Abstract
The 16S rRNA methyltransferase ArmA is a worldwide emerging determinant that confers high-level resistance to most clinically relevant aminoglycosides. We report here the identification and characterization of a multidrug-resistant Salmonella enterica subspecies I.4,12:i:− isolate recovered from chicken meat sampled in a supermarket on February 2009 in La Reunion, a French island in the Indian Ocean. Susceptibility testing showed an unusually high-level resistance to gentamicin, as well as to ampicillin, expanded-spectrum cephalosporins and amoxicillin-clavulanate. Molecular analysis of the 16S rRNA methyltransferases revealed presence of the armA gene, together with blaTEM-1, blaCMY-2, and blaCTX-M-3. All of these genes could be transferred en bloc through conjugation into Escherichia coli at a frequency of 10−5 CFU/donor. Replicon typing and S1 pulsed-field gel electrophoresis revealed that the armA gene was borne on an ∼150-kb broad-host-range IncP plasmid, pB1010. To elucidate how armA had integrated in pB1010, a PCR mapping strategy was developed for Tn1548, the genetic platform for armA. The gene was embedded in a Tn1548-like structure, albeit with a deletion of the macrolide resistance genes, and an IS26 was inserted within the mel gene. To our knowledge, this is the first report of ArmA methyltransferase in food, showing a novel route of transmission for this resistance determinant. Further surveillance in food-borne bacteria will be crucial to determine the role of food in the spread of 16S rRNA methyltransferase genes worldwide.
INTRODUCTION
Aminoglycosides are used for the treatment of a wide range of infections due to both Gram-negative and Gram-positive bacteria. Resistance to these antimicrobials is usually due to the production of aminoglycoside-modifying enzymes, which are able to acetylate, phosphorylate, or adenylate the antibiotic molecule (8). Since 2003, the 16S rRNA methyltransferases have emerged in Gram-negative pathogens as an acquired aminoglycoside resistance mechanism that confers high-level resistance to most clinically relevant aminoglycosides (13, 17). Seven different 16S rRNA methyltransferase genes have been described thus far: armA, rmtA, rmtB, rmtC, rmtD, rmtE, and npmA (9, 10, 35). armA and rmtB are the most prevalent among Enterobacteriaceae. Except for the identification of armA and rmtB in animal isolates from Spain and China (5, 11, 17) and rmtC in Salmonella enterica from food in the United Kingdom (23), all methyltransferase genes described to date were identified in human clinical samples (36). Although rmtC has been reported in the bacterial chromosome integrated through the ISEcp1 mobile element (23), in most cases these genes have been related to large conjugative plasmids in association with extended-spectrum β-lactamases (ESBLs) or, more recently, carbapenemases such as KPC-2 (25) or NDM-1 (29). Although the genetic plasticity of these conjugative elements is high, armA has been always identified in the transposable element Tn1548 (14, 16).
In order to determine whether food animal products are a source of 16S rRNA methyltransferases, we studied the presence of these genes in bacteria isolated from food in La Reunion Island. We report here a multidrug-resistant Salmonella enterica isolate, 09CEB904SAL, recovered from food and producing the ArmA methyltransferase. The isolate was identified as S. enterica I.4,12:i:−, a monophasic variant of S. enterica serovar Typhimurium. Molecular characterization of 09CEB904SAL revealed the presence of Salmonella genomic island 1 (SGI1) (2, 19, 27) and of the virulence plasmid S. Typhimurium pSLT (31). In addition, three β-lactamase genes—blaTEM-1, blaCMY-2, and blaCTX-M-3—were detected, the latter borne on a broad-host-range IncP conjugative plasmid together with the armA methyltransferase gene. To our knowledge, this is the first report of ArmA methyltransferase from food. This reflects a novel transmission route for armA and confirms presence of 16S rRNA methyltransferases in the East of Africa.
(An initial report of this study was presented in the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy in September 2009 in San Francisco, CA.)
MATERIALS AND METHODS
Bacterial strains and antimicrobial susceptibility testing.
