Abstract
Objectives:
This study was aimed to evaluate the chemical composition, antioxidant potential in vitro and in vivo, anti-inflammatory, and antinociceptive activity of turmeric oil.
Materials and Methods:
Chemical analysis of turmeric oil was done by gas chromatography/mass spectrometry. Antioxidant activities in vitro was done by six different methods and in vivo antioxidant activity was determined by measuring superoxide generation from macrophages treated with phorbol-12-myristate-13-acetate (PMA) as well as determining antioxidant level after feeding the oil orally for one month. Anti-inflammatory activity was studied in mice using carrageenan, dextran, and formalin. Antinociceptive activity was evaluated by using acetic acid-induced writhing movement in mice.
Results:
The main constituent of essential oil of turmeric was found to be ar-turmerone (61.79%), curlone (12.48%), and ar-curcumene (6.11%). Turmeric oil was found to have in vitro antioxidant activity and IC50 for scavenging superoxides, hydroxyl radicals, and lipid peroxidation were 135 μg/ml, 200 μg/ml, and 400 μg/ml, respectively. The ferric-reducing activity for 50 μg of turmeric essential oil was found to be 5 mM. Intraperitoneal administration of oil was found to inhibit PMA-induced superoxide radicals elicited by macrophages. Oral administration of turmeric oil for one month to mice significantly increased superoxide dismutase, glutathione, and glutathione reductase enzyme levels in blood and glutathione-S-transferase and superoxide dismutase enzymes in liver. Turmeric oil showed significant reduction in paw thickness in carrageenan, dextran-induced acute inflammation, and formalin-induced chronic inflammation. The drug produced significant antinociceptive activity (P < 0.001) at all doses studied.
Conclusions:
These results demonstrated that turmeric oil has potential health benefits as it can scavenge the free radicals and produce significant anti-inflammatory and antinociceptive activities.
Keywords: Anti-inflammatory, antinociceptive, antioxidant, Curcuma longa, gas chromatography/mass spectrometry
Introduction
Essential oils present in plants possess chemical constituents mainly consisting of terpenoids, which have low molecular weight. This allows its easy transport across cell membranes to induce different biological activities. They are also gaining interest as natural antioxidants and food preservatives because of their safety compared with synthetic food additives.[1] Curcuma longa L. is one of the main spices belonging to the family of Zingiberaceae, used to enhance the color, aroma, and flavor of food in most regions of Southern Asia. Turmeric has long been known to play a significant role in Ayurveda, Chinese medicines, and traditional household treatments. Turmeric essential oil is extracted from the rhizome of the plant by steam distillation. Free radicals produced due to overproduction of reactive oxygen species induced by exposure to external oxidant substances or due to a failure in the defense mechanisms form the causative agents of several diseases including arthritis, hemorrhagic shock, heart disease, ageing, Parkinson's disease, amyotrophic lateral sclerosis, altered immunity, gastric ulcer, inflammation, cataract, and cancer. It is possible to reduce the risks of chronic diseases and prevent disease progression by either enhancing the body's natural antioxidant defense or by supplementing with dietary antioxidants. Turmeric oil consists of a unique set of sesquiterpenoids considered to have significant pharmacological activities. Antifungal, insect repellent, antibacterial, antimutagenic, and anticarcinogenic activities[2–4] of turmeric oil have been reported. The present study is aimed to evaluate the antioxidant, anti-inflammatory, and antinociceptive properties of turmeric oil.
Materials and Methods
Drugs
Essential oil of turmeric prepared by steam distillation was procured from Kancore Ingredients Limited, Angamali, Kerala. In order to get a uniform suspension of spices oil for in vitro studies, the oil was dissolved in hexane (1 mg/ml) and 10 μl of Triton X 100 (Sigma-Aldrich) was added and further evaporated to dryness and finally made up to 10 ml with distilled water. For oral administration, oil was dissolved in paraffin oil.
