Abstract
Background
Hydrogen sulfide (H2S) displays vasodilative, anti-oxidative, anti-inflammatory and cytoprotective activities. Impaired production of H2S contributes to the increased intrahepatic resistance in cirrhotic livers. The study aimed to investigate the roles of H2S in carbon tetrachloride (CCl4)-induced hepatotoxicity, cirrhosis and portal hypertension.
Methods and Findings
Sodium hydrosulfide (NaHS), a donor of H2S, and DL-propargylglycine (PAG), an irreversible inhibitor of cystathionine γ-lyase (CSE), were applied to the rats to investigate the effects of H2S on CCl4-induced acute hepatotoxicity, cirrhosis and portal hypertension by measuring serum levels of H2S, hepatic H2S producing activity and CSE expression, liver function, activity of cytochrome P450 (CYP) 2E1, oxidative and inflammatory parameters, liver fibrosis and portal pressure. CCl4 significantly reduced serum levels of H2S, hepatic H2S production and CSE expression. NaHS attenuated CCl4-induced acute hepatotoxicity by supplementing exogenous H2S, which displayed anti-oxidative activities and inhibited the CYP2E1 activity. NaHS protected liver function, attenuated liver fibrosis, inhibited inflammation, and reduced the portal pressure, evidenced by the alterations of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), hyaluronic acid (HA), albumin, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and soluble intercellular adhesion molecule (ICAM)-1, liver histology, hepatic hydroxyproline content and α-smooth muscle actin (SMA) expression. PAG showed opposing effects to NaHS on most of the above parameters.
Conclusions
Exogenous H2S attenuates CCl4-induced hepatotoxicity, liver cirrhosis and portal hypertension by its multiple functions including anti-oxidation, anti-inflammation, cytoprotection and anti-fibrosis, indicating that targeting H2S may present a promising approach, particularly for its prophylactic effects, against liver cirrhosis and portal hypertension.
Introduction
Hydrogen sulfide (H2S) has displayed many physiological and pathological activities [1]. Administration of H2S limited myocardial infarct size caused by ischemia reperfusion injury (IRI) [2], [3], suppressed development of gastric ulcer [4], alleviated hypoxic pulmonary hypertension [5], attenuated neuronal injury [6], and prevented the development of hypertension [7]. An H2S-releasing molecule, GYY4137, protected endotoxic shock by decreasing the production of proinflammatory cytokines including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) [8]. The multiple actions of H2S mainly involve inhibiting oxidative stress [9], production of lipid peroxidation and inflammatory factors [4], and activating ATP sensitive potassium (KATP) channels [10].
H2S has also shown regulatory effects on hepatic physiology and pathology [11]. H2S is endogenously produced in mammalian tissues by cystathionine γ-lyase (CSE) and cystathionine β-synthase (CBS) [1], but CBS accounts for only 3% of H2S production in livers [12]. CSE-derived H2S contributes to hepatic arterial buffer response, mediates vasorelaxation of the hepatic artery via activation of KATP channels [13], and modulates biliary bicarbonate excretion [14]. H2S has exhibited anti-inflammatory and cytoprotective activities against hepatic IRI [15], and protected acetaminophen-induced hepatotoxicity in mice [16]. Diallyl trisulfide, an H2S-releasing chemical, protected from carbon tetrachloride (CCl4)-induced liver injury [17], [18].
The reduction of H2S production and CSE expression is related to the development of increased intrahepatic resistance and portal hypertension in a rat model of liver cirrhosis induced by CCl4 [19], and H2S counteracts the impaired vasodilation and hepatic stellate cell contraction, which contribute to the dynamic component of portal hypertension [20]. These two studies have focused on the regulatory effects of H2S in already established portal hypertension mainly by its vasodilative activities via activating KATP channels. As mentioned in one report [20], liver fibrosis represents the main causative factor in portal hypertension in cirrhosis, but the role of H2S in the development of cirrhosis remains unclear. CCl4 has been widely used to induce liver injury and cirrhosis in animal models, as it is rapidly metabolized into the trichloromethyl radical by cytochrome P450 (CYP) 2E1, and the reactive intermediate attacks membrane lipids, resulting in the formation of lipid peroxide molecules and necrosis of hepatocytes [21]. Given that H2S displays anti-oxidative, anti-inflammatory and cytoprotective activities, an anti-fibrotic effect against pulmonary fibrosis [22], and a regulatory effect on hypoxic pulmonary hypertension [23] and portal hypertension [19], [20], we hypothesized that H2S might have a protective effect against CCl4-induced acute hepatotoxicity, and the resulting liver cirrhosis and portal hypertension.
Methods
Ethics Statement
All the procedures and care administered to the animals have been approved by the institutional ethic committee, under a permit of animal use (SYXK20020009) in the First Affiliated Hospital of Harbin Medical University, compliance with the Experimental Animal Regulations by the National Science and Technology Commission, China.
Animals
Male Wistar rats (weighing 210-230 g) were supplied by the Animal Research Center at the First Affiliated Hospital of Harbin Medical University, Harbin, China. They were housed in the animal facility with a 12-h-light/-dark cycle and the temperature was maintained at 22-23°C.