09CEB904SAL, an S. enterica isolate, was recovered from chicken meat sampled during a control by the retailer in a supermarket on February 2009 in La Reunion, a French island in the Indian Ocean (22). The isolate was serotyped on the basis of the somatic O antigen and phase 1 and phase 2 flagellar H antigens, as specified by the White-Kauffmann-Le Minor scheme (18) using agglutination tests with antisera (obtained from Bio-Rad [Marnes-la-Coquette, France] and AES [Bruz, France]) according to an in-house method certified by the French Accreditation Committee (accreditation no. 1-0245). The absence of flagellar phase 2 antigens was confirmed following phase inversion method as recommended by EFSA (12). Escherichia coli K802N was used as the recipient strain for plasmid conjugation experiments. Antimicrobial susceptibility tests were first performed by disk diffusion method as recommended by the Clinical and Laboratory Standards Institute (CLSI) (6). MICs were performed, and results were interpreted according to CLSI guidelines (7).
DNA analysis and manipulation.
ESBL genes were detected by using Check KPC ESBL (Check-Points B.V., Wageningen, The Netherlands), a microarray using Clondiag technology (Jena, Germany) capable of detecting blaKPC and groups of blaCTX-M and discriminate ESBLs from non-ESBLs for blaTEM and blaSHV.
SGI1 was detected by amplifying the right and left junction and the class1 integron gene cassette as previously described (1, 26). The blaCMY-2 gene was detected as previously described (28). PCRs for all of the methylase genes were performed with the primers listed in Table 1. Plasmid DNA extraction was performed using a Plasmid Midi kit (Qiagen, Inc., Chatworth, CA). Tn1548 mapping was carried out using plasmid extractions as a template and by performing overlapping PCRs with nine pairs of designed primers along the transposon (Table 1 and Fig. 2). The PCR products were purified and subsequently sequenced with their corresponding primers.
Table 1.
Primers used in this study
| Primer | Sequence (5′–3′) | Positiona or amplicon size (bp) | Source or reference |
|---|---|---|---|
| Tn1F | GGCACTGTTGCAAATAGTCGG | 10326–10346 | This study |
| Tn1R | TTGCTGCTTGGATGCCCGAGG | 11981–12001 | This study |
| Tn2F | CCGGGTGACGCACACCGTGGA | 11784–11804 | This study |
| Tn2R | TCATTTACCAACTGACTTGAT | 12863–12883 | This study |
| Tn3F | TTATTCGCTTTGTGAAAGGCG | 12837–12857 | This study |
| Tn3R | TCCAGACGGCCACATTGGAGG | 14817–14837 | This study |
| Tn4F | TCAACGACCTCCTCCAATGTG | 14807–14827 | This study |
| Tn4R | ACCGCATGGGTTGTGGCATCC | 16787–16807 | This study |
| Tn5F | GGATGCCACAACCCATGCGGT | 16787–16807 | This study |
| Tn5R | ACAATAAGATTGTTGTACTTT | 18813–18833 | This study |
| Tn6F | TATGGGCAGGGCGAAGCGCTA | 18792–18812 | This study |
| Tn6R | TTTGAGTAAACTACTCTTTCC | 20773–20793 | This study |
| Tn7F | CGTATTGGTCTTGTGGGTGAT | 20743–20763 | This study |
| Tn7R | GTGAAATCTGCCCATAGAACA | 22722–22742 | This study |
| Tn8F | GGGAAAATGATTTGAAAATTA | 22589–22609 | This study |
| Tn8R | CTGCTATTGTGCCTTTAATTA | 24589–24609 | This study |
| Tn9F | TTGTTCTTCTAACCTAGTAAT | 24572–24592 | This study |
| Tn9R | CACTGTTGCAAAGTTAGCGAT | 25584–25604 | This study |
| armAF | CAAATGGATAAGAATGATGTT | 774 | 13 |
| armAR | TTATTTCTGAAATCCACT | 774 | 13 |
| rmtA.F | ATGAGCTTTGACGATGCCCTA | 756 | This study |
| rmtA.R | TCACTTATTCCTTTTTATCATG | 756 | This study |
| rmtB.F | ATGAACATCAACGATGCCCT | 769 | 36 |
| rmtB.R | CCTTCTGATTGGCTTATCCA | 769 | 36 |
| rmtC.F | CGAAGAAGTAACAGCCAAAG | 711 | 10 |
| rmtC.R | ATCCCAACATCTCTCCCACT | 711 | 10 |
| rmtD.F | CGGCACGCGATTGGGAAGC | 401 | 10 |
| rmtD.R | CGGAAACGATGCGACGAT | 401 | 10 |
| rmtE.F | ATGAATATTGATGAAATGGTTGC | 818 | 9 |
| rmtE.R | TGATTGATTTCCTCCGTTTTTG | 818 | 9 |
| U7-L12 | ACACCTTGAGCAGGGCAAAG | SGI1 left junction | 2 |
| LJ-R1 | AGTTCTAAAGGTTCGTAGTCG | SGI1 left junction | 2 |
| 104-RJ | TGACGAGCTGAAGCGAATTG | SGI1 right junction | 2 |
| C9L2 | AGCAAGTGTGCGTAATTT | SGI1 right junction | 2 |
| L1 | GGCATCCAAGCAGCAAG | 5′CS of class 1 integron | 26 |
| R1 | AAGCAGACTTGACCTGA | 3′CS of class 1 integron | 26 |
That is, the position in plasmid pMUR050 (AY522431).
