuF-aadA2 cassette array; tni402, transposition region of Tn402; IRi, 25-bp IR at intI1 end; IRt, 25-bp IR at tni end. The chrA-mph(A) module (indicated in panel D) is found after position 1593 of the 3′-CS and consists of part of a chromate resistance transposon (IRchr and chrA), 123 bp of the IRt end of tni402, IS6100, and the mph(A)-mrx-mphR(A) macrolide resistance region (29). Dashed lines represent the IncFII backbone. The nqrC-like (putative Na+-translocating NADH-quinone reductase) and scsD (secreted copper sensitivity repressor) genes are part of an ∼10-kb region found in several IncF plasmids that is apparently derived from the Citrobacter koseri chromosome (GenBank accession no. CP000822). Arrows labeled HR and dotted lines indicate where homologous recombination explains differences between structures. (A) pC15-1a MRR (AY458016). DRs flanking the ISEcp1-blaCTX-M-15 transposition unit are shown. The left-hand boundary with the IncFII backbone is defined by a remnant of IS1. (B) Related MRRs in pJIE186, pEK516 (EU935738; rearranged version; see Fig. S3B in the supplemental material), and pJIE100. (C) pJIE098 MRR. (D) pJIE118/pJIE157/pJIE224 MRR. The IRi-tnp21 boundary is the same as in Tn21 (AF071413). (E) pJIE286 and pEK499 MRRs. The separate region shown on the right is in pEK499 (EU935739) only. (F) Related MRRs in pJIE085, pJIE134, and pJIE250. IS26 at the end of chrA-mph(A) truncates a region derived from IncQ plasmids that includes sul2 and a truncated Tn5393, carrying the strAB genes, and ends with IRstr (48). This truncates Tn1721, carrying tetAR(A), which is followed by part of tni402 and a region flanked by two copies of IS26 apparently derived from a larger region found in pU302L (AY333434) and pCTX-M3 (AF550415), in which ISCfr1 and orf69 are complete. The pJIE085 and pJIE134 MRRs end with a complex multi-IS structure (details in Fig. S5A in the supplemental material), while pJIE250 has IS26 only, truncating the cjrA gene found in several plasmids. (G) pJIE101 MRR. resP is identical to the genes in IncN plasmids. IS26 at the end of chrA-mph(A) forms part of composite transposon Tn4352 (46), the IS26-tni402 boundary is in >30 sequences in GenBank, the IRt-mer21 boundary is the same as in Tn21, and the mer21-IRTEM boundary is seen in other plasmid sequences (25, 30).
A few additional IncF plasmids from E. coli carrying blaCTX-M-15 have now also been completely sequenced. The IncFII plasmid pEK516 (GenBank accession no. EU935738) from an ST131 isolate of United Kingdom strain D (44) is closely related to pC15-1a and has a similar MRR, while another IncFII plasmid, pEC_B24 (GU371926), isolated in Belgium, has a simpler MRR and a different backbone (38). The IncFII and IncFIA plasmids pEK499 (EU935739) from the United Kingdom (44) and pEC_L8 (GU371928) and pEC_L46 (GU371929) from Belgium (38) are all from ST131 isolates and have related backbones, and all share MRR components with pC15-1a, but the pEC_L plasmids appear to have been subject to extensive rearrangements (38).
We previously identified blaCTX-M-15 as the dominant ESBL gene in Enterobacteriaceae from western Sydney, Australia (50). Transconjugants from 11 different E. coli isolates had blaCTX-M-15 and one or more IncF replicons, and here we have determined the structure of the MRRs in the plasmids that they carry. The modular, mosaic nature of MRRs enabled mapping by reference to pC15-1a and other resistance plasmids and limited sequencing. Plasmid backbones were also compared by restriction digestion and sequencing of IncF replicon targets.
(Part of this work was presented at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy [poster C1-103], Washington, DC, 25 to 28 October 2008.)
MATERIALS AND METHODS
Bacterial strains.
Transconjugants carrying blaCTX-M-15 and IncF replicons from clinical E. coli isolates with distinct pulsed-field gel electrophoresis (PFGE) types (n = 11) from a collection from western Sydney, Australia, recovered from December 2005 to August 2006 (50) were selected for study. All 11 original isolates were subjected to multilocus sequence typing (MLST; http://mlst.ucc.ie/mlst/dbs/Ecoli [43]). Cefotaxime (CTX) and ceftazidime (CAZ) MICs were determined by Etest (AB Biodisk, Solna, Sweden).
Plasmid analysis.