Acute hepatotoxicity experiment
The rats received phenobarbital sodium (0.35 g/L) in drinking water for 3 days, followed by a single i.p. injection of CCl4 (Nanjing Chem. Ltd., Nanjing, China) diluted in equal volume of paraffin oil at a dose of 3 ml/kg body weight. Then the rats were randomly assigned to 3 groups (each group had 6 rats): control, NaHS and PAG, receiving an i.p. injection of 1 ml of physiological saline, NaHS (Sigma-Aldrich) solution (14 µmol/kg body weight), or PAG (Sigma-Aldrich) solution (50 mg/kg body weight), respectively. The injection was repeated every 6 h. The rats were killed 48 h after CCl4 administration. Six untreated rats served as healthy controls. Blood was collected via cardiac puncture, and serum was prepared by centrifugation at 2000 × g for 10 min, and stored at -80°C. Livers were divided into two parts, one fixed in 10% buffered formalin, the other stored at -80°C.
Induction of liver cirrhosis
Liver cirrhosis was induced by CCl4 as described previously [20], [21]. Briefly, rats received phenobarbital sodium (0.35 g/L) in drinking water for 3 days, followed by i.p. injections of 1 ml/kg body weight of CCl4 diluted in an equal volume of paraffin oil twice a week for 12 weeks.
Prevention experiment
Thirty-six rats receiving i.p. injections of CCl4 as above were randomly assigned into 3 groups (each had 12 rats): saline, NaHS and PAG, receiving i.p. injections of 1 ml of saline, NaHS solution (10 µmol/kg body weight) and PAG solution (30 mg/kg body weight), respectively, every two days for 12 weeks, starting on the same day of CCl4 administration. 300 µl of blood samples were collected by tail tipping at indicated time points. At completion of the experiments, the rats were weighed, and the portal pressure measured as below, and 300 µl of blood sample was collected from the portal vein. The spleen was weighed as % of bodyweight and used as a parameter for portal hypertension. Blood was collected via cardiac puncture, and then the liver and spleen were collected. Serum and liver samples were prepared and stored as above.
Measurement of portal pressure
The methodology has been described previously [24]. Briefly, under anesthesia by an i.p. injection of sodium pentobarbital (50 mg/kg body weight), rats underwent laparotomy, and a PE-tube (23G, 0.6×30 mm) was inserted over the ileocolic vein and advanced toward the confluence of the portal and splenic veins. This cannula was used to monitor portal pressure for 5 min by a Medlab-Ug4Cs bio-signal processing system (Nanjing Medease Science and Technology co., Ltd, Nanjing, China).
Treatment experiment
Rats with liver cirrhosis induced by 12-week administration of CCl4 as above were randomly assigned to 3 groups (each had 6 rats): saline, NaHS and PAG, receiving daily injection of 1 ml of saline, NaHS solution (10 µmol/kg) and PAG solution (30 mg/kg), respectively, for 5 days. At completion of the experiments, the portal pressure measured, and then serum and liver samples were prepared.
Measuring H2S production in livers and H2S concentration in sera
The methods have been described previously [15], [25]. All samples were assayed in triplicate and H2S was calculated against a calibration curve of NaHS (0.122–250 µmol/L). The H2S producing activity was expressed as µmole of H2S formed/g tissues.
RT-PCR analysis
The analysis of CES mRNA by RT-PCR has been described previously [15]. A pair of primers 5′- GAC CTC AAT AGT CGG CTT CGT TTC -3′ and 5′- CAG TTC TGC GTA TGC TCC GTA ATG -3′ to generate a 618 bp product of CSE mRNA. A 497 bp PCR product was amplified from glyceraldehyde 3-phosphate dehydrogenase (GAPDH) cDNA as an internal control, with a pair of primers: 5′-GAAGGTGAAGGTCGGAGT-3′ and 3′-TGAAACCATAGCACCTTCC-5′. PCR products underwent a 2% agarose gel electrophoresis, and the density of each band was evaluated using the gel image analyzer (UV ChemiDOC, USA). The relative density of mRNA levels was calculated as the following formula: band density/ GAPDH band density.
Measurement of serum parameters
The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), hyaluronic acid (HA) and albumin were measured with an auto-biochemical analyzer (Toshiba, Japan). The serum levels of TNF-α, IL-6, interleukin-1β (IL-1β) and soluble intercellular adhesion molecule-1 (ICAM-1) were measured with ELISA kits (BPB Biomedicals, Inc., USA).
Measurement of CYP2E1 activity in livers
The CYP2E1 activity was assayed using p-nitrophenol (Sigma-Aldrich) as a substrate as described previously [26].
Measurement of hepatic malondialdehyde (MDA) and glutathione (GSH)
Liver tissue was homogenized in a buffer containing 9 volumes of 0.15 mol/L KCl-1.0 mmol/L EDTA to obtain 1∶10 (w/v) homogenates. Homogenates were then centrifuged at 10,000 g (4°C) for 30 min to collect the supernatant for determining the concentrations of MDA, GSH and total protein. MDA was evaluated by the thiobarbituric acid reactive substances (TBARS) method [27]. The final concentration of MDA was expressed as µmol/g protein. GSH were measured using commercially available kits (Jiancheng Institute of Biotechnology, Nanjing, China) and expressed as nmol/mg protein. Protein concentration of liver homogenate was determined by the Bradford method, using bovine serum albumin as a standard [28].
Measurement of hepatic hydroxyproline
Hepatic hydroxyproline was measured as described previously [29].
Histological analysis
Formalin-fixed liver specimens were embedded in paraffin, sectioned, stained with hematoxylin and eosin (HE) or Masson, and examined under light microscopy. Quantifying liver fibrosis was performed by measuring blue pixels of the images taken from Masson-stained sections. Ten photographs (200 × magnification) were randomly taken from each liver at fixed exposure time and conditions. The pictures were saved as JPEG (Joint photographic experts group). The histogram function in Adobe Photoshop CS4 was used to count the blue pixels, and the numbers of red pixels were recorded for each picture.