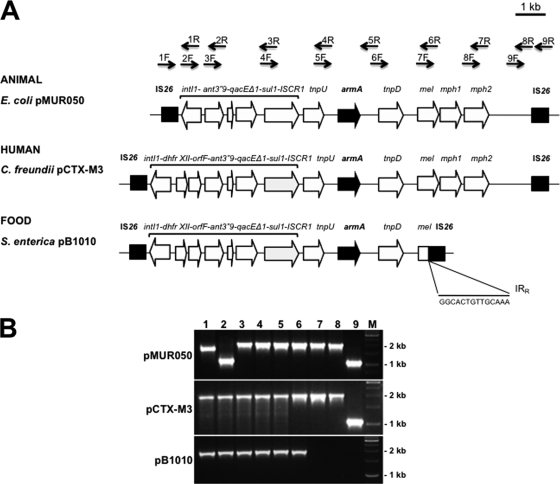
Fig. 2.
(A) Genetic structure of Tn1548 reported from an animal isolate (pMUR050), a human isolate (pCTX-M-3), and a food isolate of Salmonella enterica 09CEB904SAL (pB1010). Black arrows in the upper part of the panel represent pairs of primers designed for the mapping of Tn1548. (B) PCR fragments obtained in Tn1548 mapping with plasmids from an animal isolate (pMUR050), a human isolate (pCTX-M-3), and a food isolate (pB1010). Lanes 1 to 9 represent the corresponding pairs of primers. M, molecular weight marker. The PCR results for lanes 7, 8, and 9 are negative in pB1010.
Conjugation experiments.
Conjugation experiments were carried out in brain heart infusion (BHI) broth with 09CEB904SAL as the donor and E. coli K802N (which is resistant to nalidixic acid) as the recipient. Both strains were grown separately in BHI with moderate shaking until the donor strain reached an optical density at 600 nm of 0.9. Then, 1 ml of the donor culture, 1 ml of the recipient culture, and 1 ml of fresh BHI were mixed in a sterile flask, followed by incubation for 2 to 3 h at 37°C without shaking. Transconjugants were selected on BHI agar plates with nalidixic acid (50 μg/ml) and either ampicillin or gentamicin (50 μg/ml) to select for plasmid-encoded aminoglycosides and β-lactam resistance. Putative transconjugants were confirmed not to be nalidixic acid-resistant mutant donors by antimicrobial susceptibility testing and subsequent comparison with the donor and recipient resistance phenotype. armA PCR and S1 pulsed-field gel electrophoresis (S1-PFGE) were performed with the transconjugants. The conjugation frequency for each plasmid was calculated as the percentage of grown transconjugants bearing each plasmid versus the total conjugation frequency of the donors. To assess the possibility for the conjugation of more than one plasmid, 100 colonies grown using ampicillin-selected conjugation were replicated onto selective plates of gentamicin and amoxicillin-clavulanate in order to determine transconjugants with a plasmid bearing armA, blaCMY-2, or both genes.
Plasmid analysis.