DNA was prepared for S1 nuclease digestion (3) by embedding bacterial cells (109 CFU/ml) in 1% InCert agarose plugs (FMC Bioproducts, Rockland, ME) and treating them with 1 mg/liter proteinase K (Invitrogen, Carlsbad, CA) in ESP buffer (0.5 M EDTA, pH 9, 1% N-lauroylsarcosine) at 55°C overnight. After they were washed (once in saline, four times in 1× TE [Tris-EDTA]), the plugs were equilibrated in 200 μl 1× S1 nuclease buffer (room temperature, 20 min; Promega, Madison, WI) and incubated with 8 U S1 nuclease (Promega) in 100 μl buffer (37°C, 45 min), and then 10 μl 0.5 M EDTA (pH 8) was added. PFGE was carried out in 1% PFGE-grade agarose (Bio-Rad, Hercules, CA) or SeaPlaque agarose (GTG Lonza, Rockland, ME) in a CHEF DRII system at 14°C and 6 V/cm with a switch angle of 120° and with a switch time of 5 to 40 s (12 h), followed by 3 to 8 s (8 h), using a MidRange I PFG marker (New England BioLabs, Ipswich, MA). Plasmid bands cut from gels were destained in 1 ml 1× Tris-acetate-EDTA (TAE) buffer at room temperature for 30 min and held overnight at 4°C in 50 μl fresh 1× TAE buffer before fragments were used as templates for replicon PCR. Plasmid DNA was extracted from transconjugants by alkaline lysis (36) and digested with HpaI according to the manufacturer's instructions (New England BioLabs).
PCR amplification.
Selected primers and reaction conditions are given in Table S1 in the supplemental material. Lysates prepared by incubating a few colonies from a fresh CHROMagar Orientation (CHROMagar, Paris, France) plate in 100 μl water at 95°C for 10 min and centrifuging (1 min, 16,000 × g) were used as templates. An Expand Long Template PCR system (Roche Diagnostics, Mannheim, Germany) was used according to the manufacturer's instructions. For inverse PCR (27), outward-facing primers were used with a template created by restricting plasmid DNA with an appropriate enzyme (New England BioLabs) and self-ligating with T4 DNA ligase (New England BioLabs). The FII and FIA amplicons obtained here encompass the regions used in the IncF replicon sequence typing (RST) scheme (41), while the sequences of two IncFIB amplicons were combined to enable RST classification (see Table S1 in the supplemental material).
Characterizing MRRs carrying blaCTX-M-15.
PCR mapping was carried out with reference to the pC15-1a MRR and other available MRR sequences (see Fig. S1 and S2 and Table S1 in the supplemental material). ApaI or SalI (New England BioLabs) fragments carrying blaCTX-M-15 from selected plasmids (see Fig. S1 and S2 in the supplemental material) were ligated to pBC SK (Stratagene, La Jolla, CA) digested with the same enzyme and used to transform E. coli DH5α with selection on chloramphenicol (20 μg/ml) and CTX (8 μg/ml). Sequence adjacent to IRTEM in pJIE098 and pJIE101 was obtained using a DNA Walking Speedup kit (Seegene USA, Rockville, MD) and nested primers (see Table S1 in the supplemental material). Direct sequencing of pJIE100 DNA with a primer facing out of the IRR end of IS26 gave superimposed sequences. Subtraction of the sequence already identified next to one copy of IS26 gave the sequence adjacent to the other copy. Searches with these sequences identified matching regions in GenBank, and primers were designed to confirm links between regions. Selected regions [e.g., boundaries between potentially mobile segments, aac(6′)-Ib genes, and replicon PCR products] were sequenced.
DNA sequencing and analysis.
DNA prepared using PureLink (Invitrogen) Quick Plasmid Miniprep (cloned DNA) or PCR purification (amplicons) kits or treated with 0.65 M NaCl and 5% (wt/vol) polyethylene glycol 8000 for 30 min at 4°C (whole-plasmid DNA) was sequenced at the Westmead DNA facility or Macrogen (Seoul, South Korea). Sequences were assembled and analyzed with Lasergene software (DNAStar Inc., Madison, WI), the Gene Construction Kit program (Textco BioSoftware Inc., West Lebanon, NH), and programs available through http://www.ncbi.nlm.nih.gov/.
Nucleotide sequence accession numbers.
GenBank accession no. EU418920 for pJIE098 has been extended. Partial sequences of other plasmids are available under the following GenBank accession numbers: pJIE100, EU418921; pJIE101, EU418922; pJIE118, EU418924; pJIE134, EU418925; pJIE186, EU418930; pJIE250, EU418932; pJIE085, GU264002; and pJIE286, GU264003.
RESULTS AND DISCUSSION
Strains and plasmids carrying blaCTX-M-15.
The different E. coli isolates carrying blaCTX-M-15 were identified as ST131 (n = 7) or ST405 (n = 4; Table 1) by MLST. S1 nuclease digestion followed by PFGE (data not shown) of one transconjugant from each isolate revealed a single plasmid in each case (designated pJIE followed by the isolate number), ranging in size from ∼70 to ∼220 kb (Table 1). Replicon PCR (8, 28) using excised plasmid bands as templates indicated that each plasmid carried all of the replicons previously detected in the corresponding transconjugant (Table 1). The MRR in each plasmid was characterized, and HpaI digest patterns of plasmids and the sequences of IncF replicon PCR products were also compared.
Table 1.