Western Blot analysis
The methodology has been described previously [15]. The Abs against CSE or α-smooth muscle actin (α-SMA) (Santa Cruz Biotechnology, Inc. Santa Cruz, CA) were used. The levels of proteins were normalized with respect to band density of β-actin, as an internal control.
Statistics
Results were expressed as mean values ± standard deviation (SD). A one-way analysis of variance (ANOVA) followed by the post-hoc Dunnett's test was used for evaluating statistical significance (SPSS 17.0). A value of P<0.05 was considered significant.
Results
H2S protects livers from CCl4-induced acute toxicity
Administration of NaHS significantly attenuated, while PAG further raised, the elevated serum levels of ALT and AST by CCl4 (Table 1). The impaired liver function by CCl4 and the protective effects of H2S were supported by the histological alterations (Figure S1). The liver sections from healthy control rats showed normal histology (Figure S1A). CCl4-treated livers had focal midzonal necrosis and ballooning degeneration (Figure S1B). NaHS attenuated the necrosis and vacuolization (Figure S1C), while PAG further aggravated the hepatotoxicity and caused more inflammatory cells infiltration (Figure S1D). NaHS also significantly reduced the activity of CYP2E1, the major enzyme to metabolize CCl4, in livers from CCl4-treated rats, compared with saline, while either CCl4 or PAG had no effects on its activity (Table 1). CCl4-treated rats had a significant higher level of hepatic MDA (a marker of lipid peroxidation) and a significantly lower level of hepatic GSH, compared to healthy controls. NaHS significantly attenuated the increase of hepatic MDA, and restored hepatic GSH, but PAG only had slight effects on hepatic MDA and GSH, in CCl4-treated rats, compared with saline (Table 1).
Table 1. Serum ALT and AST, and hepatic CYP2E1, MDA and GSH in hepatotoxicity experiment*.
| Healthy control | CCl4 | |||
| saline | NaHS | PAG | ||
| (n = 6) | (n = 6) | (n = 6) | (n = 6) | |
| Serum ALT (IU/L) | 36.2±7.5 | 245.8±36.5a | 149.6±25.7a,b | 268.7±46.9a,b |
| Serum AST (IU/L) | 78.9±11.4 | 657.0±96.1a | 427.6±102.3a,b | 835.2±133.5a,b |
| Liver CYP2E1(µmol/g protein) | 2.8±0.2 | 2.7±0.4 | 1.4±0.3a,b | 3.1±1.0 |
| Liver MDA (µmol/g protein) | 1.6±0.3 | 5.9±1.2a | 3.8±0.7a,b | 6.3±1.5a |
| Liver GSH (µmol/g protein) | 11.5±2.4 | 5.7±1.8a | 10.7±3.0a,b | 4.6±1.1a |
*The blood and liver samples were collected from the rats 48 h after CCl4 administration. Data are expressed as means ± SD, and statistical analysis was performed by one-way ANOVA analysis followed by the post-hoc Dunnett's test.
Significant difference (P<0.05) from healthy controls.
Significant difference (P<0.05) from saline -treated rats.
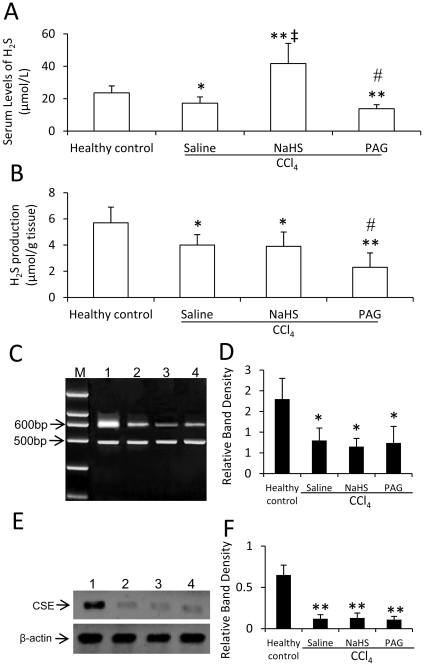
Serum levels of H2S, production of H2S and CSE expression in the acute experiment
The serum levels of H2S in CCl4 + saline-treated rats were significantly lower than that in healthy controls (P<0.05) (Fig. 1A), supported by that CCl4 significantly (P <0.05) reduced hepatic H2S producing activity (Fig. 1B). Hepatic expressions of CSE mRNA (Fig. 1C and D) and protein (Fig. 1E and F) were significantly (Both P<0.05) lower in CCl4-treated rats than that in healthy controls. NaHS significantly (P<0.001) increased the levels of H2S (Fig. 1A), but had no effect on hepatic H2S producing activity (Fig. 1B) and the expression of CSE mRNA (Fig. 1C and D) and protein (Fig. 1E and F), in CCl4-treated rats, compared saline. However, PAG significantly (Both P<0.05) reduced the levels of H2S (Fig. 1A) and H2S producing activity (Fig. 1B), but had no effect on the expression of CSE mRNA (Fig. 1C and D) and protein (Fig. 1E and F), in CCl4-treated rats, compared with saline.
Figure 1. Serum levels of H2S, H2S production and CSE expression in CCl4-induced acute hepatotoxicity.