In order to determine plasmid size, agarose gel plugs of total DNA were prepared according to the PulseNet PFGE protocol for the subtyping of E. coli O157:H7, Salmonella spp., and Shigella spp. (30). These plugs were digested with S1 nuclease (Promega, Madison, WI) according to the manufacturer's instructions. Plugs were loaded into a 1% agarose gel. PFGE was performed as previously described (32) with the following modifications: a running time of 21 h, a temperature of 14°C, a field strength of 6 V/cm, an included angle of 120°, an initial pulse time of 2.2 s, and a final pulse time of 63.8 s. The gels were stained with Sybr Safe (Invitrogen, Paisley, United Kingdom) for 20 min, destained in MilliQ water, and photographed under UV light. Lambda-ladder PFGE marker (New England Biolabs, Ipswich, MA) was used for molecular weight determinations. A PCR method based on five multiplex and three simplex PCRs was performed in order to determine the plasmid incompatibility group (4).
Nucleotide sequence accession number.
A partial sequence of the Tn1548 variant observed in the present study has been deposited in GenBank under accession number HQ728525.
RESULTS AND DISCUSSION
Identification and characterization of S. enterica 09CEB904SAL.
In February 2009, microbial analyses of food isolates in supermarkets from La Reunion Island identified a chicken meat sample with the presence of S. enterica 09CEB904SAL. This Salmonella strain was serotyped as S. enterica subspecies I.4,12:i:−. A complete antimicrobial profile showed that the bacterium possessed an unusual high-level multidrug resistance profile to ampicillin, amoxicillin-clavulanate, cephalothin, cefotaxime, ceftazidime, cefoxitin, streptomycin, kanamycin, gentamicin, sulfonamides, trimethoprim, tetracycline, and chloramphenicol (Table 2).
Table 2.
MICs, resistance genes, and plasmids
| Strain | MIC (mg/liter)a |
Resistance gene(s) | Plasmid(s) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMC | AMP | CTX | CAZ | CIP | GEN | KAN | SXT | TET | TMP | |||
| 00CEB904SAL | >128 | >32 | >4 | >16 | 0.06 | >256 | >128 | >1,024 | >64 | >32 | armA, blaCTX-M-3, blaTEM-1, blaCMY-2 | pB1008, pB1009, pB1010 |
| K802N | 2 | 4 | 0.12 | 0.5 | 0.25 | 0.5 | ≤4 | ≤8 | ≤1 | ≤0.5 | None | None |
| BB1065 | 4 | >32 | >4 | >16 | 0.25 | >256 | >128 | >1,024 | 64 | >32 | armA, blaCTX-M-3, blaTEM-1 | pB1010 |
| BB1064 | >128 | >32 | 4 | 16 | 0.25 | 0.5 | ≤4 | ≤8 | ≤1 | ≤0.5 | blaCMY-2 | pB1008 |
| BB1063 | >128 | >32 | >4 | 16 | 0.25 | >256 | >128 | >1,024 | >64 | >32 | armA, blaCTX-M-3, blaTEM-1, blaCMY-2 | pB1008, pB1010 |
AMC, amoxicillin-clavulanate; AMP, ampicillin; CTX, cefotaxime; CAZ, ceftazidime; CIP, ciprofloxacin; GEN, gentamicin; KAN, kanamycin; SXT, trimethoprim-sulfamethoxazole; TET, tetracycline; TMP, trimethoprim.
PCR for the SGI1 showed that this isolate bore the canonical right and left junctions from S. Typhimurium (Table 1). Integron cassette analysis identified two genes cassettes of 1,000 and 1,200 bp, which have been previously described in DT104 S. Typhimurium (2). PFGE analysis, performed as previously described (24), showed only 71% similarity with the subspecies I.4,12:i:− profile of the European epidemic clone STYMXB.0131 (24).
Identification and characterization of resistance determinants.
