Abstract
Objective:
To investigate the preventive and curative role of ascorbic acid on doxorubicin (dox)-induced myocardial toxicity in rats.
Materials and Methods:
Animals were divided into five groups of six animals each. Group I served as normal control and received saline 5 ml/kg/day intraperitoneal (i.p.) for a period of 15 days. Group II animals received ascorbic acid 20 mg/kg per oral (p.o.) for 15 days as a pretreatment control (PR). Group III animals received dox 2.5 mg/kg body weight (b.w.), i.p., in six equal injections for two weeks for a total cumulative dose of 15 mg/kg b.w. Group IV animals received ascorbic acid 20 mg/kg p.o. for 15 days as a pretreatment followed by dox 2.5 mg/kg b.w., i.p., in six equal injections for two weeks for a total cumulative dose of 15 mg/kg body weight. Group V animals received dox 2.5 mg/kg b.w., i.p., in six equal injections for two weeks for a total cumulative dose of 15 mg/kg b.w. followed by ascorbic acid 20 mg/kg p.o for 15 days as post-treatment control (CR). The biochemical parameters such as tissue glutathione (GSH), malondialdehyde (MDA), catalase (CAT), and superoxide dismutase (SOD), and enzyme biomarkers such as creatine phosphokinase (CPK), lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were monitored.
Results:
Pretreatment with ascorbic acid (20 mg/kg p.o.) significantly protected the myocardium from the toxic effect of dox (PR), by increasing the levels of antioxidant enzymes such as GSH, SOD, and CAT toward normal and decreased the levels of MDA, CPK, LDH, AST, and ALT as compared with dox-treated rats. Post-treatment with ascorbic acid to dox-treated group (CR) significantly increased the levels of tissue GSH, SOD, CAT and significantly decreased the level of MDA as compared with dox-treated group. It also reduced the severity of cellular damage of the myocardium as confirmed by histopathology. The restoration of the endogenous antioxidant system clearly depicts that ascorbic acid produced its protective effect by scavenging the reactive oxygen species.
Conclusion:
The results obtained in this study provide evidence for the usefulness of the ascorbic acid as a cardioprotective agent.
Keywords: Ascorbic acid, cardiotoxicity, doxorubicin, free radicals
Introduction
Doxorubicin (dox), an anthracycline, is widely used as antineoplastic agent and shows a broad range of antitumor activity, including soft tissue sarcoma, breast cancer, small cells carcinoma of the lung, and acute leukemia. A number of mechanisms have been proposed for cardiotoxic effects of dox, including free radical-induced myocardial injury, lipid peroxidation,[1] mitochondrial damage, iron-dependent oxidative damage to macromolecules, vasoactive amine release,[2] myocyte damage induced by intracellular calcium overload, oxidation of fatty acids leading to the depression of energy metabolism in the cardiac tissue, impairment in myocardial adrenergic signaling/regulation, and the cellular toxicity.[3]
Moreover, increased oxidative stress and release of reactive oxygen radicals, including super oxide anion (O2−) and other reactive oxygen intermediates as well as antioxidant deficits, have been suggested to play a major role in dox-induced cardiomyopathy and heart damage.[4] In recent years, it has been observed that there is a growing interest in uses of natural antioxidants as a protective strategy against the cardiovascular-related problems such as ischemia-reperfusion and dox-induced cardiotoxicity.
A review of literature reveals that ascorbic acid is a potent water-soluble antioxidant that scavenges reactive oxygen and nitrogen species.[5] Ascorbic acid deficiency is characterized by increased oxidative stress and tissue injury including oxidant-induced necrotic cell death.[6] It prevents oxidative modification of both cytosolic and membrane component of cells. In view of above facts, the present study was undertaken to find out the preventive and curative role of ascorbic acid against dox-induced cardiomyopathy in rats.
Materials and Methods
Animals
Wistar rats of either sex weighing 150 to 200 g were used (n = 6 in each group). Animals were acclimatized for one week to laboratory conditions (temperature, 23 ± 2°C; humidity, 50 ± 5%; and 12-hour light-dark cycle) before study. The animal care and handling was carried out according to the guidelines set by CPCSEA. Animals were provided free access to food and water ad libitum. The study was approved by the Institutional Animal Ethical Committee.