Healthy rats, or CCl4-treated rats receiving administrations of saline, NaHS or PAG, were killed 48 h after CCl4 administration, blood and liver samples were collected. The levels of H2S in sera (A) and H2S producing activity in livers (B) were measured. (C-F) The expression of CSE mRNA (C, D) and protein (E, F) was detected in liver tissues from healthy rats (lane 1), or CCl4-treaed rats receiving administration of saline (lane 2), NaHS (lane 3) or PAG (lane 4), by RT-PCR (C, D) or Western blot analysis (E, F), respectively. M, DNA marker. (D) The density of each band from (C) was measured and compared to that of the internal control, GAPDH. (F) The density of each band from (E) was measured and compared to that of the internal control, β-actin. Results are expressed as mean ± SD (n = 6). Compared to the healthy controls, a significant difference is denoted by “*” , and a highly significant difference by “**” (P<0.001). Compared to rats treated with CCl4 + saline, a significant increase is denoted by “‡”, and a significant reduction is denoted by “#”.
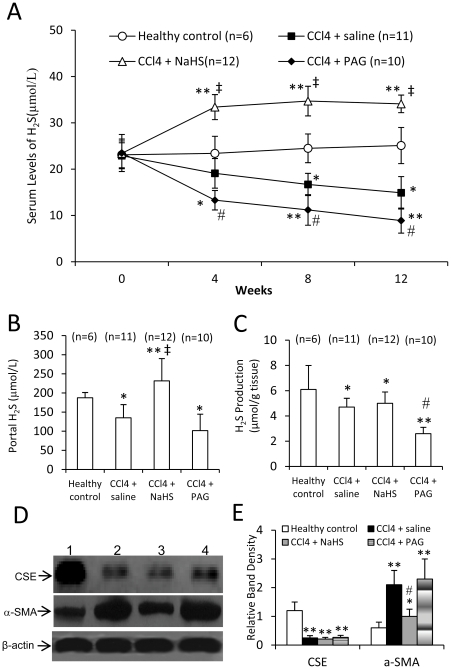
H2S production in cirrhotic rats induced by CCl4
Among the rats in the prevention experiment, one from the CCl4 + saline group and two from the CCl4 + PAG group died before completion of the experiment, and were excluded from the study. Blood samples were collected at indicated time points to measure the serum levels of H2S in rats. The healthy rats had a stable serum level of H2S, while the H2S levels in CCl4-induced cirrhotic rats receiving injection of saline declined at week 4 after CCl4 administration, and continued dropping as the H2S levels were even lower at week 8 and 12 than that at week 4 (Fig. 2A). However, NaHS significantly (P<0.05) elevated the serum level of H2S at week 4 after CCl4 administration in CCl4-induced cirrhotic rats, compared with saline, and serum H2S was maintained at a higher level at week 8 and 12 (Fig. 2A). Administration of PAG resulted in significantly (All P<0.05) lower serum levels of H2S in CCl4-induced cirrhotic rats, than saline, at all the three time points (Fig. 2A). The portal serum levels of H2S at the completion of experiment was significantly (P<0.05) lower in CCl4-induced cirrhotic rats receiving injection of saline than the healthy controls (Fig. 2B). However, NaHS significantly (P<0.05) elevated, but PAG did not significantly (P>0.05) decrease, the portal serum levels of H2S, in CCl4-induced cirrhotic rats, compared with saline (Fig. 2B). We further examined the H2S producing activity (Fig. 2 C) in livers from CCl4-induced cirrhotic rat receiving injections of saline, NaHS or PAG, and the similar results were obtained as shown in the acute hepatotoxicity experiment (Fig. 1B), supported by the results of hepatic CES expression (Fig. 2D and E), where CCl4 significantly downregulated, while NaHS or PAG had no effect on, the expression of CES.
Figure 2. Serum H2S levels, H2S production, and expression of CSE and α-SMA in the prevention experiment.
The rats were treated with CCl4 + saline, CCl4 + NaHS or CCl4 + PAG for 12 weeks. Untreated rats served as healthy controls. (A) Blood samples were collected at indicated time points, and the serum levels of H2S were measured. The statistical comparison between two groups was done at the respective time point. (B-E) The rats were killed at the completion of experiment. Blood samples were collected from portal vein, and livers harvested. The levels of H2S in sera from the portal vein (B), and H2S producing activity in livers (C) were measured. (D) The expression of CSE and α-SMA was detected in livers from healthy controls (lane 1), or rats treated saline+CCl4 (lane 2), NaHS+CCl4 (lane 3) or PAG+CCl4 (lane 4) by Western blot analysis. (E) The density of each band was measured and compared to that of the internal control, β-actin. Results are expressed as mean ± SD. n, number of samples. Compared to the healthy controls, a significant difference is denoted by “*”, a highly significant difference by “**” (P<0.001). Compared to saline + CCl4-treated rats, a significant increase is denoted by “‡”, and a significant reduction is denoted by “#”.
H2S attenuates the impaired liver function and elevated inflammatory factors in cirrhotic rats
Cirrhotic rats induced by 12-week administration of CCl4 had significantly higher levels of ALT, AST, HA and lower level of albumin, than healthy controls (Table 2). However, simultaneous administration of NaHS resulted in significantly lower levels of ALT, AST and HA, and higher level of albumin, while PAG led to significantly higher levels of AST and HA, slight increase of ALT level and slight reduction of albumin level, in CCl4-induced cirrhotic rats, compared with saline (Table 2).