Conjugation experiments were performed using 09CEB904SAL as the donor, E. coli K802N as the recipient, and gentamicin, ampicillin, and nalidixic acid as the selection markers. Analysis of the transconjugants revealed presence of two different phenotypes corresponding with two types of transconjugants: (i) transconjugant BB1063 expressing high-level resistance to gentamicin, ampicillin, amoxicillin-clavulanate, cefotaxime, ceftazidime, cefoxitin, and sulfonamides (Table 2) and (ii) transconjugant BB1064 showing only high-level resistance to ampicillin, amoxicillin-clavulanate, and ceftazidime but not to aminoglycosides or sulfonamides. In order to study the high-level resistance to aminoglycosides and beta-lactams, primers targeting all of the 16S rRNA methyltransferase genes, as well as the main ESBL genes, were used with plasmid extractions of the wild-type bacterium and transconjugants as a template (Table 1). KPC ESBL microarray and conventional PCR and sequencing (21) confirmed the presence of a blaCTX-M-3 gene and a non-ESBL blaTEM-1 gene. DNA fragments corresponding to armA, blaCMY-2, blaTEM-1, and group 1 blaCTX-M-3 were amplified from the wild-type strain and sequenced. Concordantly, a non-ESBL blaTEM-1 and a group 1 blaCTX-M-3 were identified from the wild-type and transconjugant BB1063, whereas only blaCMY-2 was amplified from transconjugant BB1064, suggesting the presence of two different plasmids in 09CEB904SAL. To the best of our knowledge, this is the first report of armA gene in an isolate from food origin. Previously, armA has been identified from several Gram-negative pathogens as one of the most prevalent 16S rRNA methyltransferase genes (20, 37). To date, most 16S rRNA methyltransferase-producing Gram-negative bacteria were recovered from human clinical samples. However, in the case of armA, it has also been found in porcine and chicken E. coli isolates from Spain and China, respectively (11, 17).
Plasmid analysis.
As mentioned before, conjugation experiments suggested that 09CEB904SAL bore at least two transferable plasmids. S1-PFGE analyses revealed the presence of three plasmids of approximately 50, 80, and 110 kb that were named pB1008, pB1009, and pB1010, respectively (Fig. 1). The plasmids were classified according to a PCR-based replicon typing protocol (4). The 110-kb plasmid (pB1010) belonged to the IncP incompatibility group, whereas the 50-kb (pB1008) and 80-kb (pB1009) plasmids were not typeable using this methodology. Interestingly, this is the first time that the armA gene has been documented on an IncP plasmid, despite the fact that IncP plasmids are broad-host-range plasmids, and they have been widely described since the 1980s among several genus of Proteobacteria generally associated with antibiotic resistance determinants (33, 34). This finding suggests that further spread of armA to other strains could be enhanced from this genetic platform, since genes linked to IncP plasmids have a large host range, including Pseudomonas spp. or even Gram-positive bacteria. To precisely calculate the conjugation frequency of plasmid pB1010 bearing armA, blaTEM-1, and blaCTX-M-3 and plasmid pB1008 bearing blaCMY-2, a conjugation experiment using ampicillin was performed. Transconjugants were replicated onto gentamicin and amoxicillin-clavulanate plates, since resistance to the latter is due to blaCMY-2. Plasmid pB1010 was transferred into E. coli at a frequency of 9 × 10−4 (giving rise to transconjugant BB1065), whereas pB1008 was transferred at 4 × 10−4 per donor CFU. The two plasmids were cotransferred at a frequency of 10−5 per donor CFU. No resistance determinant was identified on plasmid pB1009, but the spvC gene, which is characteristic of pSLT, the virulence plasmid of S. Typhimurium, was detected by PCR (3) in 09CEB904SAL, with plasmids pB1008, pB1009, and pB1010, but not in the transconjugants (which lack pB1009), suggesting that pB1009 could be a pSLT derivative.
Fig. 1.