Characteristics of strains and plasmids carrying blaCTX-M-15
| Plasmida | Strain ST | Plasmid characteristics |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Size (kb) | blaTEM | aac(3)-IIe | aac(6′)-Ib-cr | blaOXA-30 | tetA(A) | MRRb | HpaI typec | Replicon classified by RST | ||
| pC15-1a | ST131? | 92 | 1b | + | + | + | + | 1a | A1 | F2:A−:B− |
| pJIE186 | ST131 | ∼90 | 1b | + | + | + | + | 1b | A1 | F2:A−:B− |
| pEK516 | ST131 | 64 | 1b | + | + | + | + | 1b | A2 | F2:A−:B− |
| pJIE100 | ST131 | ∼75 | 1b | − | − | − | − | 1c | A3 | F2:A−:B− |
| pJIE098 | ST405 | ∼145 | 1cd | + | − | − | +h | 2 | B | F31:A4:B1 |
| pJIE118 | ST131 | ∼140 | − | + | + | + | + | 3a | C1 | F2i:A1:B− |
| pJIE157 | ST131 | ∼140 | − | + | + | + | + | 3a | C2 | F2i:A1:B− |
| pJIE224 | ST131 | ∼140 | − | + | + | + | + | 3a | C3 | F2i:A1:B− |
| pEK499 | ST131 | 118 | 1be | − | + | + | + | 3b | F2:A1:B− | |
| pJIE286 | ST131 | ∼170 | − | − | + | + | + | 3c | D | F2:A1:B− |
| pEC_L8 | ST131 | 119 | 1b | − | +g | +g | + | 4 | F2i:A1:B− | |
| pEC_L46 | ST131 | 145 | 1bf | − | + | + | − | 5 | F2i:A1:B− | |
| pJIE085 | ST405 | ∼160 | − | + | + | + | +g | 6a | E1 | F1j:A1:B2 |
| pJIE134 | ST405 | ∼155 | − | + | + | + | +g | 6b | E2 | F1j:A1:B2 |
| pJIE250 | ST405 | ∼160 | − | + | + | + | +g | 6c | E3 | F1j:A1:B2 |
| pJIE101 | ST131 | ∼220 | 1b | + | + | + | + | 7 | F | F22:A1:B20 + N |
Plasmids with names in boldface were isolated as part of this study. The sequences of previously published plasmids are available under the following GenBank accession numbers: pC15-1a, AY458016; pEK516, EU935738; pEK499, EU935739; pEC_L8, GU371928; and pEC_L46, GU371929.
MRRs that could be derived from one other by simple insertions, deletions, or rearrangements are given the same number but different letters.
Similar HpaI patterns are given the same letter but different numbers.
blaTEM-1c was erroneously designated blaTEM-1i in reference 50.
The blaTEM-1b gene is missing 53 bp at the 3′ end.
The blaTEM-1b gene is missing 83 bp at the 5′ end, which is found elsewhere on the plasmid.
Two copies of the gene indicated are present.
The tetA(A) gene in pJIE098 is truncated by IS26 at the 5′ end.
Only 95% identity to pC15-1a/pEK516/pEK499 over the longer region amplified here with primer pair OR1/CA1 (see Table S1 in the supplemental material).
ISEc12 is inserted in RepFII of pJIE085, pJIE134, and pJIE250, while ISEc23 is inserted in RepFII of pRSB107, in each case increasing the size of the amplicon obtained with the OR1/CA1 primer pair (see Table S1 in the supplemental material) by ∼2.5 kb.
The MRRs of all 11 pJIE plasmids are related to the pC15-1a MRR.
The IRR end of ISEcp1 had been detected upstream of blaCTX-M-15 in all original isolates (50), and the expected 48-bp spacer was identified in all 11 pJIE plasmids. ISEcp1 was intact in all except pJIE286, where truncation by IS26 (Fig. 1E) has left only 24 bp of the IRR end adjacent to blaCTX-M-15. This apparently common arrangement (11, 12, 23, 45, 47) separates blaCTX-M-15 from the ISEcp1 promoter, resulting in reduced resistance to cephalosporins (23, 45), as observed for the JIE286 transconjugant (CTX MIC of 24 μg/ml and CAZ MIC of 3 μg/ml versus CTX and CAZ MICs of >256 μg/ml against other isolates).
The pJIE186 MRR is almost identical to the pC15-1a MRR, except for a rearrangement. The other 10 pJIE plasmids have related MRRs, but various regions are missing compared with pJIE186 and/or extra modules (all previously identified in other MRRs) are present (Fig. 1). One end of the MRR in all 11 plasmids has the same boundary with the plasmid backbone as in pC15-1a.
IS26-mediated rearrangements and deletions.
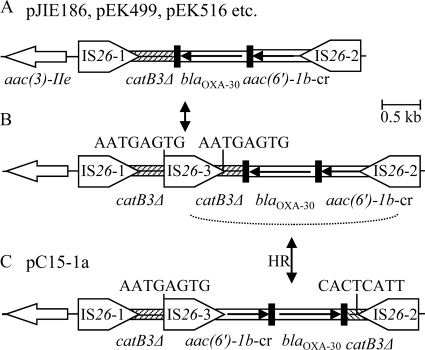
In pC15-1a, the aac(6′)-Ib-cr and blaOXA-30 gene cassettes are associated with two fragments of the catB3 cassette and three IS26 elements (Fig. 2C). In the 9 pJIE plasmids that include this region (Fig. 1), aac(6′)-Ib-cr-blaOXA-30-catB3Δ is associated with two copies of IS26 and is in the opposite orientation (Fig. 2A). This configuration is also found in pEK499, pEK516, pEC_L8, and pEC_L46, although the catB3 cassette is incorrectly named catB4 (38, 44). Insertion of an extra copy of IS26 and homologous recombination between IS26 elements in opposite orientations (Fig. 2) could explain the generation of the pC15-1a configuration, and it seems unlikely that these events would have happened in the reverse order.