Table 2. Serum biochemicals and inflammatory factors in cirrhotic rats*.
| Healthy control | CCl4 + saline | CCl4 + NaHS | CCl4 + PAG | |
| (n = 6) | (n = 11) | (n = 12) | (n = 10) | |
| ALT (IU/L) | 34.4±8.9 | 198.8±26.7a | 137.6±20.3a,b | 208.5±36.4a |
| AST(IU/L) | 80.3±12.5 | 466.7±46.4a | 317.5±78.2a,b | 515.0±97.6a,b |
| HA (µg/L) | 113.5±20.1 | 482.6±51.4a | 296.3±32.1a,b | 549.0±47.3a,b |
| Albumin (g/L) | 472.5±43.8 | 314±51.6a | 443.7±80.5a,b | 294.3±32.8a |
| TNF-α (ng/L) | 43.9±6.8 | 143.5±21.4a | 109.3±22.6a,b | 176.3±28.5a,b |
| IL-1β(ng/L) | 20.3±5.0 | 75.4±10.2a | 50.6±9.8a,b | 80.2±17.4a |
| IL-6 (ng/L) | 87.0±15.9 | 297.1±55.4a | 216.4±42.1a,b | 320.6±51.4a |
| ICAM-1(ng/L) | 69.2±8.7 | 180.8±19.7a | 153.6±22.8a,b | 207.2±31.3a,b |
*Data are expressed as means ± SD, and statistical analysis was performed by one-way ANOVA analysis followed by the post-hoc Dunnett's test.
Significant difference (P<0.05) from healthy controls.
Significant difference (P<0.05) from CCl4+saline-treated rats.
Cirrhotic rats had significantly higher levels of proinflammatory cytokines including TNF-α, IL-1β and IL-6, and soluble ICAM-1 (Table 2), which have been shown to play important roles in development of cirrhosis. However, simultaneous administration of NaHS resulted in significantly lower levels of all the four parameters in CCl4-induced cirrhotic rats than saline, while PAG led to significantly higher levels of TNF-α and soluble ICAM, and slight increase of IL-1β and IL-6, in CCl4-induced cirrhotic rats, compared with saline (Table 2).
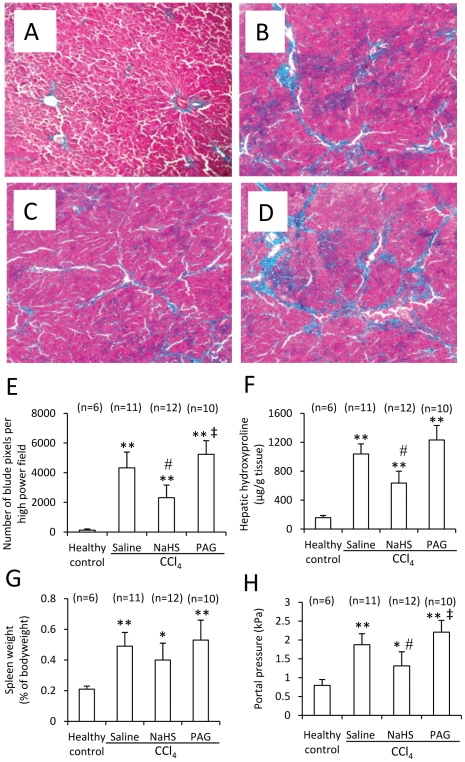
H2S attenuates CCl4-induced liver cirrhosis and portal hypertension
The Masson-stained liver sections from healthy controls had almost no collagen fibers of blue color (Fig. 3A), but those from CCl4-induced cirrhotic rats injected with saline had abundant and widespread fibers of blue color (Fig. 3B). However, the liver sections from CCl4-induced cirrhotic rats injected with NaHS had fewer fibers (Fig. 3C), while those from PAG-injected cirrhotic rats (Fig. 3D) had even more fibers, compared with those from saline-injected cirrhotic rats. The numbers of blue pixels in Masson-stained liver sections were measured to quantify collagen fibers. CCl4 highly significantly (P<0.001) increased the number of blue pixels in liver sections (Fig. 3E). However, administration of NaHS resulted in a significant (P<0.05) lower number of blue pixels, but PAG, a significant (P<0.05) higher number of blue pixels, in CCl4-induced cirrhotic rats, compared with saline (Fig. 3E). We further measured hydroxyproline, a marker of fibrosis, and found that CCl4-treated rats had significantly (P<0.001) higher hepatic hydroxyproline content than the healthy controls. However, administration of NaHS significantly (P<0.05) reduced, but PAG did not significantly (P>0.05) increase, the hepatic hydroxyproline contents in CCl4-induced cirrhotic rats, compared with saline (Fig. 3F). Next, we detected hepatic expression of α-SMA, another marker of liver fibrosis, and found that the CCl4-treated rats had significantly higher hepatic expression of α-SMA, compared to the healthy controls. However, administration of NaHS significantly (P<0.05) downregulated, but PAG did not significantly (P>0.05) increase, hepatic expression of α-SMA in CCl4-induced cirrhotic rats, compared with saline (Fig. 2D and E).
Figure 3. Histology of liver cirrhosis, hepatic hydroxyproline, spleen weight and portal pressure in the prevention experiment.
Representative illustrations (200 × magnification) of Masson-stained liver sections were taken from healthy controls (A), or rats treated with CCl4 + saline (B), CCl4 + NaHS (C) or CCl4 + PAG (D), for 12 weeks. (E) The numbers of blue pixels of each image of the above Masson-stained liver sections were counted, and the average number of blue pixels for each liver was calculated. (F) The level of hydroxyproline in livers taken from the above rats was measured. (G) Each spleen was weighed as % of bodyweight. (H) The portal pressure of each was measured. Data were expressed mean ± SD. n, number of samples. Compared to the healthy controls, a significant difference is denoted by “*”, and a highly significant difference, by “**” (P<0.001). Compared to saline + CCl4-treated rats, a significant increase is denoted by “‡”, and a significant reduction is denoted by “#”.