Animals
Swiss albino and Balb/C mice (20–25 g) were used for antioxidant, anti-inflammatory, and antinociceptive study. They were purchased from Small Animal Breeding Station, Mannuthy, Kerala, and housed in well-ventilated cages under controlled conditions of light and humidity and provided with normal mouse chow (SaiDurga Food and Feeds, Bangalore, India) and water ad libitum. All the animal experiments were done as per the instructions prescribed by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India, and implemented through the Institutional Animal Ethics Committee.
Chemicals and Reagents
Dextran and carrageenan were purchased from Sigma Aldrich; Chemical Company, U.S.A. Nitrobluetetrazolium (NBT), glutathione, glutathione oxidized, nicotinamide adenine dinucleotide phosphate reduced, and 5-5′dithiobis 2-niotrobenzoic acid were purchased from Sisco Research Laboratories Pvt. Ltd., Mumbai, India. 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azobis-3-ethylbenzthiozoline-6-sulphonic acid (ABTS) were purchased from Sigma Aldrich (St.Louis, USA) and phorbol-12-myristate-13-acetate (PMA) was a gift from Dr. Allen Conney, USA. All other chemicals and reagents used were of analytical reagent grade.
GCMS Analysis of Essential Oil
The turmeric oil was subjected to gas chromatography/mass spectrometry (GCMS) analysis using a Hewlett-Packard gas chromatograph (Model 6890) coupled with a quadruple mass spectrometer (Model HP 5973) using a HP – 5MS capillary column (5% phenylmethylsiloxane; 30 × 320 × 0.25 Um). The interphase, ion source, and selective mass detector temperatures were maintained at 243°C, 230°C, and 150°C, respectively. Helium was used as a carrier gas at a flow rate of 1.4 ml/min. For the turmeric oil, the oven temperature was programmed linearly at 60°C, and then increased from 60°C to 243°C at the rate of 3°C/min.
Determination of Antioxidant Activity of Essential Oil In Vitro
In vitro antioxidant activity was determined by different methods. Superoxide radical scavenging activity was determined by the NBT reduction method.[5] Hydroxyl radical scavenging activity was measured by studying the competition between deoxyribose and test compound for hydroxyl radicals generated from the Fe3+/ascorbate/EDTA/H2O2 system.[6] The percentage inhibition of lipid peroxidation was determined by comparing the results of the test compound with that of the control. The determination of DPPH radical scavenging activity was done by the method of Aquino et al.[7] ABTS radical scavenging activity of turmeric oil was determined using ferryl myoglobin/ABTS protocol.[8] The ferric reducing antioxidant power assay was measured at low pH.[9] Values were expressed as millimoles of ferric chloride formed.
In vivo Antioxidant Effect of Essential Oil on Phorbol-12-myristate-13-acetate-induced Superoxide Radical Generation
In vivo antioxidant effect was studied by the inhibition of superoxide generation by macrophages activated with PMA in mice. Female Balb/C mice were treated with different concentrations (10, 50, and 250 mg/kg b.wt) of turmeric oil for 5 days. Peritoneal macrophages elicited by 5% sodium caseinate were activated on the fifth day by injecting PMA (100 ng/animal, i.p). After 3 hours, macrophages were harvested and superoxide generation was measured.[10]
Determination of Effect of Essential Oil on in vivo Antioxidant Enzyme Levels
Female Balb/C mice (4-6 weeks) weighing 20 to 25 g were divided into four groups of five animals and they were treated orally with turmeric oil dissolved in paraffin oil at different doses for 30 days.
Group I: Normal
Group II: Control treated with paraffin oil only
Group III: 100 mg turmeric oil/kg body weight (b.wt)
Group IV: 500 mg turmeric oil/kg b.wt
At the end of the experiment, all animals were sacrificed. Blood was collected by heart puncture and liver was excised and washed in ice-cold TrisHCl buffer (0.1 M, pH-7.4). Cytosolic samples of liver homogenate were prepared by centrifuging at 10 000 rpm for 30 minutes at 4°C.