Preparation of Drug and Mode of Administration
dox was procured from Khandelwal Labs (Mumbai, India). The required amount of drug was dissolved in saline and animals were administered with 2.5 mg/kg body weight intraperitoneally. Ascorbic acid was purchased from S.D. Fine Chemicals, Dharwad. The required amount of ascorbic acid (20 mg/kg) was dissolved in distilled water and administered orally.
Experimental Protocol
Animals were divided into five groups of six animals each. Group I served as normal control and received saline 5 ml/kg/day i.p for a period of 15 days. Group II animals received ascorbic acid 20 mg/kg p.o. for 15 days as a pretreatment control (PR). Group III animals received dox 2.5 mg/kg b.w., i.p., in six equal injections for two weeks for a total cumulative dose of 15 mg/kg b.w. Group IV animals received ascorbic acid 20 mg/kg p.o. for 15 days as a pretreatment followed by dox 2.5 mg/kg b.w., i.p., in six equal injections for two weeks for a total cumulative dose of 15 mg/kg body weight. Group V animals received dox 2.5 mg/kg b.w., i.p., in six equal injections for two weeks for a total cumulative dose of 15 mg/kg b.w. followed by ascorbic acid 20 mg/kg p.o for 15 days as post-treatment control (CR).[7]
Estimation of Serum Biomarkers
After 36 hours of the last treatment, orbital blood samples were obtained under light ether anesthesia using heparinized microcapillaries for the estimation of cardiac biomarkers creatine phosphokinase (CPK)[8] and lactate dehydrogenase (LDH).[9] After experimental period, blood was withdrawn from retro-orbital sinus, serum was separated by centrifugation and used for estimation of marker enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT).[10,11]
Estimation of Oxidative Stress
A portion of heart was taken from all the groups and a 30% w/v homogenate was prepared in 0.9% buffered KCl (pH 7.4) for the estimation of glutathione (GSH),[12] superoxide dismutase (SOD),[13] catalase (CAT),[14] and malondialdehyde (MDA).[15] The remaining portion of the heart tissue was used for histopathological studies.
Histopathological Studies
Myocardial tissue from all the groups was subjected to histopathological studies. The tissue was fixed using 10% formalin solution in phosphate buffer, and sections were prepared using paraffin blocks and stained with hematoxylin and eosin after dewaxing. The sections were observed for histopathological changes.
Statistical Analysis
The results were expressed as the mean ± SEM and analyzed using one-way ANOVA followed by Dunnett's multiple comparison tests. Data were computed for statistical analysis using the Graph Pad Prism Software.
Results
Heart Weight, Body Weight, and Ratio of Heart Weight to Body Weight
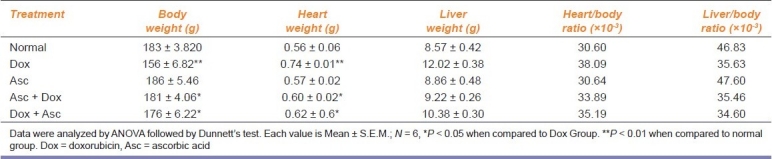
The changes in the heart weight, body weight, and ratio of the heart weight to body weight are shown in Table 1. The heart weight in dox-treated rats was significantly increased compared with normal rats. The heart weight in ascorbic acid-treated rats was not changed significantly as compared with the normal rats. The heart weight in PR and CR groups was significantly decreased as compared with dox-treated rats. The body weight in dox-treated rats was significantly decreased compared with the normal rats. The body weight in PR and CR groups was significantly increased to be nearly the same as in normal rats.
Table 1.
Effect of ascorbic acid on heart weight, body weight, and ratio of heart weight to body weight in rats
Serum Enzyme Levels
Rats administered with dox had significantly increased levels of CPK, LDH, AST, and ALT as compared with normal animals [Table 2]. In PR and CR groups, there was significant decrease (P < 0.001) in the levels of these enzymes.