The spleen weight and portal pressure were used as parameters for portal hypertension. The spleen weight of CCl4–treated rats was highly significantly (P<0.001) higher than that of healthy controls (Fig. 3G). However, administration of neither NaHS nor PAG resulted in significant difference in spleen weight of CCl4-induced cirrhotic rats, compared with saline (Fig. 3G). CCl4-induced cirrhotic rats had a significantly (P<0.001) higher portal pressure than the healthy controls (Fig. 3H). However, administration of NaHS resulted in a significant (P<0.05) lower level of portal pressure, while PAG, a significant (P<0.05) higher level of portal pressure, in CCl4-induced cirrhotic rats, than saline (Fig. 3H).
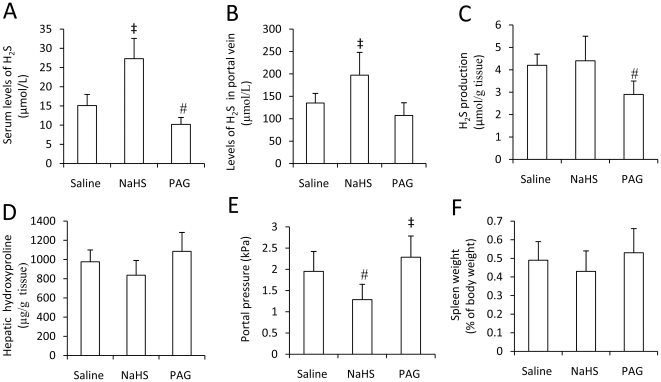
The therapeutic effect of H2S on liver cirrhosis and portal hypertension
Given that H2S displayed a preventive effect against CCl4-induced liver cirrhosis and portal hypertension, we next investigated whether H2S could have a similar therapeutic effect in already established liver cirrhosis. Liver cirrhosis was induced in rats by 12-week administration of CCl4 as above, and then saline, NaHS or PAG was administered to the cirrhotic rats, respectively, for 5 days. Administration of NaHS significantly (Both P<0.05) elevated the levels of H2S in sera from the peripheral blood (Fig. 4A) and portal vein (Fig. 4B), but again had no effect on H2S production activity in livers (Fig. 4C). PAG significantly (P <0.05) reduced the levels of H2S in sera from the systemic circulation (Fig. 4A), marginally significantly (P = 0.07) reduced the levels of H2S in the portal vein (Fig. 4B), and significantly (P <0.05) reduced the H2S production in livers (Fig. 4C). NaHS or PAG had no significantly influence on the histological alterations of livers (Data not shown), and the hepatic hydroxyproline in NaHS or PAG-treated rats were not significant different from the saline-injected rats (Fig. 4D). However, administration of NaHS significantly (P<0.05) reduced the portal pressure, while PAG significantly (P<0.05) elevated the portal pressure, compared with saline (Fig. 4E), but their effects on spleen weight were not significant, compared with saline (Fig. 4F).
Figure 4. H2S levels and production, hepatic hydroxyproline, portal pressure and spleen weight in the treatment experiment.
Liver cirrhosis was induced in rats as in Figure 3, and then the rats were assigned to three groups (Each group had 6 rats), and received daily injection of saline, NaHS or PAG, respectively, for 5 days, and then killed. (A) Blood samples were collected and the serum levels of H2S were measured. (B) Blood samples were collected from portal vein to measure the levels of H2S in portal vein (B). The hepatic H2S producing activity (C) and hydroxyproline contents (D) were measured. The portal pressure was measured (E), and each spleen was weighed as % of bodyweight (F). Results are expressed as mean ± SD. A significant increase from saline-treated rats is denoted by “‡”, and a significant reduction by “#”.
Discussion
CCl4 has been widely used as a chemical model to induce hepatotoxicity, liver cirrhosis and portal hypertension, since it is metabolized into trichloromethyl radical, leading to increased lipid peroxidation, depletion of GSH, impaired hepatic anti-oxidant activity and necrosis of hepatocytes [21], [30]. The present study has demonstrated that CCl4 downregulated the expression of CSE, the major enzyme accounting for 97% of H2S production in livers [1], [12], [19], resulting in decreased hepatic H2S producing activity. Administration of NaHS, a donor of H2S, attenuated CCl4-induced acute hepatotoxicity, evidenced by the reduction of serum levels of AST and ALT, and attenuation of histopathological alterations, in accordance with the previous reports on its cytoprotective effects against myocardial IRI [2], endotoxin-induced cardiac injury [31], hepatic IRI [15], acetaminophen-induced hepatotoxicity [16], or gastric mucosa damage caused by stress [4], nonsteroidal anti-inflammatory drugs [32] or oxidative stress [33]. The protective effects of H2S may rely on its anti-oxidative activity by reducing the production of lipid peroxides, as NaHS inhibited the activity of CYP2E1, one of the major enzymes metabolizing CCl4 [21]. The vasodilative activity of H2S may also contribute this protective activity, as H2S-induced vasorelaxation [34] can improve microcirculation of livers, which help livers to get rid of excessive lipid peroxides. In addition, though not investigated herein, the anti-apoptotic activity of H2S as demonstrated against hepatic IRI [15], may also contribute to this action.
The effects of CCl4 on H2S production were shown to be accumulative as the serum levels of H2S declined in a time-dependent course. NaHS had no effect on either hepatic CSE expression or H2S producing activity, and led to a relatively higher but stable level of H2S in sera, implying that the body has the ability to adjust endogenous production of H2S or get rid of excessive H2S, which has been recognized as a “toxic gas” in environmental pollution [35]. In both the acute and prevention experiments, PAG, an irreversible inhibitor of CSE, significantly suppressed the H2S producing activity and reduced the H2S levels, thus showing opposite effects to NaHS on liver function, lipid peroxides, and liver fibrosis, but had no impact on CSE expression, in accordance with previous reports [3], [15].