Fig. 2.
IS26-mediated rearrangement of the cassette array containing aac(6′)-Ib-cr, blaOXA-30, and catB3Δ. Components are generally represented as described in the legend to Fig. 1. (A) Cassette array region in MRRs of pJIE186 and eight other pJIE plasmids, pEK499, pEK516, pEC_L8, and pEC_L46; (B) insertion of IS26-3 into the catB3Δ cassette would generate 8-bp DRs characteristic of IS26 transposition (9); (C) subsequent homologous recombination between IS26-2 and IS26-3 (dotted line) would invert the intervening region to give the pC15-1a configuration, in which the 8-bp sequence adjacent to IRL of IS26-3 is the reverse complement of the 8 bp adjacent to IRR of IS26-2.
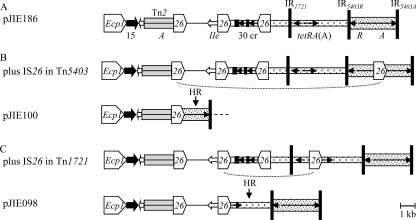
pJIE286 and pEK499 lack the aac(3)-IIe region found between directly oriented copies of IS26 (Fig. 1D and E), and its presence/absence is readily explained by homologous recombination inserting/deleting a circular molecule containing this region plus one IS26 element (29). pJIE098 and pJIE100 have larger deletions with one end corresponding to IRR of IS26, suggesting insertion of an additional copy of IS26 followed by homologous recombination between directly oriented IS26 elements (Fig. 3B and C). The variable associations between blaCTX-M-15 and other resistance genes observed in a number of isolates and plasmids (6, 18, 20, 22, 23, 37, 39) are presumably explained by similar events, particularly in the case of closely related plasmids (21).
Fig. 3.
Deletions in MRRs caused by insertion of additional copies of IS26 and homologous recombination. Components are generally represented as described in the legend to Fig. 1. (A) Part of the pJIE186 MRR; (B) insertion of an extra copy of IS26 in Tn5403 and homologous recombination with the IS26 element interrupting the IRtnp end of Tn2 (dotted line) would result in the pJIE100 structure; (C) insertion of an extra copy of IS26 in Tn1721 and homologous recombination with the IS26 element next to aac(3)-IIe would result in the pJIE098 structure.
pJIE186 and pJIE100 are closely related to pC15-1a (Canada) and pEK516 (United Kingdom).
Upstream of blaCTX-M-15 the pJIE186 and pC15-1a MRRs have the same boundary with the IncFII backbone (Fig. 1A and B), while in pJIE100 the MRR has a different boundary (Fig. 1B), probably due an IS26-mediated deletion. pJIE186 and pJIE100 have IncFII replicons only and gave amplicons that are identical to both pC15-1a and pEK516 (corresponding to RST allele F2). The predicted pC15-1a and pEK516 HpaI patterns differ (see Fig. S3A in the supplemental material), despite high sequence identity, due to a rearrangement and a deletion (see Fig. S3B in the supplemental material). pJIE186 is indistinguishable from pC15-1a and pJIE100 matches pC15-1a with the appropriate regions deleted, suggesting that these four IncFII plasmids are closely related.
The pJIE098 MRR may have been created by site-specific recombination in Tn2.
One end of the pJIE098 MRR is defined by the boundary between IRTEM and the IncFII backbone (Fig. 1C), but pJIE098 has blaTEM-1c (15), which has 3 nucleotide differences from blaTEM-1b (see Fig. S4A in the supplemental material). Other differences from Tn2 suggest that this structure in pJIE098 has arisen by resolvase-mediated site-specific recombination between Tn2a, carrying blaTEM-1c (1), and Tn2, containing ISEcp1-blaCTX-M-15 (see Fig. S4A in the supplemental material). As Tn3-like transposons exhibit transposition immunity—i.e., the presence of one copy in a DNA molecule significantly reduces insertion of a second copy (16)—these two transposons may have originally been on different plasmids, helping to explain the multiple replicons in pJIE098.
The HpaI pattern of pJIE098 differs from the patterns of the other 10 pJIE plasmids (see Fig. S4B in the supplemental material). The IncFIB amplicon matches pRSB107 (GenBank accession no. AJ851089) (40), designated RST allele B1. The IncFII (F31) and IncFIA (A4) amplicons did not exactly match any sequences in GenBank, but plasmids carrying blaCTX-M-15 from E. coli strains from Italy and the United Kingdom were also F31:A4:B1 (41), suggesting that similar plasmids are present in other locations.
pJIE118, pJIE157, and pJIE224 are closely related.
In the apparently identical MRRs of pJIE118, pJIE157, and pJIE224 the IRTEM end of Tn2 is truncated by IS26, which is followed by a class 1 integron with the dfrA17-aadA5 cassette array and the chrA-mph(A) module (29) (Fig. 1D). Slight differences between the HpaI patterns suggest differences in the backbones of these plasmids (see Fig. S3C in the supplemental material), but their IncFIA amplicons are identical, corresponding to allele A1 found in most IncFIA plasmids, including pEC_L8 and pEC_L46 (41). The IncFII amplicons of pJIE118, pJIE157, and pJIE224 are also all 100% identical to pEC_L8 and pEC_L46, and all these plasmids would be classified as F2 by RST. However, there are significant differences (∼95% identity) from pC15-1a and pEK516, also classified as F2, in the longer region amplified here. Homologous recombination in the ∼3-kb region between the Tn5403 end of the MRR and the IncFII replicon could explain this association of the same MRR-backbone boundary with variant replicons.
pJIE286 is related to pEK499 from the United Kingdom.
