Abstract
Objective:
The role of ovarian hormones and nitric oxide on morphine-induced antinociception and their interaction have been widely investigated. The results of previous study showed that nitric oxide synthase inhibition differently affects morphine-induced antinociception in male and female rats. The present study was carried out to evaluate the different effects of chronic administration of L-arginine (LA) and L-NAME (LN) on morphine-induced antinociception in ovariectomized (OVX) and naive female rats.
Materials and Methods:
Sixty female rats were randomly divided into six groups (n = 10) as follows: (1) sham, (2) OVX, (3) sham-LA (4) sham-LN (5) OVX-LA, and (6) OVX-LN. The animals of sham-LA and OVX-LA groups received daily injection of 200 mg/kg LA (i.p.) during 6 weeks, while in sham-LN and OVX-LN groups, the animals were treated with 10 mg/kg LN (i.p.). The animals of sham and OVX groups received 2 ml/kg saline (i.p.) instead of LA and LN. Finally, all the animals were tested on the hot plate test (52 ± 0.2°C; cut-off time 80 seconds) for evaluating the antinociceptive effects of morphine. The hot plate test was performed as three base records with a 15-min interval before the injection of morphine (10 mg/kg; s.c.) following which it was repeated every 15 min after injection. Analgesic effect of morphine was quantified as maximal percent effect (MPE). Base reaction latency times (seconds) before the injection of morphine and MPE after the injection of morphine were compared using repeated-measure analysis of variance (ANOVA) followed by post-hoc Tukey's test. Differences were considered statistically significant when P < 0.05.
Results:
Before injection of morphine, there was no significant difference observed between sham and OVX groups in three recorded base reaction latency times. The base reaction latency times in sham-LA group were significantly higher than those of sham group (P < 0.001). In sham-LN group, the base reaction latency times were nonsignificantly lower than those of sham group (P = 0.095). There was no significant difference between OVX-LA group and OVX group. In OVX-LN group, three base reaction latency times were nonsignificantly lower than those of OVX group (P = 0.077). MPE in sham-LN group was higher than that of sham group (P < 0.05); however, there was no significant difference between sham-LA and sham groups.
Conclusion:
It is concluded that NO has a role in pain perception and the analgesic effect of morphine. The effect of NO might be differing in the presence or absence of ovarian hormones, but further investigations need to be done.
Keywords: L-arginine, L-NAME, morphine, ovariectomy, pain, hot plate, rat, analgesia
Introduction
There is evidence that the prevalence of some disorders such as migraine, fibromyalgia and temporomandibular joint disorder, which are accompanied by chronic pain, is higher in females compared to males.[1] A direct or indirect effect of gonadal hormones on pain perception has been reported.[2] Presence of the estrogen receptors in brain areas which are related to pain confirms that sex hormones contribute to pain perception or even analgesia.[3] Regarding the fact that androgen and estrogen receptors are present in periaqueductal gray (PAG) which is involved in descending pain inhibition and morphine antinociception,[4] it seems that sex hormones have some effects on the opioid system.
Nitric oxide (NO), an important modulator of neuronal functions,[5] is synthesized from L-arginine (LA) by NO synthase (NOS).[6] NO is involved in nociceptive processes.[6] It has been shown that NOS inhibition potentiates carrageenan-induced hyperalgesia.[7] In contrast, it has been reported that administration of LA facilitates the nociceptive tail flick reflex.[8] The role of NO on analgesic effects of morphine has also been reported. Spinal cord NOS inhibition has been found to enhance the antinociception mediated by mu, delta and kappa opioid receptors.[9]
The interaction between estrogen and NO system may be responsible for the morphine-induced antinociception. It has been well documented that estrogen influences the NO system in both peripheral and nervous tissues.[10] It has been observed that estrogen increases endothelial NOS (eNOS) activity and expression.[10] There is evidence showing that estrogen influences neuronal NOS (nNOS) mRNA, the number of nitrergic neurons and NO production in the brain.[10]
Interaction of both sex hormones and NO with other neurotransmitters such as gamma-aminobutyric acid (GABA), acetylcholine, serotonin and dopamine, which have some roles in antinociceptive properties of morphine and pain perception, has been widely documented.[11] The results of a previous study showed that NOS inhibition differently affects morphine-induced antinociception in male and female rats.[12] Therefore, the aim of this study was to evaluate different effects of chronic administration of LA (the precursor of NO) and L-NAME (LN; NOS inhibitor) on morphine-induced antinociception in ovariectomized (OVX) and naive female rats.