Table 2.
Effect of ascorbic acid on serum biomarkers in doxorubicin exposed rats

Antioxidant Status
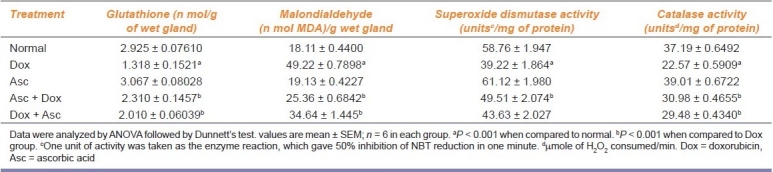
Effect of dox on tissue lipid peroxidation, antioxidant, and antioxidant enzymes is shown in Table 3. The MDA level was increased; GSH, SOD, and CAT levels were significantly decreased in dox-treated group as compared with normal animals. In PR and CR groups, there was a significant increase (P < 0.001) in the levels of GSH, SOD, and CAT as compared with dox, whereas a significant decrease was noted in the MDA level.
Table 3.
Effect of ascorbic acid on glutathione, malondialdehyde, superoxide dismutase, and catalase in Doxorubicin-treated rat hearts
Histopathological Observation
The histology of the heart tissue from the Group 1 animals showed normal morphological appearance [Figure 1a], whereas in dox group, disruption or loss of myofibrils and vacuolization of the cytoplasm, enlarged swollen mitochondria, patchy necrosis, and inflammatory cells were observed [Figure 1b]. The histology of heart tissues from group 3 showed a lesser loss of myofibrils, vacuolization of the cytoplasm and inflammatory cells [Figure 1c]. The histology of heart tissues from Group 4 showed moderate loss of myofibrils and vacuolization of the cytoplasm [Figure 1d].
Figure 1a.
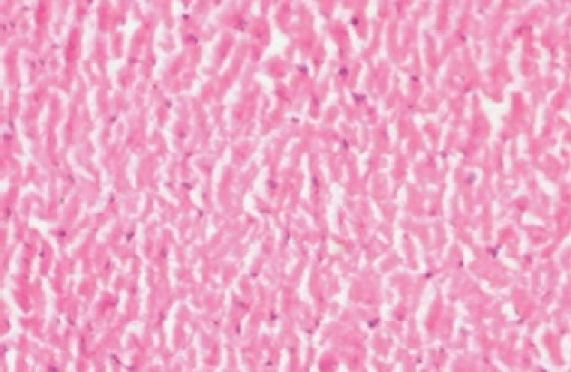
Photomicrograph of rat heart of normal showing regular morphology
Figure 1b.
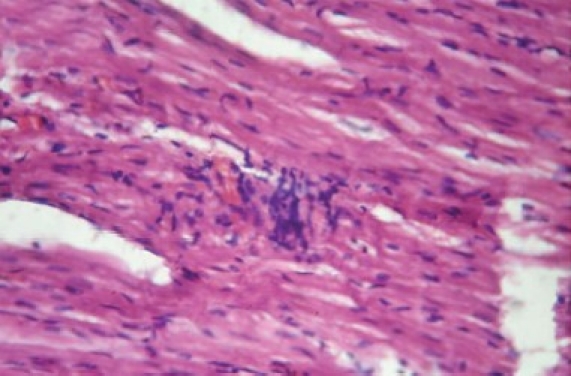
Photomicrograph of rat heart treated with doxorubicin showing extensive vacuolization and myofi bril loss
Figure 1c.
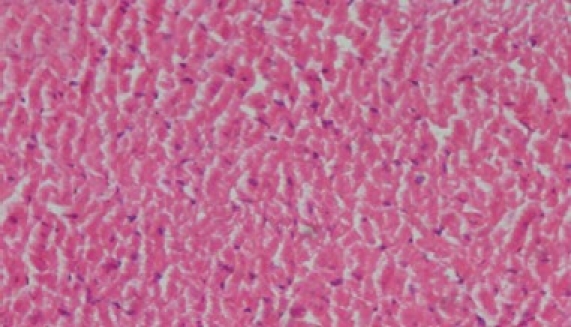
Photomicrograph of rat heart treated with ascorbic acid + doxorubicin (PR) showing less extensive vacuolization and no myofi bril loss
Figure 1d.