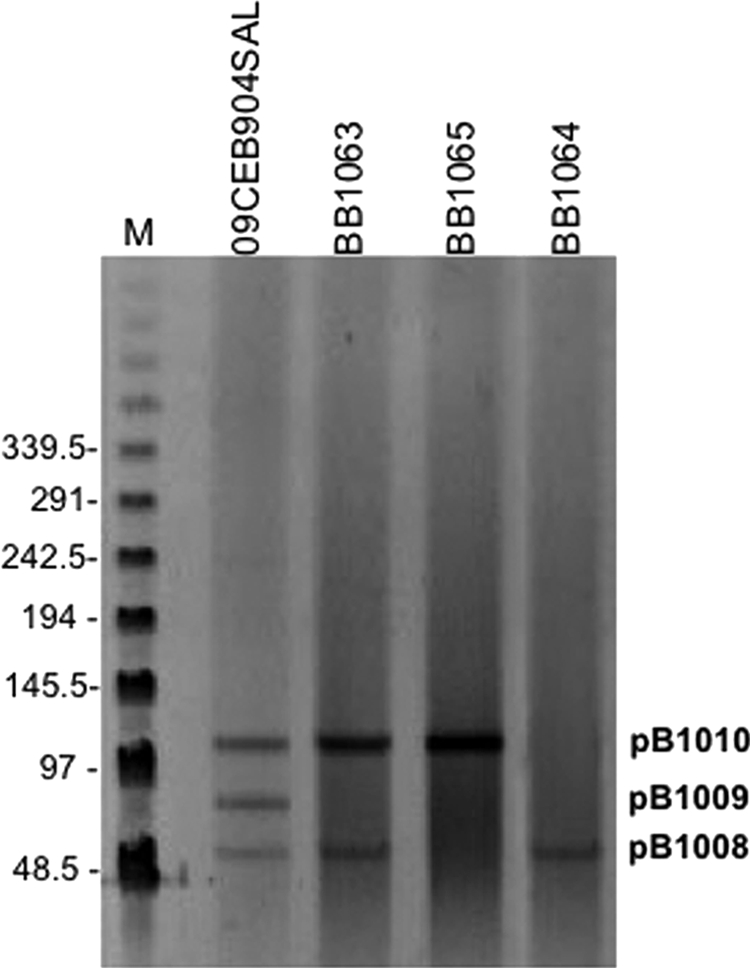
Plasmid profile of the strains used in the present study. M, lambda ladder molecular marker. The numbers on the left show molecular weights in kilobase pairs. Salmonella enterica 09CEB904SAL and transconjugants BB1063, BB1065, and BB1064 are indicated in lanes 2 to 5, respectively. Each band represents one plasmid. S. enterica 09CEB904SAL bears pB1008, pB1009, and pB1010. The image reveals three types of transconjugants, one with pB1008 alone, one with pB1010 alone, and one with both pB1008 and pB1010.
Mapping of Tn1548.
Tn1548 is the genetic platform that mobilizes armA between plasmids (14, 16). For this reason, a PCR mapping method for Tn1548 was developed by designing nine pairs of primers that amplify overlapping PCR products. PCR fragments for the expected size were amplified from the control plasmids pCTX-M3 and pMUR050 (15, 17). In Salmonella 09CEB904SAL, Tn1548 harbors the dhfrXII trimethoprim resistance cassette, like the strains bearing armA identified in clinical settings (13), whereas in the first E. coli isolate described with armA and in animals, Tn1548 lacked this cassette (16). Furthermore, in 09CEB904SAL, PCRs for the amplification of the downstream region of the transposon, corresponding to the pairs 7, 8, and 9 were negative. Suspecting the possibility of a deletion in that region, a long PCR with primers Tn6F and Tn9R was performed. An amplicon of ∼3 kb was obtained, instead of the 7-kb amplicon that would correspond to the complete Tn1548 element. Subsequent sequencing of this DNA fragment with the primers Tn6F and Tn9R revealed insertion of an IS26 element with its IRR region interrupting the macrolide resistance genes downstream armA. These data show that Tn1548 is a dynamic element that is ultimately responsible for the spread of armA.
Overall, these results confirm the presence of armA in East Africa and imply a novel route of transmission for this emerging resistance determinant. Further surveillance in food-borne bacteria will be crucial to determine the role of food products in the spread of the 16S rRNA methyltransferase genes worldwide.
ACKNOWLEDGMENTS
This study was supported by project BIO2010/20204 from the Ministry of Science and Innovation (MICINN), by work package 29 from the MedVetNet Network of Excellence (FOOD-CT-2004-506122), and by the Programa de Vigilancia Sanitaria 2009 AGR/4189 of the Comunidad de Madrid (Madrid, Spain). L.H. and B.G. acknowledge the Comunidad de Madrid and the Universidad Complutense de Madrid for their respective fellowships.
We thank Jeremy Vivancos and Isabelle Henry for help in the local investigation, Marie Bugarel for helpful advice and technical support, and Natalia Montero for excellent technical assistance.
Footnotes
Published ahead of print on 22 August 2011.