The total protein was estimated by the method of Lowry et al.[11] Hemoglobin was estimated by cyanmethemoglobin using Drabkin's method.[12] Whole blood was used for determination of superoxide dismutase by NBT reduction method,[5] catalase was estimated by measuring the rate of decomposition of hydrogen peroxide at 240 nm,[13] and glutathione levels was estimated by the method of Moron et al.[14] Serum was used for glutathione reductase estimation by the method of Racker.[15] Liver homogenate was used for assessing the activities of superoxide dismutase, catalase, glutathione, and glutathione reductase as given above, as well as glutathione peroxidase activity based on the degradation of H2O2 in the presence of (Glutathione reduced) GSH.[16] Glutathione-S-transferase (GST) was measured based on the rate of increase in conjugate formation between GSH and 1-chloro-2,4-nitrobenzene.[17]
Carrageenan-induced Acute Inflammatory Model
Female Balb/C mice were divided into five groups comprising of six animals in each groups. Group I with carrageenan alone was kept as control and group II was treated with standard drug—diclofenac (10 mg/kg). Group III, IV, and V were treated with different concentrations of turmeric essential oil (100, 500, and 1 000 mg/kg body weight) intraperitoneally for 5 consecutive days. One hour after the fifth dose of extract administration, acute inflammation was induced in the hind paw of mice by subplantar injection of 0.02 ml freshly prepared 0.1% suspension of carrageenan in normal saline.[18] The paw thickness was measured using vernier calipers and recorded every hour up to 6th hour and finally at 24th hour.
Dextran-induced Acute Inflammatory Model
Female Balb/C mice were divided into five groups comprising of six animals in each. Group I with dextran alone was kept as control and group II was treated with standard reference drug—diclofenac (10 mg/kg). Group III, IV, and V were administered with different concentrations of turmeric oil (100, 500, and 1 000 mg/kg body weight) intraperitoneally for 5 consecutive days. One hour after the fifth dose, acute inflammation was induced by subplantar injection of 0.2 ml freshly prepared 1% suspension of dextran in 0.1% carboxymethyl cellulose.[19] The paw thickness was measured using vernier calipers andrecorded every hour up to 6th hour, and finally at 24 hours in presence and absence of turmeric oil.
Formalin-induced Chronic Inflammatory Model
Female Balb/C mice were divided into five groups. Group I was kept as control and group II was treated with standard drug—diclofenac (10 mg/kg). Group III, IV, and V were treated with different concentrations of turmeric oil (100, 500, and 1 000 mg/kg body weight) intraperitoneally for 5 consecutive days. On the fifth day, chronic inflammation was induced by subplantar injection of freshly prepared 0.02 ml of 2% formalin on the right hind paw in all animals.[20] The paw thickness was measured using vernier calipers for 6 consecutive days after injection of formalin.
Antinociceptive Activity of Turmeric Oil
A total of 30 male Balb/C mice were divided into five groups and treated as follows: Group I served as control and group II received aspirin (100 mg/kg b.wt); group III, IV, and V received 100, 500, and 1000 mg/kg body weight of the turmeric oil, respectively. After the 30-minute pretreatment, each group was administered (10 ml/kg) with 0.6% acetic acid. Mice were then placed in observation boxes and antinociceptive activity was expressed as the percentage inhibition of abdominal constrictions compared with control animals.
Statistical Analysis
The statistical significance was compared between control and experimental groups by one way analysis of variance (ANOVA) followed by appropriate post hoc test (Dunnett multiple comparison test) using Graph pad in Stat software; P < 0.05 was considered as significant. All values are expressed as mean ± S.D.