The present study has for the first time demonstrated the anti-fibrotic activity of H2S against liver cirrhosis, evidenced by reduced number of collagenous fibers in livers, and hepatic hydroxyproline content and expression of α-SMA. Besides the direct mechanism on fibrogenesis, the protective effect of H2S on hepatic injury also contributes to its anti-fibrotic activity. In addition, NaHS inhibited the production of inflammatory factors including TNF-α, IL-1β, IL-6, soluble ICAM-1. The anti-inflammatory effect, particular inhibition of IL-6 production by H2S, can contribute to its anti-fibrotic activity, as IL-6 is upregulated in cirrhotic livers and contributes to the fibrogenic process in an autocrine/paracrine manner [36].
The regulation of sinusoidal resistance depends on the contraction of hepatic stellate cells (HSCs) around sinusoidal endothelial cells [19]. It has been demonstrated that H2S is an autocrine mediator involved in regulating HSCs contraction and maintaining portal venous pressure by targeting KATP channels [19]. H2S can counteract the impaired vasodilation and HSC contraction, thus reducing portal hypertension, cirrhotic livers [20]. The results presented herein further support the effects of H2S on portal hypertension as administration of NaHS showed prophylactic and therapeutic effects in reducing portal hypertension. The protective effects of H2S on liver cirrhosis may also attributes to this effect in the prevention experiment, as liver fibrosis represents the main causative factor in portal hypertension [20]. However, H2S did not show significant therapeutic effects on liver cirrhosis in the treatment experiment, based on the histological alterations and hepatic hydroxyproline contents in cirrhotic rats, indicating H2S may not have the ability to reverse the process of liver fibrosis.
In conclusion, the present study has for the first time systemically investigated the potential protective role of H2S on CCl4-induced acute hepatotoxicity, the prophylactic effect of H2S on long-term CCl4-induced cirrhosis and portal hypertension, and the therapeutic effect of H2S on portal hypertension, by its multiple functions including anti-oxidation, anti-inflammation, cytoprotection and anti-fibrosis. The results indicate that targeting H2S may present a potent approach, particularly for its prophylactic effects, against liver cirrhosis and portal hypertension. Preclinical trials by applying some promising H2S-relesasing chemicals have already been launched though at their infancy. For instance, diallyl trisulfide, a stable H2S donor and organic polysulfide compound [37], has been shown to protect from carbon tetrachloride (CCl4)-induced liver injury [17], [18]. However, cautions must be taken as H2S has been recognized as a “toxic gas” [35], and more preclinical trials are required before H2S-releasing agents reach the clinics for use in preventing liver cirrhosis and portal hypertension.
Supporting Information
Histology of hepatotoxicity induced by CCl4. Representative illustrations (200 × magnification) of HE-stained liver sections were taken from healthy Wistar rats (A), or CCl4-treaed rats receiving administration of saline (B), NaHS (C) or PAG (D).
(TIF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Grants from the National Natural Scientific Foundation of China (30872987 and 30973474)(www.nsfc.gov.cn), and Heilongjiang Provincial Scientific and Technology Bureau, China (QC06C075)(http://www.hljkjt.gov.cn). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Łowicka E, Betowski J. Hydrogen sulfide (H2S) - the third gas of interest for pharmacologists. Pharmacol Rep. 2007;59:4–24. [PubMed] [Google Scholar]
- 2.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Y, Yao X, Zhang Y, Li W, Kang K, et al. The protective role of hydrogen sulfide in cardiac ischemia reperfusion-induced injury in diabetic rats. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2010.07.012. doi: 10.1016/j.ijcard.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Lou LX, Geng B, Du JB, Tang CS. Hydrogen sulphide-induced hypothermia attenuates stress-related ulceration in rats. Clin Exp Pharmacol Physiol. 2008;35:223–228. doi: 10.1111/j.1440-1681.2007.04812.x. [DOI] [PubMed] [Google Scholar]
- 5.Chunyu Z, Junbao D, Dingfang B, Hui Y, Xiuying T, et al. The regulatory effect of hydrogen sulfide on hypoxic pulmonary hypertension in rats. Biochem Biophys Res Commun. 2003;302:810–816. doi: 10.1016/s0006-291x(03)00256-0. [DOI] [PubMed] [Google Scholar]
- 6.Zhang LM, Jiang CX, Liu DW. Hydrogen sulfide attenuates neuronal injury induced by vascular dementia via inhibiting apoptosis in rats. Neurochem Res. 2009;34:1984–1992. doi: 10.1007/s11064-009-0006-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhong G, Chen F, Cheng Y, Tang C, Du J. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J Hypertens. 2003;21:1879–1885. doi: 10.1097/00004872-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Salto-Tellez M, Tan CH, Whiteman M, Moore PK. GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free Radic Biol Med. 2009;47:103–113. doi: 10.1016/j.freeradbiomed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Kimura Y, Goto Y, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 10.Zhong GZ, Li YB, Liu XL, Guo LS, Chen ML, et al. Hydrogen sulfide opens the KATP channel on rat atrial and ventricular myocytes. Cardiology. 2010;115:120–126. doi: 10.1159/000260073. [DOI] [PubMed] [Google Scholar]