REFERENCES
- 1. Amar C. F., et al. 2008. Real-time PCRs and fingerprinting assays for the detection and characterization of Salmonella genomic island-1 encoding multidrug resistance: application to 445 European isolates of Salmonella, Escherichia coli, Shigella, and Proteus. Microb. Drug Resist. 14:79–92 [DOI] [PubMed] [Google Scholar]
- 2. Boyd D., et al. 2001. Complete nucleotide sequence of a 43-kilobase genomic island associated with the multidrug resistance region of Salmonella enterica serovar Typhimurium DT104 and its identification in phage type DT120 and serovar Agona. J. Bacteriol. 183:5725–5732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bugarel M., Granier S. A., Weill F. X., Fach P., Brisabois A. 2011. A multiplex real-time PCR assay targeting virulence and resistance genes in Salmonella enterica serotype Typhimurium. BMC Microbiol. 11:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carattoli A., et al. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228 [DOI] [PubMed] [Google Scholar]
- 5. Chen L., et al. 2007. Emergence of RmtB methylase-producing Escherichia coli and Enterobacter cloacae isolates from pigs in China. J. Antimicrob. Chemother. 59:880–885 [DOI] [PubMed] [Google Scholar]
- 6. Clinical and Laboratory Standards Institute 2006. M2–A9: performance standards for antimicrobial disk susceptibility tests; approved standards, 9th ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7. Clinical and Laboratory Standards Institute 2011. M100–S21: performance standards for antimicrobial susceptibility testing; 21st informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8. Davies J., Wright G. D. 1997. Bacterial resistance to aminoglycoside antibiotics. Trends Microbiol. 5:234–240 [DOI] [PubMed] [Google Scholar]
- 9. Davis M. A., et al. 2010. Discovery of a gene conferring multiple-aminoglycoside resistance in Escherichia coli. Antimicrob. Agents Chemother. 54:2666–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doi Y., Arakawa Y. 2007. 16S rRNA methylation: emerging resistance mechanism against aminoglycosides. Clin. Infect. Dis. 45:88–94 [DOI] [PubMed] [Google Scholar]
- 11. Du X. D., et al. 2009. Plasmid-mediated ArmA and RmtB 16S rRNA methylases in Escherichia coli isolated from chickens. J. Antimicrob. Chemother. 64:1328–1330 [DOI] [PubMed] [Google Scholar]
- 12. EFSA 2010. Scientific opinion on monitoring and assessment of the public health risk of “Salmonella Typhimurium-like” strains. EFSA J. 8:48 [Google Scholar]
- 13. Galimand M., Courvalin P., Lambert T. 2003. Plasmid-mediated high-level resistance to aminoglycosides in Enterobacteriaceae due to 16S rRNA methylation. Antimicrob. Agents Chemother. 47:2565–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galimand M., Sabtcheva S., Courvalin P., Lambert T. 2005. Worldwide disseminated armA aminoglycoside resistance methylase gene is borne by composite transposon Tn1548. Antimicrob. Agents Chemother. 49:2949–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Golebiewski M., et al. 2007. Complete nucleotide sequence of the pCTX-M3 plasmid and its involvement in spread of the extended-spectrum beta-lactamase gene blaCTX-M-3. Antimicrob. Agents Chemother. 51:3789–3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gonzalez-Zorn B., et al. 2005. Genetic basis for dissemination of armA. J. Antimicrob. Chemother. 56:583–585 [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez-Zorn B., et al. 2005. armA and aminoglycoside resistance in Escherichia coli. Emerg. Infect. Dis. 11:954–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grimont P. A. D., Weill F. X. 2007. Antigenic formulae of the Salmonella serovars. WHO Collaborating Centre for Reference and Research on Salmonella, World Health Organization, Geneva, Switzerland [Google Scholar]
- 19. Guerra B., Junker E., Miko A., Helmuth R., Mendoza M. C. 2004. Characterization and localization of drug resistance determinants in multidrug-resistant, integron-carrying Salmonella enterica serotype Typhimurium strains. Microb. Drug Resist. 10:83–91 [DOI] [PubMed] [Google Scholar]