Results
Composition of Turmeric Oil
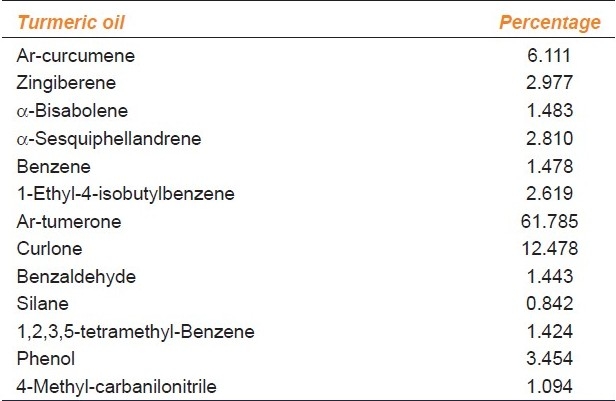
Compounds were identified by GCMS analysis of turmeric oil and are given in Table 1. The major ingredient of turmeric oil was ar-turmerone (61.79%), curlone (12.48%), and ar-curcumene (6.11%).
Table 1.
Chemical composition of turmeric oil
Antioxidant Activities of Essential Oil from Spices In Vitro
The turmeric oil was found to scavenge superoxide, hydroxyl radicals, and inhibit tissue lipid peroxidation. The concentration of turmeric oil needed for 50% scavenging (IC50) of superoxides was 135 μg/ml and for hydroxyl radicals was 200 μg/ml. IC50 for inhibition of lipid peroxidation was 400 μg/ml. The ferric-reducing activity for 50 μg of turmeric oil was found to be 5 mM. Turmeric oil also possessed mild DPPH radical scavenging ability and IC50 was more than 1 000 μg/ml. Turmeric oil showed only a mild capacity to scavenge ABTS radical [Figure 1].
Figure 1.
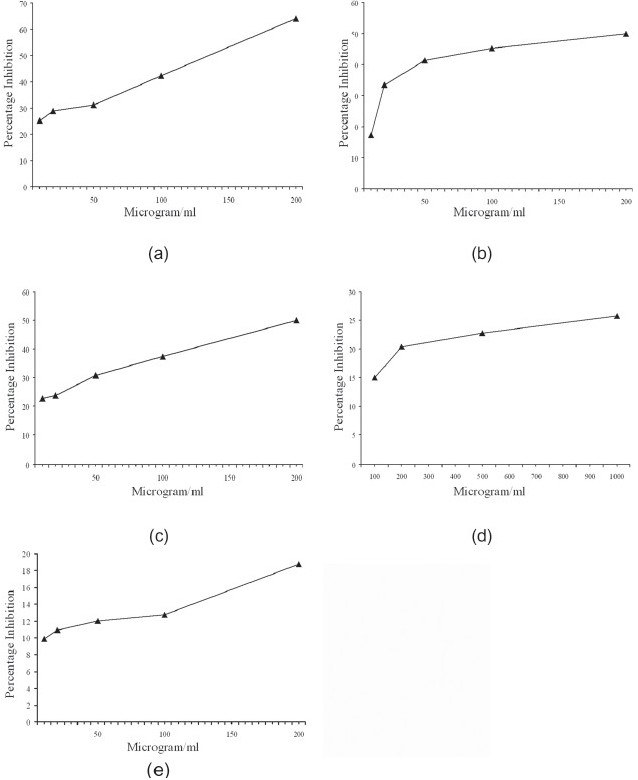
In vitro free radical scavenging activity of turmeric oil. (a) Superoxide radical scavenging activity. (b) Hydroxyl radical scavenging activity. (c) Inhibition of lipid peroxidation. (d) DPPH radical scavenging activity. (e) ABTS radical scavenging activity
Effect of Turmeric Oil on Phorbol-12-myristate-13-acetate-induced Superoxide Radical Generation
Superoxide radicals generated during the activation of PMA in sodium caseinate-induced macrophages in vivo were found to be scavenged by turmeric oil. The percentage inhibition was 22.86%, 18.25%, and 14.17% for turmeric oil at 250, 50, and 10 mg/kg b.wt, respectively.