The pJIE286 MRR is closely related to the pJIE118/pJIE157/pJIE224 MRR, except that the aac(3)-IIe region, blaTEM-1b, and most of ISEcp1 are missing (Fig. 1D and E). Like these three plasmids, pJIE286 has allele A1 but a different HpaI pattern (see Fig. S3C in the supplemental material), and the IncFII amplicon is 100% identical to pC15-1a/pEK516 rather than the pEC_L plasmids. pEK499 has the same MRR as pJIE286 but also includes the remainder of ISEcp1 and blaTEM-1b in another inserted region (Fig. 1E). pEK499 also has the same replicon combination as pJIE286, but much of the backbone is 99% identical to the pEC_L8 and pECL_46 backbones. This suggests that the IncFII replicon could have been acquired (along with part of the MRR) from a pC15-1a-like plasmid by homologous recombination (see Fig. S3D in the supplemental material). pJIE286 is conjugative, while pEK499 is smaller (Table 1) and nonconjugative, due to a deletion in the tra region (44), and PCR confirmed that the region including blaTEM-1b in pEK499 is not present in pJIE286, but these two plasmids may nevertheless have been generated from the same ancestor by different events.
The pJIE085, pJIE134, and pJIE250 MRRs: homologous recombination in IS26.
The pJIE085 MRR includes a class 1 integron with dfrA17-aadA5 and chrA-mph(A) plus several other common MRR modules (29), and one end is defined by a complex multi-IS structure that includes IS26 (Fig. 1F; see S4A in the supplemental material). The relationship between this structure and the pJIE134 MRR is readily explained by insertion/release of a circular molecule by homologous recombination in IS26 (Fig. 1F). A region adjacent to ISEcp1 is missing from the pJIE250 MRR compared with the pJIE085 and pJIE134 MRRs, possibly explained by an ISEcp1-mediated deletion. IS26 only, rather than the multi-IS structure, defines the end of the MRR in pJIE250, and the adjacent backbone region is different, suggesting an IS26-mediated deletion. These variations between MRRs explain some of the differences between large fragments in the HpaI restriction patterns of these plasmids (see Fig. S4B and C in the supplemental material), suggesting that their backbones are closely related.
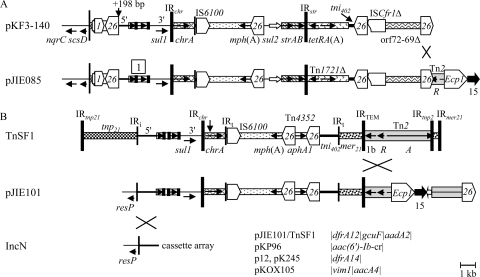
The pJIE085 MRR matches most of the MRR in pKF3-140 from a Klebsiella pneumoniae isolate from China (49) (Fig. 4A). Recombination between IS26 elements that define the end of this match could have brought these regions together, or the two smaller MRRs could have been created from the larger one by the reverse process. The RST formula of pKF3-140 (F1:A2:B2) is different from that of pJIE085, pJIE134, and pJIE250 (F1:A1:B2), but alleles A1 and A2 have only 1 nucleotide difference, and there is only one additional difference in the longer IncFIA region amplified, suggesting a close relationship between these four plasmids.
Fig. 4.
Recombination between common MRR modules. Components are generally represented as described in the legend to Fig. 1. Crosses indicate regions where homologous recombination could have taken place. (A) Relationship between the pJIE085 and pKF3-140 MRRs. An extra 198 bp of the 5′-CS is present in pKF3-140 compared with pJIE085, as indicated. The IS26 element truncating orf69 and Tn2 in pJIE085 marks the end of the match to the pKF3-140 MRR. (B) Relationship between the pJIE101 MRR, TnSF1, and IncN plasmids. The vertical arrow in TnSF1 indicates a 521-bp insert not present in any other examples of chrA-mph(A). The cassette arrays in pJIE101, TnSF1, and IncN plasmids are indicated. The pJIE101 IncN replicon is 100% identical to p12 (14) over the region amplified. Sequences referred to in this diagram are from the following GenBank accession numbers: pKF3-140, FJ876827; TnSF1, AF188331; pKP96, EU195449; p 12, FJ223605; pKOX105, HM126016; and pK245, DQ449578.
The pJIE101 MRR: homologous recombination in Tn2.