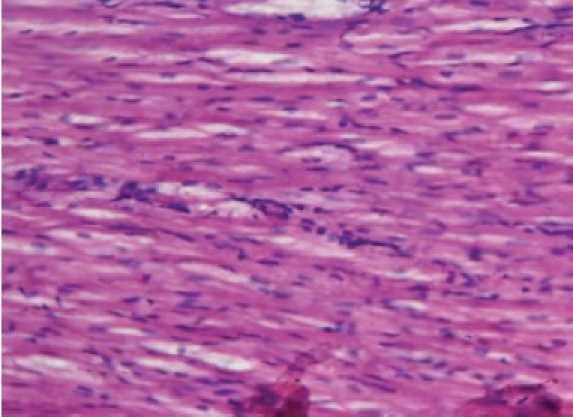
Photomicrograph of rat heart treated with doxorubicin + ascorbic acid (CR) showing less extensive vacuolization and myofi bril loss as compared with doxorubicin treated rats
Discussion
In the present study, ascorbic acid was investigated for its influence on dox-induced myocardial toxicity. The gross anatomical changes of the heart treated with dox showed a typical chronic response including ventricular dilatation, cardiac hypertrophic, inflammatory cells, and overall enlargement. Another effect of dox-induced cardiotoxicity is characterized by decreased body weight and increase in the heart weight.[16] The results of the present study confirmed the earlier findings that dox administration causes decrease in the body weight and increase in heart weight. However, all these changes are found to be inhibited in preventive and curative models. Chronic treatment with dox induced severe biochemical changes as well as oxidative damage in the heart tissues. The experimental evidence suggests the generation of free radicals in the heart tissue by chronic administration of dox.[17] The generated reactive oxygen species (ROS) such as superoxide radicals and hydroxyl radicals have a potential to cause damage to various intracellular components. A deficiency of oxygen supply or glucose may damage the myocardial cells and the cell membrane becomes more permeable and ruptures, resulting in leakage of enzymes.[18] Ascorbic acid was found to inhibit the dox-induced CPK and LDH release in the serum of rats. It is widely reported that dox causes lipid peroxidation and disruption of cardiac myocytes, which can lead to increased release of CPK in the serum. In PR and CR groups, there was inhibition of CPK and LDH release which resulted in either complete reversal or considerable recovery of the serum enzyme activities. The present results are in agreement with those of Koti et al.[19] The levels of AST and ALT in dox-treated groups showed a significant increase as compared with normal. The increased level of these enzymes indicates myocardial injury. Mild elevations of AST have been associated with liver injury or myocardial infarction.[20] Higher the activity of AST, the larger is the injury size.[21] These results imply that dox when taken for long period of time could cause both liver and heart injury. A typical myocardial injury gives an AST/ALT ratio greater than 1. The large doses of dox over a long period of time are likely to lead to myocardial damage.
In PR and CR groups, AST and ALT levels significantly decreased as compared with dox-treated groups. Present results suggest that treatment with ascorbic acid which is responsible for maintenance of normal architectural integrity of cardiac muscle may inhibit myocardial damage.
The mechanism of cardiotoxicity induced by dox is not clearly understood from the present study. Large body of evidence indicates toward the formation of oxygen free radicals, which can damage cells by lipid peroxidation. Cardiac tissue damage may be due to increased oxidative stress and depletion of antioxidants as reported earlier.[22]
In our study, dox-treated rats showed increase in MDA levels and decrease in GSH, SOD, and CAT levels in heart tissue confirming the cardiac damage.[23] Depletion of GSH in rat heart tissue due to enhanced lipid peroxidation and excessive lipid peroxidation can cause increased GSH consumption. Significant increase in the GSH, SOD, and CAT activities and decrease in lipid peroxidation in heart tissue of PR and CR groups support the above hypothesis that this increase is possibly required to overcome excessive oxidative stress caused by dox. The cardioprotective activity of ascorbic acid was further confirmed by histopathological studies.