- 20. Gurung M., et al. 2010. Emergence of 16S rRNA methylase gene armA and cocarriage of blaIMP-1 in Pseudomonas aeruginosa isolates from South Korea. Diagn. Microbiol. Infect. Dis. 68:468–470 [DOI] [PubMed] [Google Scholar]
- 21. Gutierrez B., et al. 2009. Novel genetic environment of qnrB2 associated with TEM-1 and SHV-12 on pB1004, an IncHI2 plasmid, in Salmonella Bredeney BB1047 from Spain. J. Antimicrob. Chemother. 64:1334–1336 [DOI] [PubMed] [Google Scholar]
- 22. Henry I., et al. 2009. Proceedings of the International Symposium on Salmonella and Salmonellosis, St. Malo, France, p. 283–284 [Google Scholar]
- 23. Hopkins K. L., Escudero J. A., Hidalgo L., Gonzalez-Zorn B. 2010. 16S rRNA methyltransferase RmtC in Salmonella enterica serovar Virchow. Emerg. Infect. Dis. 16:712–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hopkins K. L., et al. 2010. Multiresistant Salmonella enterica serovar 4,[5],12:i:− in Europe: a new pandemic strain? Eur. Surveill. 15:19580. [PubMed] [Google Scholar]
- 25. Jiang Y., et al. 2010. Complete nucleotide sequence of Klebsiella pneumoniae multidrug resistance plasmid pKP048, carrying blaKPC-2, blaDHA-1, qnrB4, and armA. Antimicrob. Agents Chemother. 54:3967–3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levings R. S., Partridge S. R., Lightfoot D., Hall R. M., Djordjevic S. P. 2005. New integron-associated gene cassette encoding a 3-N-aminoglycoside acetyltransferase. Antimicrob. Agents Chemother. 49:1238–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mulvey M. R., Boyd D. A., Olson A. B., Doublet B., Cloeckaert A. 2006. The genetics of Salmonella genomic island 1. Microbes Infect. 8:1915–1922 [DOI] [PubMed] [Google Scholar]
- 28. Mulvey M. R., et al. 2005. Molecular characterization of cefoxitin-resistant Escherichia coli from Canadian hospitals. Antimicrob. Agents Chemother. 49:358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poirel L., Revathi G., Bernabeu S., Nordmann P. 2010. Detection of NDM-1-producing Klebsiella pneumoniae in Kenya. Antimicrob. Agents Chemother. 55:934–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ribot E. M., et al. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 3:59–67 [DOI] [PubMed] [Google Scholar]
- 31. Sanderson K. E., Hessel A., Rudd K. E. 1995. Genetic map of Salmonella typhimurium, edition VIII. Microbiol. Rev. 59:241–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. San Millán A., et al. 2007. Beta-lactam resistance in Haemophilus parasuis is mediated by plasmid pB1000 bearing blaROB-1. Antimicrob. Agents Chemother. 51:2260–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schlüter A., Szczepanowski R., Pühler A., Top E. M. 2007. Genomics of IncP-1 antibiotic resistance plasmids isolated from wastewater treatment plants provides evidence for a widely accessible drug resistance gene pool. FEMS Microbiol. Rev. 31:449–477 [DOI] [PubMed] [Google Scholar]
- 34. Thomas C. M., Smith C. A. 1987. Incompatibility group P plasmids: genetics, evolution, and use in genetic manipulation. Annu. Rev. Microbiol. 41:77–101 [DOI] [PubMed] [Google Scholar]
- 35. Wachino J., et al. 2007. Novel plasmid-mediated 16S rRNA m1A1408 methyltransferase, NpmA, found in a clinically isolated Escherichia coli strain resistant to structurally diverse aminoglycosides. Antimicrob. Agents Chemother. 51:4401–4409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yan J. J., et al. 2004. Plasmid-mediated 16S rRNA methylases conferring high-level aminoglycoside resistance in Escherichia coli and Klebsiella pneumoniae isolates from two Taiwanese hospitals. J. Antimicrob. Chemother. 54:1007–1012 [DOI] [PubMed] [Google Scholar]
- 37. Zhou Y., et al. 2010. Distribution of 16S rRNA methylases among different species of Gram-negative bacilli with high-level resistance to aminoglycosides. Eur. J. Clin. Microbiol. Infect. Dis. 29:1349–1353 [DOI] [PubMed] [Google Scholar]