The pJIE101 MRR includes a class 1 integron and chrA-mph(A), but with the dfrA12-gcuF-aadA2 cassette array (Fig. 1G). The whole of the region upstream of ISEcp1 matches TnSF1, and homologous recombination between Tn2 elements in a pJIE186-like MRR and a TnSF1-like structure would link these regions to create the MRR seen in pJIE101 (Fig. 4B).
pJIE101 apparently has an IncN replicon in addition to IncFII, FIA, and FIB. Consistent with this one end of the MRR, marked by IRi (the 25-bp IR at the intI1 end of class 1 integrons), has the same boundary with the resP region as in several IncN plasmids with class 1 integrons and different cassette arrays (Fig. 4B). Homologous recombination, in this case, between two copies of the 5′ conserved sequence (5′-CS), again readily explains the association of the IncN resP region with the remainder of the MRR. Other plasmids also have both IncF and IncN replicons: pEC_L46 (38) includes a region matching part of the IncN plasmid 9 (GenBank accession no. FJ223607) (14) that contains the IncN replicon, and pGSH500 from K. pneumoniae also has both IncFII-like and IncN-like replicons (28).
The pJIE101 HpaI pattern differs from those of the other 10 pJIE plasmids (see Fig. S4C in the supplemental material), and the IncFIA amplicon matches many plasmids in GenBank, while the IncFII and IncFIB amplicons had no exact matches. The same RST combination as pJIE101 (F22:A1:B20) was also detected in plasmids carrying blaCTX-M-15 from E. coli strains from Italy and the United Kingdom (41), which apparently do not have an IncN replicon.
Concluding remarks.
In the IncF plasmids from Sydney studied here, the blaCTX-M-15 gene is located in closely related MRRs that are also related to those on plasmids from several other geographic locations. Differences in the combinations of certain resistance genes co-occurring with blaCTX-M-15 appear to reflect deletions/insertions of parts of a common MRR, often mediated by IS26, rather than the presence of significantly different MRRs. We found no evidence of movement of a discrete MRR between different plasmids; rather, it appears that different MRRs have been constructed by recombination between elements that are common in these regions (IS26 and Tn2) acting as adaptors. The location of blaCTX-M-15 in large MRRs that also carry common modules and mobile elements that enable recombination and reassortment may be a principal contributor to its success.
Mapping of MRRs containing blaCTX-M-15 was possible because all of the components identified had been seen before in sequences available in GenBank, often in the same combinations with the same boundaries, for example, sul2-strAB (>50 sequences), the IS26-tni402 boundary in pJIE101 (>30 sequences), and the chrA-mph(A) module (>10 examples). The two cassette arrays identified also appear to be very common (31), suggesting that they are traveling as part of larger structures. This further illustrates that MRRs are mosaics of modules already available in the gene pool (2, 29, 42).
One boundary of the MRR with plasmid backbone was the same in all plasmids studied here and in other available sequences, even in plasmids carrying different combinations and variants of IncF replicons. Recombination in plasmid backbones, in addition to MRRs, thus appears to contribute to the creation of multiple replicon IncF plasmids. Such mosaicism hampers the development of robust methods for classifying and comparing IncF plasmids. Depending on the target sequences chosen, RST-type classification schemes can underestimate diversity (e.g., F2 plasmids may have differences over a longer region) and single nucleotide changes may overemphasize differences (e.g., IncFIA of pKF3-140). RST of IncF plasmids may thus be a useful starting point for grouping plasmids and identifying similarities between those from different locations, but it needs to be combined with other methods (restriction digests, MRR mapping) to characterize plasmids.
Use of such a combination of methods should allow identification of the most informative sets of multi-IncF plasmids to completely sequence, to aid in understanding the processes involved in their generation and evolution, and in developing improved classification schemes. Characterization of IncF plasmids carrying blaCTX-M-15 from a range of locations may shed light on how such plasmids spread, and characterization of plasmids from different strain types may help to elucidate relationships between particular plasmids and strains, suggested here by differences between pJIE plasmids from ST405 and ST131 isolates. Characterization of many plasmids carrying blaCTX-M-15 from places where they are long established and/or highly prevalent may help to determine whether variety increases with time or whether a limited number of dominant structures/plasmids emerge, which has important implications for our understanding of natural selection within the mobile antibiotic resistance gene pool.
Supplementary Material
ACKNOWLEDGMENTS
We thank Andrew Ginn for technical assistance.
Z.Z. was supported by an Endeavor International Postgraduate Research Scholarship from the Australian Government Department of Education, Science and Training. S.R.P. was partly supported by grant 512396 from the Australian National Health and Medical Research Council. This study made use of the E. coli MLST database (http://mlst.ucc.ie/mlst/dbs/Ecoli), which is currently supported by a grant from the Science Foundation of Ireland (05/FE1/B882).
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 22 August 2011.