Effect of Administration of Turmeric Oil on Antioxidant Enzymes and Glutathione
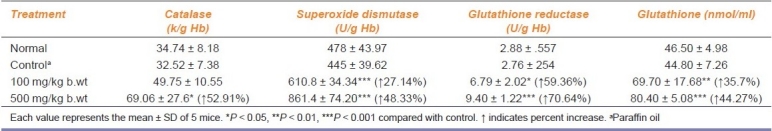
Antioxidant enzymes in blood and serum of mice were increased after administration of turmeric oil (100 and 500 mg/kg b.wt) for a period of 30 days [Table 2]. Catalase was found to be increased in all animals treated with 100 and 500 mg/kg b.wt (P < 0.05) of turmeric oil (P < 0.05). The turmeric oil administration significantly elevated superoxide dismutase (P < 0.001) at both concentrations. Glutathione level was also found to be increased in all treated groups and significant increase was shown by turmeric oil at 100 mg/kg b.wt (P < 0.01) and 500 mg/kg b.wt (P < 0.001). Glutathione reductase was elevated by the administration of turmeric oil (P < 0.05) in all animals treated with 100 mg/kg b.wt, and highest activity was shown by turmeric oil at 500 mg/kg b.wt (P < 0.001).
Table 2.
Effect of oral administration of turmeric oil on antioxidant systems in blood
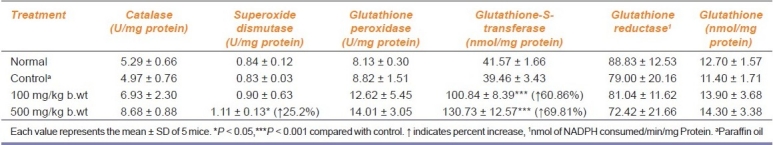
Turmeric oil also showed a significant effect on the antioxidant enzymes in liver tissue of mice after treatment for 30 days [Table 3]. Catalase did not change significantly in any of the treated groups. Superoxide dismutase activity was elevated in turmeric oil treated with 500 mg/kg b.wt (P < 0.01). The level of glutathione peroxidase enzyme was increased by turmeric oil at 100 and 500 mg/kg b.wt. Even though glutathione was found to be increased, it was not found significant. The level of glutathione reductase was found to be unaltered in all the treated groups. GST level was found to be elevated significantly in animals treated with turmeric oil (P < 0.001).
Table 3.
Effect of oral administration of turmeric oil on antioxidant systems in liver
Carrageenan-induced Acute Inflammatory Model
Carrageenan-induced acute inflammation model paw edema was significantly reduced at 3rd hour by 61.1% in animals treated with 1 000 mg/kg b.wt. turmeric essential oil when compared with control group. At doses of 500 and 100 mg/kg b.wt., turmeric oil produced 50% and 33.3% inhibition, respectively, at third hour [Figure 2]. The inhibition percentage in standard (diclofenac, 10 mg/kg) group was 55.56%.
Figure 2.