Materials and Methods
Animals and Chemicals
Eight-week-old female Wistar rats (200 ± 10 g) were obtained from the Razi Vaccine and Serum Research Institute of Razi Institute for Vaccine and Serum Production (Mashhad, Iran). The animals were housed as 4–5 in number per standard cage at room temperature (24 ± 1°C) on a 12-h light/dark cycle. Rats were provided ad libitum access to food and water. Rats were given 1 week to adapt to this new environment before any procedure was initiated. Animal handling and all related procedures such as nociceptive tests were approved by the Mashhad Medical University Committee on Animal Research.
The drugs used were L-arginine, L-NAME (Sigma, St. Louis, MO, USA) and morphine sulfate (TEMAD Ltd., Teheran, Iran). All the drugs were dissolved in saline solution.
Surgery
Rats were ovariectomized under ketamine anesthesia (150 mg/kg, i.p.).[13] Anesthesia was confirmed by reduced respiratory rate and no response to gentle pinching of foot pad. Ventral incision was made through the skin of the flank of the rat and ovaries, and ovarian fats were removed. Ovaries were isolated by ligation of the most proximal portion of the oviduct before removal.[13] The animals were reversed to their cages to recover from surgery. After 6 weeks, the animals were tested in hot plate.
Nociceptive Test
To assess nociceptive responses, hot plate method was used. In hot plate method, the rats were placed on a hot plate with temperature setting controlled at 52 ± 0.2°C. Cut-off time was 80 seconds.[12] Nociceptive response is defined as licking or moving fore paws or moving hind paws. Time duration between placing the animals on hot plate and licking fore paws or moving hind paws was considered as the reaction time. The hot plate test was performed as three base records with an interval of 15 min before the injection of morphine (10 mg/kg; s.c.)[12] following which it was repeated five times every 15 min after injection.[12] Data were expressed as time latency or as percentage of maximum possible effect (%MPE) which was calculated as follows:
%MPE = (latency after drug administration – baseline latency)/(80 – baseline latency) ×100.
Procedure
Sixty rats were divided into six groups (n = 10) as follows: (1) sham, (2) OVX, (3) sham-LA, (4) sham-LN, (5) OVX-LA, and (6) OVX-LN.
The animals of sham-LA and OVX-LA groups received 200 mg/kg LA[12] every day during 6 weeks. The animals of sham-LN and OVX-LN groups received 10 mg/kg LN[12] every day during 6 weeks. The animals of sham and OVX groups received 2 ml/kg saline instead of LA and LN every day during 6 weeks. Finally, all the animals were tested on the hot plate test for evaluating the antinociceptive effects of morphine.
Statistical Analysis
All data were presented as mean ± SEM of reaction time. Base reaction latency times (seconds) before the injection of morphine and MPE after the injection of morphine were compared using repeated-measure analysis of variance (ANOVA) followed by post-hoc Tukey's test. Differences were considered statistically significant when P < 0.05.
Results
The Chronic Effects of L-Arginine and L-NAME on Base Reaction Latency Times
Before the injection of morphine, the three recorded base reaction latency times in sham groups were 27.09 ± 3.07, 35.17 ± 3.49 and 28.19 ± 2.42 seconds. The animals of OVX groups had latency times of 27.54 ± 1.7, 37.65 ± 5.01 and 21.61 ± 4.1 seconds before the injection of morphine; there was no significant difference between the two groups with repeated-measure ANOVA test [Figure 1].
Figure 1.