REFERENCES
- 1. Bailey J. K., Pinyon J. L., Anantham S., Hall R. M. 2011. Distribution of the blaTEM gene and blaTEM-containing transposons in commensal Escherichia coli. J. Antimicrob. Chemother. 66:745–751 [DOI] [PubMed] [Google Scholar]
- 2. Baquero F. 2004. From pieces to patterns: evolutionary engineering in bacterial pathogens. Nat. Rev. Microbiol. 2:510–518 [DOI] [PubMed] [Google Scholar]
- 3. Barton B. M., Harding G. P., Zuccarelli A. J. 1995. A general method for detecting and sizing large plasmids. Anal. Biochem. 226:235–240 [DOI] [PubMed] [Google Scholar]
- 4. Baudry P. J., et al. 2009. Mechanisms of resistance and mobility among multidrug-resistant CTX-M-producing Escherichia coli from Canadian intensive care units: the 1st report of QepA in North America. Diagn. Microbiol. Infect. Dis. 63:319–326 [DOI] [PubMed] [Google Scholar]
- 5. Bonnet R. 2004. Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob. Agents Chemother. 48:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyd D. A., et al. 2004. Complete nucleotide sequence of a 92-kilobase plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. Antimicrob. Agents Chemother. 48:3758–3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cantón R., Coque T. M. 2006. The CTX-M β-lactamase pandemic. Curr. Opin. Microbiol. 9:466–475 [DOI] [PubMed] [Google Scholar]
- 8. Carattoli A., et al. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228 [DOI] [PubMed] [Google Scholar]
- 9. Chandler M., Mahillon J. 2002. Insertion sequences revisited, p. 306–367In Craig N. L., Cragie R., Gellert M., Lambowitz A. M.(ed.), Mobile DNA. ASM Press, Washington, DC [Google Scholar]
- 10. Coque T. M., et al. 2008. Dissemination of clonally related Escherichia coli strains expressing extended-spectrum β-lactamase CTX-M-15. Emerg. Infect. Dis. 14:195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cullik A., Pfeifer Y., Prager R., von Baum H., Witte W. 2010. A novel IS26 structure is surrounding blaCTX-M genes in different plasmids of German clinical isolates of Escherichia coli. J. Med. Microbiol. 59:580–587 [DOI] [PubMed] [Google Scholar]
- 12. Diestra K., et al. 2009. Characterization of plasmids encoding blaESBL and surrounding genes in Spanish clinical isolates of Escherichia coli and Klebsiella pneumoniae. J. Antimicrob. Chemother. 61:60–66 [DOI] [PubMed] [Google Scholar]
- 13. Eckert C., Gautier V., Arlet G. 2006. DNA sequence analysis of the genetic environment of various blaCTX-M genes. J. Antimicrob. Chemother. 57:14–23 [DOI] [PubMed] [Google Scholar]
- 14. Gootz T. D., et al. 2009. Genetic organization of transposase regions surrounding blaKPC carbapenemase genes on plasmids from Klebsiella strains isolated in a New York City hospital. Antimicrob. Agents Chemother. 53:1998–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goussard S., Courvalin P. 1999. Updated sequence information for TEM β-lactamase genes. Antimicrob. Agents Chemother. 43:367–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grindley N. D. F. 2002. The movement of Tn3-like elements: transposition and cointegrate resolution, p. 272–302In Craig N. L., Craigie R., Gellert M., Lambowitz A. M.(ed.), Mobile DNA II. ASM Press, Washington, DC [Google Scholar]
- 17. Hopkins K. L., et al. 2006. Replicon typing of plasmids carrying CTX-M or CMY β-lactamases circulating among Salmonella and Escherichia coli isolates. Antimicrob. Agents Chemother. 50:3203–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karisik E., et al. 2006. Molecular characterization of plasmids encoding CTX-M-15 β-lactamases from Escherichia coli strains in the United Kingdom. J. Antimicrob. Chemother. 58:665–668 [DOI] [PubMed] [Google Scholar]
- 19. Lartigue M. F., Poirel L., Aubert D., Nordmann P. 2006. In vitro analysis of ISEcp1B-mediated mobilization of naturally occurring β-lactamase gene blaCTX-M of Kluyvera ascorbata. Antimicrob. Agents Chemother. 50:1282–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lavollay M., et al. 2006. Clonal dissemination of a CTX-M-15 β-lactamase-producing Escherichia coli strain in the Paris area, Tunis, and Bangui. Antimicrob. Agents Chemother. 50:2433–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leflon-Guibout V., et al. 2004. Emergence and spread of three clonally related virulent isolates of CTX-M-15-producing Escherichia coli with variable resistance to aminoglycosides and tetracycline in a French geriatric hospital. Antimicrob. Agents Chemother. 48:3736–3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Machado E., et al. 2006. Dissemination in Portugal of CTX-M-15-, OXA-1-, and TEM-1-producing Enterobacteriaceae strains containing the aac(6′)-Ib-cr gene, which encodes an aminoglycoside- and fluoroquinolone-modifying enzyme. Antimicrob. Agents Chemother. 50:3220–3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Montesinos I., et al. 2010. Molecular characterization of plasmids encoding CTX-M-15 extended-spectrum β-lactamase associated with the ST131 Escherichia coli clone in Belgium. J. Antimicrob. Chemother. 65:1828–1830 [DOI] [PubMed] [Google Scholar]
- 24. Nicolas-Chanoine M. H., et al. 2008. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 61:273–281 [DOI] [PubMed] [Google Scholar]