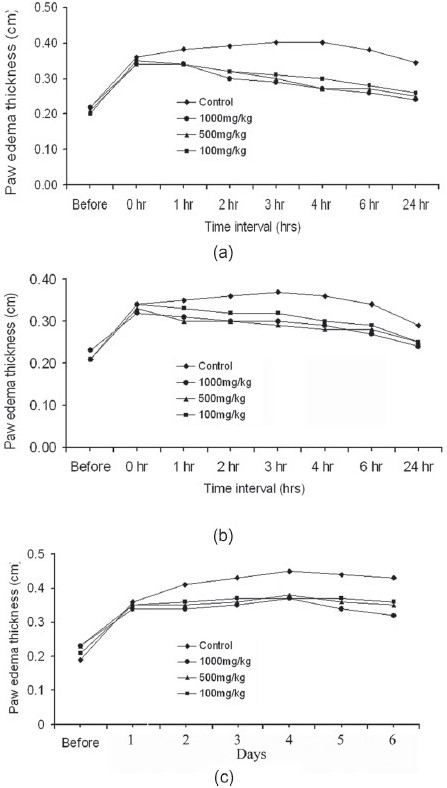
Effect of turmeric oil on carrageenan-, dextran-, and formalin-induced paw edema. (a) Carrageenan model. (b) Dextran model. (c) Formalin model
Dextran-induced Acute Inflammatory Model
The turmeric oil at a dose of 1 000 mg/kg b.wt significantly reduced the paw thickness by 58.8% in the 3rd hour when compared with the control group [Figure 2]. At doses of 500 and 100 mg/kg b.wt., turmeric oil reduced the paw edema by 47.1% and 35.3%, respectively. The percentage inhibition in standard (diclofenac, 10 mg/kg) group was 52.94%.
Formalin-induced Chronic Inflammatory Model
Turmeric essential oil inhibited the paw edema in a significant manner in acute inflammatory models [Figure 2]. The percentage inhibition of paw edema was 34.17% (P < 0.01), 41.67% (P < 0.01), and 50% (P < 0.001) in 100, 500, and 1 000 mg/kg of turmeric oil-treated groups. The percentage inhibition of paw edema of turmeric oil was comparable with diclofenac at 10 mg/kg (54.80%).
Antinociceptive Activity of Turmeric Oil by Writhing Test
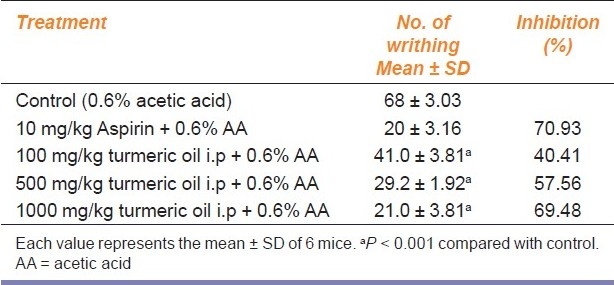
Intraperitoneal administration of turmeric oil produced a marked reduction in the number of writhings induced by acetic acid and the effects were also compared with that of aspirin. Turmeric essential oil significantly reduced the nociceptive response at 100, 500, and 1 000 mg/kg body weight by 40.41% (P < 0.001), 57.56% (P < 0.001), and 69.48% (P < 0.001), respectively [Table 4]. Standard group which received aspirin showed 70.93% (P < 0.001) of inhibition in writhing movement.
Table 4.
Study of antinociceptive activity of turmeric oil by acetic acid-induced writhing test
Discussion
The GCMS of essential oil of Curcuma longa L. revealed that the major compound present in the essential oil was ar-turmerone. The significant antioxidant activity shown by turmeric oil may be due to the presence of its principle constituent ar-turmerone. There are some reports in literature indicating the in vitro antioxidant potential of turmeric oil[2,4] which may be due to the presence of phenyl ring portion and a 13-unsaturated ketone function of ar-turmerone.[21] Antioxidant activity of the spices essential oil can be used for reducing rancidity in food. The antioxidant properties of turmeric oil can be utilized by food industry as alternatives to synthetic food preservatives.