The possible action of ascorbic acid is mediated through scavenging physiologically relevant reactive oxygen and nitrogen species. These include free radicals such as hydroxyl radicals, aqueous radicals, superoxide anion, and nitrogen dioxide, as well as nonradical species such as hypochlorus acid, ozone, singlet oxygen, nitrosating species (N2O3/N2O4), nitroxide, and peroxynitrite. In addition to scavenging of ROS and reactive nitrogen species, ascorbic acid can regenerate other small molecule antioxidants, such as a-tocopherol, GSH, urate, and β-carotene, from their respective radical species.[24,25]
At cellular level, ascorbic acid has been reported to mitigate the deleterious effect of ROS directly by increasing antioxidant enzyme activities of cells and indirectly by reducing oxidized form of vitamin E and GSH.[26–28] Antioxidant and free radical scavenger properties of ascorbic acid possibly prevent the effects of oxidative stress.[29] Preservation of intracellular ascorbic acid levels minimizes the peroxynitrite-mediated injury which is attributable to the beneficial effect of ascorbic acid.[30] Ascorbic acid protects dox-induced biochemical and histological changes in the cardiac tissue of rats either by restoring endogenous antioxidant activity or as a antioxidant or both. A similar action of scavenging of dox-induced free radicals by ascorbic acid in the present study cannot be ruled out.
Finally, we conclude that the cardiotoxicity induced by dox is in relationship with oxidative stress. Our study suggests that the ascorbic acid could be used as an antioxidant during or after dox therapy. Ascorbic acid shows a greater protective effect in preventive as compared with curative model. The prophylactic use of ascorbic acid in cardiomyopathy appears to be promising because of its low cost and strong antioxidant property.
Acknowledgments
The authors thank Principal, K.L.E. University's College of Pharmacy, Hubli, India, for providing the necessary facilities to carry out the work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Singal PK, Segstro RJ, Singh RP, Kutryk MJ. Changes in lysosomal morphology and enzyme morphology and enzyme activities during the development of Adriamycin induced cardiomyopathy. Can J Cardiol. 1985;1:139–47. [PubMed] [Google Scholar]
- 2.Bristow MR, Sageman WS, Scott RH, Billingham ME, Bowden RE, Kernoff RS, et al. Acute and chronic cardiovascular effect of doxorubicin in dog. J Cardiovasc Phamacol. 1980;2:487–515. doi: 10.1097/00005344-198009000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Wold LE, Aberle NS, 2nd, Ren J. Doxorubicin induces cardiomyocytes dysfunction via P38 MAP kinase dependant oxidative stress mechanism. Cancer Detect Prev. 2005;29:294–9. doi: 10.1016/j.cdp.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Hanna HA, Fatima M, Gamal AE, Senot HD. Cardioprotective activities on doxorubicin induced cardiotoxicity. Bioorg Med Chem. 2005;13:1847–57. doi: 10.1016/j.bmc.2004.10.066. [DOI] [PubMed] [Google Scholar]
- 5.Premkumar K, Bowlus CL. Ascorbic acid does not increase the oxidative stress induced by dietary iron in C3H mice. J Nutr. 2004;134:435–8. doi: 10.1093/jn/134.2.435. [DOI] [PubMed] [Google Scholar]
- 6.Lou AS, Broun, Frank LH, Dean PJ. Ascorbic deficiency and oxidative stress in alveolar type II cell. Am J Physiol Lung Cell Mol Physiol. 1997;273:782–8. [Google Scholar]
- 7.Ayaz SA, Bhandari K, Pillai K. Influence of DL and α lipoic acid and Vitamin E against doxorubicin induced biochemical and histological changes in cardiac tissue of rat. Indian J Pharmacol. 2005;37:294–9. [Google Scholar]
- 8.Rosalki SB. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967;69:696–705. [PubMed] [Google Scholar]