- 25. Novais A., et al. 2010. International spread and persistence of TEM-24 is caused by the confluence of highly penetrating Enterobacteriaceae clones and an IncA/C2 plasmid containing Tn1696::Tn1 and IS5075-Tn21. Antimicrob. Agents Chemother. 54:825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Novais A., et al. 2006. Emergence and dissemination of Enterobacteriaceae isolates producing CTX-M-1-like enzymes in Spain are associated with IncFII (CTX-M-15) and broad-host-range (CTX-M-1, -3, and -32) plasmids. Antimicrob. Agents Chemother. 51:796–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ochman H., Gerber A. S., Hartl D. L. 1988. Genetic applications of an inverse polymerase chain reaction. Genetics 120:621–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Osborn A. M., da Silva Tatley F. M., Steyn L. M., Pickup R. W., Saunders J. R. 2000. Mosaic plasmids and mosaic replicons: evolutionary lessons from the analysis of genetic diversity in IncFII-related replicons. Microbiology 146:2267–2275 [DOI] [PubMed] [Google Scholar]
- 29. Partridge S. R. 2011. Analysis of antibiotic resistance regions in Gram-negative bacteria. FEMS Microbiol. Rev. 35:820–855 [DOI] [PubMed] [Google Scholar]
- 30. Partridge S. R., Hall R. M. 2004. Complex multiple antibiotic and mercury resistance region derived from the r-det of NR1 (R100). Antimicrob. Agents Chemother. 48:4250–4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Partridge S. R., Tsafnat G., Coiera E., Iredell J. R. 2009. Gene cassettes and cassette arrays in mobile resistance integrons. FEMS Microbiol. Rev. 33:757–784 [DOI] [PubMed] [Google Scholar]
- 32. Peirano G., Pitout J. D. 2010. Molecular epidemiology of Escherichia coli producing CTX-M β-lactamases: the worldwide emergence of clone ST131 O25:H4. Int. J. Antimicrob. Agents 35:316–321 [DOI] [PubMed] [Google Scholar]
- 33. Poirel L., Decousser J. W., Nordmann P. 2003. Insertion sequence ISEcp1B is involved in expression and mobilization of a blaCTX-M β-lactamase gene. Antimicrob. Agents Chemother. 47:2938–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Poirel L., Lartigue M. F., Decousser J. W., Nordmann P. 2005. ISEcp1B-mediated transposition of blaCTX-M in Escherichia coli. Antimicrob. Agents Chemother. 49:447–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rogers B. A., Sidjabat H. E., Paterson D. L. 2010. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J. Antimicrob. Chemother. 66:1–14 [DOI] [PubMed] [Google Scholar]
- 36. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 37. Sidjabat H. E., et al. 2009. Molecular epidemiology of CTX-M-producing Escherichia coli isolates at a tertiary medical center in western Pennsylvania. Antimicrob. Agents Chemother. 53:4733–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smet A., et al. 2010. Complete nucleotide sequence of CTX-M-15-plasmids from clinical Escherichia coli isolates: insertional events of transposons and insertion sequences. PLoS One 5:e11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Soge O. O., Queenan A. M., Ojo K. K., Adeniyi B. A., Roberts M. C. 2006. CTX-M-15 extended-spectrum β-lactamase from Nigerian Klebsiella pneumoniae. J. Antimicrob. Chemother. 57:24–30 [DOI] [PubMed] [Google Scholar]
- 40. Szczepanowski R., et al. 2005. The 120 592 bp IncF plasmid pRSB107 isolated from a sewage-treatment plant encodes nine different antibiotic-resistance determinants, two iron-acquisition systems and other putative virulence-associated functions. Microbiology 151:1095–1111 [DOI] [PubMed] [Google Scholar]
- 41. Villa L., Garcia-Fernandez A., Fortini D., Carattoli A. 2010. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J. Antimicrob. Chemother. 65:2518–2529 [DOI] [PubMed] [Google Scholar]
- 42. Walsh T. R. 2006. Combinatorial genetic evolution of multiresistance. Curr. Opin. Microbiol. 9:476–482 [DOI] [PubMed] [Google Scholar]
- 43. Wirth T., et al. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol. Microbiol. 60:1136–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Woodford N., et al. 2009. Complete nucleotide sequences of plasmids pEK204, pEK499, and pEK516, encoding CTX-M enzymes in three major Escherichia coli lineages from the United Kingdom, all belonging to the international O25:H4-ST131 clone. Antimicrob. Agents Chemother. 53:4472–4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Woodford N., et al. 2004. Community and hospital spread of Escherichia coli producing CTX-M extended-spectrum β-lactamases in the UK. J. Antimicrob. Chemother. 54:735–743 [DOI] [PubMed] [Google Scholar]
- 46. Wrighton C. J., Strike P. 1987. A pathway for the evolution of the plasmid NTP16 involving the novel kanamycin resistance transposon Tn4352. Plasmid 17:37–45 [DOI] [PubMed] [Google Scholar]
- 47. Xu L., et al. 2011. Regional survey of CTX-M-type extended-spectrum β-lactamases among Enterobacteriaceae reveals marked heterogeneity in the distribution of the ST131 clone. J. Antimicrob. Chemother. 66:505–511 [DOI] [PubMed] [Google Scholar]
- 48. Yau S., Liu X., Djordjevic S. P., Hall R. M. 2010. RSF1010-like plasmids in Australian Salmonella enterica serovar Typhimurium and origin of their sul2-strA-strB antibiotic resistance gene cluster. Microb. Drug Resist. 16:249–252 [DOI] [PubMed] [Google Scholar]
- 49. Zhao F., et al. 2010. Sequencing and genetic variation of multidrug resistance plasmids in Klebsiella pneumoniae. PLoS One 5:e10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zong Z., Partridge S. R., Thomas L., Iredell J. R. 2008. Dominance of blaCTX-M within an Australian extended-spectrum β-lactamase gene pool. Antimicrob. Agents Chemother. 52:4198–4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.