Turmeric oil displayed significant anti-inflammatory activities in the acute and chronic models of inflammation. NO, superoxide (O2–) and their reaction product peroxynitrite (ONOO_) are generated in excess during the host response against infections and inflammatory conditions. In the acute models, carrageenan-and dextran-induced paw edemas were reduced by administration of turmeric oil. Carrageenan is known for its classic biphasic effect, first phase mediated by release of histamine and serotonin during the first hour and release of kinnins up to 2.5 hour, while the second phase is mediated by release of prostaglandins, proteases, and superoxide radicals from 2.5 to 6 hours.[22] Dextran-induced paw edema is a consequence of liberation of histamine and serotonin from mast cells. In the present study, turmeric oil showed dose-dependent inhibition of second phase of carrageenan-induced paw edema, suggesting the inhibition of inflammatory mediators and scavenging of free radicals. Injection of formaldehyde (chronic inflammation) into paw of mice produced significant local inflammation. Formaldehyde-induced paw edema is mediated by an early release of substance bradykinin (neurogenic phase) followed by a tissue-mediated response involving release of histamine, 5-hydroxytryptamine, prostaglandins, and bradykinin.[23] The reduction in paw edema by turmeric oil may be due to the inhibition of these substances. Extensive research has revealed that most chronic illnesses, including cancer, diabetes, and cardiovascular and pulmonary diseases, are mediated through chronic inflammation. Thus, suppressing chronic inflammation has the potential to delay, prevent, and even treat various chronic diseases, including cancer.
Oral administration of turmeric oil reduced acetic acid-induced writhing movement. The analgesic effects of acetic acid are produced due to liberation of mediators such as histamine, serotonin, bradykinin, cytokines, and eicosanoids.[24] These factors promote increase of vascular permeability as well as reduce the threshold of nociception and stimulate the nervous terminal of nociceptive fibers.[25] Antinociceptive activity of turmeric oil is related to the reduction of the liberation of these inflammatory mediators or to the blockage of receptors resulting in peripheral antinociceptive effect. There was also significant increase in the activity of GST in the liver of all animals treated with turmeric oil. As GST is involved in carcinogen detoxification, our results indicate that the turmeric oil increase carcinogen detoxification and may reduce cancer incidence. So, in addition to imparting flavor to food, essential oils also possess potential health benefits by scavenging the free radicals formed in the body and increasing the levels of carcinogen-detoxifying enzymes.
These results indicate that in addition to imparting flavor to food, turmeric oil also possess potential health benefits by scavenging the free radicals formed in the body, and thereby act as anti-inflammatory, antinociceptive agents. Moreover, increase in the levels of GST indicates that turmeric oil can increase carcinogen detoxification in the body and thereby act as a cancer-preventing agent.
Footnotes
Source of Support: Grant from Spices Board, Cochin, India
Conflict of Interest: None declared.
References
- 1.Reische DW, Lillard DA, Eitenmiller RR. Antioxidants in food lipids. In: Ahoh CC, Min DB, editors. Chemistry, nutrition and biotechnology. New York: Marcel Dekker; 1998. pp. 423–48. [Google Scholar]
- 2.Sacchetti G, Maietti S, Muzzoli M, Scaglianti M, Manfredini S, Radice M, et al. Comparative evaluation of 11 essential oils of different origins as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005;91:621–32. [Google Scholar]
- 3.Negi PS, Jayaprakasha GK, Jaganmohan Rao L, Sakariah KK. Antibacterial activity of turmeric oil: A byproduct from curcumin manufacture. J Agri Food Chem. 1999;47:4297–300. doi: 10.1021/jf990308d. [DOI] [PubMed] [Google Scholar]