- 11.Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131:259–271. doi: 10.1053/j.gastro.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 12.Kabil O, Vitvitsky V, Xie P, Banerjee R. The Quantitative Significance of the Transsulfuration Enzymes for H2S Production in Murine Tissues. Antioxid Redox Signal. 2011;15:363–372. doi: 10.1089/ars.2010.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siebert N, Cantré D, Eipel C, Vollmar B. H2S contributes to the hepatic arterial buffer response and mediates vasorelaxation of the hepatic artery via activation of K(ATP) channels. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1266–1273. doi: 10.1152/ajpgi.90484.2008. [DOI] [PubMed] [Google Scholar]
- 14.Fujii K, Sakuragawa T, Kashiba M, Sugiura Y, Kondo M, et al. Hydrogen sulfide as an endogenous modulator of biliary bicarbonate excretion in the rat liver. Antioxid Redox Signal. 2005;7:788–794. doi: 10.1089/ars.2005.7.788. [DOI] [PubMed] [Google Scholar]
- 15.Kang K, Zhao M, Jiang H, Tan G, Pan S, et al. Role of hydrogen sulfide in hepatic ischemia reperfusion-induced injury in rats. Liver Transpl. 2009;15:1306–1314. doi: 10.1002/lt.21810. [DOI] [PubMed] [Google Scholar]
- 16.Morsy MA, Ibrahim SA, Abdelwahab SA, Zedan MZ, Elbitar HI. Curative effects of hydrogen sulfide against acetaminophen-induced hepatotoxicity in mice. Life Sci. 2010;87:692–698. doi: 10.1016/j.lfs.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Hosono-Fukao T, Hosono T, Seki T, Ariga T. Diallyl trisulfide protects rats from carbon tetrachloride-induced liver injury. J Nutr. 2009;139:2252–2256. doi: 10.3945/jn.109.109611. [DOI] [PubMed] [Google Scholar]
- 18.Fukao T, Hosono T, Misawa S, Seki T, Ariga T. Chemoprotective effect of diallyl trisulfide from garlic against carbon tetrachloride-induced acute liver injury of rats. Biofactors. 2004;21:171–174. doi: 10.1002/biof.552210135. [DOI] [PubMed] [Google Scholar]
- 19.Fiorucci S, Antonelli E, Mencarelli A, Orlandi S, Renga B, et al. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology. 2005;42:539–548. doi: 10.1002/hep.20817. [DOI] [PubMed] [Google Scholar]
- 20.Distrutti E, Mencarelli A, Santucci L, Renga B, Orlandi S, et al. The methionine connection: homocysteine and hydrogen sulfide exert opposite effects on hepatic microcirculation in rats. Hepatology. 2008;47:659–667. doi: 10.1002/hep.22037. [DOI] [PubMed] [Google Scholar]
- 21.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 22.Fang L, Li H, Tang C, Geng B, Qi Y, et al. Hydrogen sulfide attenuates the pathogenesis of pulmonary fibrosis induced by bleomycin in rats. Can J Physiol Pharmacol. 2009;87:531–538. doi: 10.1139/y09-039. [DOI] [PubMed] [Google Scholar]
- 23.Qingyou Z, Junbao D, Weijin Z, Hui Y, Chaoshu T, et al. Impact of hydrogen sulfide on carbon monoxide/heme oxygenase pathway in the pathogenesis of hypoxic pulmonary hypertension. Biochem Biophys Res Commun. 2004;317:30–37. doi: 10.1016/j.bbrc.2004.02.176. [DOI] [PubMed] [Google Scholar]
- 24.Steib CJ, Hennenberg M, Beitinger F, Hartmann AC, Bystron M, et al. Amiloride reduces portal hypertension in rat liver cirrhosis. Gut. 2010;59:827–836. doi: 10.1136/gut.2009.197756. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, et al. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005;19:623–625. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- 26.Chang TK, Crespi CL, Waxman DJ. Spectrophotometric analysis of human CYP2E1-catalyzed p-nitrophenol hydroxylation. Methods Mol Biol. 1998;107:147–152. doi: 10.1385/0-89603-519-0:147. [DOI] [PubMed] [Google Scholar]
- 27.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 28.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 29.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 30.Yuan L, Kaplowitz N. Glutathione in liver diseases and hepatotoxicity. Mol Aspects Med. 2009;30:29–41. doi: 10.1016/j.mam.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Sivarajah A, McDonald MC, Thiemermann C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock. 2006;26:154–161. doi: 10.1097/01.shk.0000225722.56681.64. [DOI] [PubMed] [Google Scholar]
- 32.Fiorucci S, Santucci L, Distrutti E. NSAIDs, coxibs, CINOD and H(2)S-releasing NSAIDs: What lies beyond the horizon. Dig Liver Dis. 2007;39:1043–1051. doi: 10.1016/j.dld.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Yonezawa D, Sekiguchi F, Miyamoto M, Taniguchi E, Honjo M, et al. A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology. 2007;241:11–18. doi: 10.1016/j.tox.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Zhao WM, Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol. 2002;283:H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- 35.Guidotti TL. Hydrogen sulphide. Occup Med (Lond) 1996;46:367–371. doi: 10.1093/occmed/46.5.367. [DOI] [PubMed] [Google Scholar]
- 36.Dranoff JA, Wells RG. Portal fibroblasts: underappreciated mediators of biliary fibrosis. Hepatology. 2010;51:1438–1444. doi: 10.1002/hep.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Predmore BL, Grinsfelder DB, Aragon JP, Elston M, Calvert JW, et al. The stable hydrogen sulphide donor, diallyl trisulfide, protects against acute myocardial infarction in mice. J Am Coll Cardiol. 2010;55:A116. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histology of hepatotoxicity induced by CCl4. Representative illustrations (200 × magnification) of HE-stained liver sections were taken from healthy Wistar rats (A), or CCl4-treaed rats receiving administration of saline (B), NaHS (C) or PAG (D).
(TIF)