- 9.Henry RJ, Chiamori N, Goiub OJ, Berkman S. Revised spectrophotometric methods for the determination of glutamic-oxalacetic transaminase, glutamic-pyruvic transaminase and lactic acid dehydrogenase. Am J Clin Path. 1960;34:381–98. doi: 10.1093/ajcp/34.4_ts.381. [DOI] [PubMed] [Google Scholar]
- 10.Sarter B. Coenzyme Q10 and Cardiovascular disease: A review. J Cardiovasc Nurs. 2002;16:9–20. doi: 10.1097/00005082-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Bradley DW, Maynard JE, Emery G. Comparison of ascorbic acid concentrations in whole blood obtained by venipuncture or by finger prick. Clin Chem. 1972;18:968–70. [PubMed] [Google Scholar]
- 12.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 13.Mishra HP, Fridovich I. The role of superoxide anion in the auto-oxidation of Epinephrine and a simple assay for superoxide dismuatse. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 14.Claiborne A. Catalase activity. In: Greenwald RA, editor. CRC Handbook of Methods for Oxygen Radical Research. Boca Raton: CRC Press; 1985. pp. 283–4. [Google Scholar]
- 15.Ohkawa H, Ohish N, Yogi K. Assay for lipid peroxidase in animal tissues by thiobarbituric acid. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Antonio A, Jose M, Jose S, Rita F, Maria N, Franklim M, et al. Endurance training attenuates doxorubicin-induced cardiac oxidative damage in mice. Int J Cardiol. 2005;100:451–60. doi: 10.1016/j.ijcard.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Hardina R, Gersl V, Klimtova I, Simunek T, Machackova J, Adamcova M. Anthracycline induced cardiotoxicity. Acta Med. 2000;43:75–82. [PubMed] [Google Scholar]
- 18.Thippeswamy AH, Shirodkar A, Koti BC, Sadiq AJ, Praveen DM, Swamy AH, et al. Protective role of Phyllantusniruri extract in doxorubicin-induced myocardial toxicity in rats. Indain J Pharmacol. 2011;43:31–5. doi: 10.4103/0253-7613.75663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koti BC, Vishwanathswamy AH, Wagawade J, Thippeswamy AH. Cardioprotective effect of lipistat against doxorubicin induced myocardial toxicity in albino rats. Indian J Exp Biol. 2009;47:41–6. [PubMed] [Google Scholar]
- 20.Stroev EA, Makarova VG. Moscow: Mir Publishers; 1989. Laboratory Manual in Biochemistry; pp. 154–6. [Google Scholar]
- 21.Kahn JC, Gueret P, Baudet M, Rocha P, Bardet J, Bourdarias JP. Clinical assessment of infarct size by serial determinations of serum creatine phosphokinase activity. Eur J Cardiol. 1979;9:21–37. [PubMed] [Google Scholar]
- 22.Hardina R, Gersl V, Klimtova I, Simunek T, Machackova J, Adamcova M. Anthracycline induced cardiotoxicity. Acta Medica. 2000;43:75–82. [PubMed] [Google Scholar]
- 23.Takacs IE, Matkovics B, Varga SI, Homolay P, Feer G, Seres T. Study of the myocardial antioxidant defense in various species. Pharmacol Res. 1992;25:177–8. [Google Scholar]
- 24.Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999;69:1086–107. doi: 10.1093/ajcn/69.6.1086. [DOI] [PubMed] [Google Scholar]
- 25.Englard S, Seifter S. The biochemical functions of ascorbic acid. Annu Rev Nutr. 1986;6:365–406. doi: 10.1146/annurev.nu.06.070186.002053. [DOI] [PubMed] [Google Scholar]
- 26.Neuzil J, Thomas SR, Stocker R. Requirement for, promotion, or inhibition by alpha-tocopherol of radical-induced initiation of plasma lipoprotein lipid peroxidation. Free Radic Biol Med. 1997;22:57–71. doi: 10.1016/s0891-5849(96)00224-9. [DOI] [PubMed] [Google Scholar]
- 27.Meister A. Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem. 1994;269:9397–400. [PubMed] [Google Scholar]
- 28.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–92. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 29.Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–8. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 30.Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, et al. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–80. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]