- 4.Jayaprakasha GK, Jena BS, Negi PS, Sakariah KK. Evaluation of Antioxidant Activities and Antimutagenicity of Turmeric Oil: A Byproduct from Curcumin Production. Z Naturforsch C. 2002;57:828–35. doi: 10.1515/znc-2002-9-1013. [DOI] [PubMed] [Google Scholar]
- 5.Mc Cord JM, Fridovich I. Superoxide dismutase enzyme function for erythrocaprein. J Biol Chem. 1969;244:6049–55. [PubMed] [Google Scholar]
- 6.Ohkawa H, Oshishi N, Yagi K. Assay for lipid peroxide in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 7.Aquino R, Morellis S, Lauro MR, Abdo S, Saija A, Tomaino A. Phenolic constituents and antioxidant activity of an extract of Anthurium vesicular leaves. J Nat Prod. 2001;64:1019–23. doi: 10.1021/np0101245. [DOI] [PubMed] [Google Scholar]
- 8.Alzoreky N, Nakahara N. Antioxidant activity of some edible Yemeni plants evaluated by Ferryl myoglobin / ABTS assay. J Food Sci Technol Res. 2001;7:144–6. [Google Scholar]
- 9.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power” - The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 10.Dwivedi PD, Verna AS, Ray PK. Induction of immune rejection of tumours by 343 protein A in mice bearing transplantable solid tissue Dalton's lymphoma tumors. ImmunopharmacolImmunotoxicol. 1992;14:105–28. doi: 10.3109/08923979209009215. [DOI] [PubMed] [Google Scholar]
- 11.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folinphenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 12.Drabkin DL, Austin M. Spectrometric studies; spectrometric constants for common haemoglobin derivatives in human, dog and rabbit blood. J Biol Chem. 1932;98:719–33. [Google Scholar]
- 13.Aebi M. Catalase estimation. In: Berg Meyer HV, editor. Methods of Enzymatic Analysis. New York: Verlag Chemie; 1974. pp. 673–84. [Google Scholar]
- 14.Moron MA, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione-S-transferase activities in rat liver. Biochim Biophys Acta. 1979;582:67–8. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 15.Racker E. Glutathionreductase (Liver and yeast) In: Sidney PC, Nathen OK, editors. Method of Enzymology. New York: Academic Press; 1955. pp. 722–5. [Google Scholar]
- 16.Hafeman DG, Sundae RA, Houestra WG. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr. 1974;104:580–7. doi: 10.1093/jn/104.5.580. [DOI] [PubMed] [Google Scholar]
- 17.Habig WH, Pabst MJ, Jakoby W B. Glutathione-S-transferase, the first enzymatic step in mercapturic formation. J Biol Chem. 1974;247:7130–9. [PubMed] [Google Scholar]
- 18.Winter CA, Risly EA, Nuss GW. Carrageenan induced edema in hind paw of the rats as assay for anti inflammatory drugs. Proc Soc Exp Bio Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 19.Maity TK, Mandal SC, Mukherjee PK. Studies on anti inflammatory effect of Cassia tora leaf extract. (fam. Leguminosae) Phytother Res. 1998;12:221–4. [Google Scholar]
- 20.Chau TT. New York: Alan R LissInc; 1989. Analgesic testing in animals models. In Pharmacological methods in the control of inflammations; pp. 448–52. [Google Scholar]
- 21.Baik KU, Jung SH, Ahn BZ. Recognition of pharmcophore of ar-turmerone for its anticancer activity. Arch Pharmacal Res. 1993;16:254–6. [Google Scholar]
- 22.Di Rosa M, Giroud JP, Willoughby DA. Studies on the mediators of acute inflammatory response induced in rats in different sites of carrageenan and turpentine. J Pathol. 1971;104:15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- 23.Wheeler-Aceto H, Cowan A. Neurogenic and tissue mediated components of formalin-induced edema: Evidence for supraspinal regulation. Agents Actions. 1991;34:264–9. doi: 10.1007/BF01993299. [DOI] [PubMed] [Google Scholar]
- 24.Deraedt R, Jougney S, Delevalcee F, Falhout M. Release of prostaglandin E and F in an algogenic reaction and its inhibition. Eur J Pharmacol. 1980;51:17–24. doi: 10.1016/0014-2999(80)90377-5. [DOI] [PubMed] [Google Scholar]
- 25.Martinez V, Thakur S, Mogil JS, Tache Y, Mayer EA. Differential effects of chemical and mechanical colonic irritation on behavioral pain response to intraperitoneal acetic in mice. Pain. 1999;81:179–86. doi: 10.1016/s0304-3959(99)00008-1. [DOI] [PubMed] [Google Scholar]