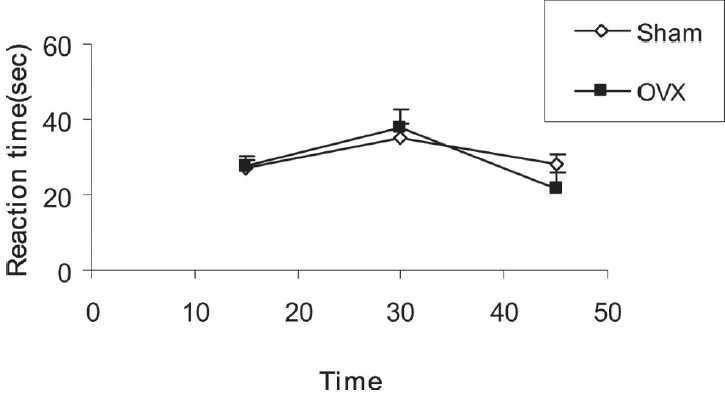
Comparison of base reaction latency times between sham and OVX groups. Data are presented as mean ± SEM (n = 10 in each group). There was no significant difference between the two groups when repeated-measure ANOVA was used
The baseline reaction latency times before the injection of morphine in sham-LA group were 19.36 ± 0.9, 20.55 ± 2.17 and 18.4 ± 2.56 seconds and were significantly higher than those of sham group (P < 0.001) [Figure 2]. In sham-LN group, the base reaction latency times were 36.99 ± 1.5, 38.14 ± 4.04 and 34.08 ± 3.83 seconds and were nonsignificantly lower than those of sham group (P = 0.095) [Figure 2].
Figure 2.
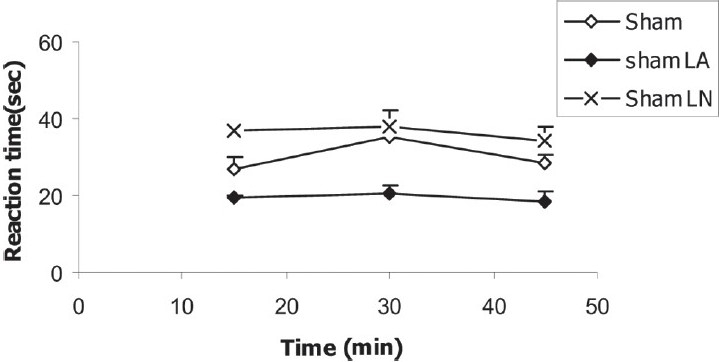
Comparison of base reaction latency times between sham, sham-LA, and sham-LN groups. Data are presented as mean ± SEM (n = 10 in each group). Repeated-measure ANOVA test showed that base reaction latency times in sham-LA group were significantly lower than those of sham group (P < 0.001). However, in sham-LN group, it was nonsignificantly higher than that of sham group (P = 0.09)
The animals of OVX-LA group had base reaction latency times of 33.55 ± 5.7, 27.44 ± 4.5 and 21.94 ± 3.5 seconds; there was no significant difference between this group and OVX group [Figure 3]. In OVX-LN group, the three base reaction latency times were 41.46 ± 4.7, 39.16 ± 3.9 and 32.61 ± 4.4 seconds and were nonsignificantly lower than those of OVX group (P = 0.077) [Figure 3].
Figure 3.
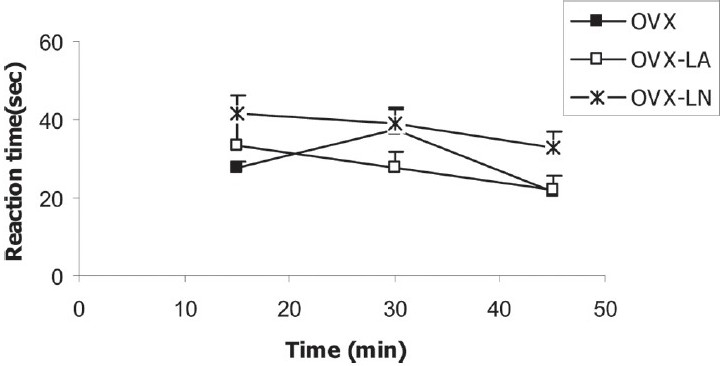
Comparison of base reaction latency times between OVX, OVX-LA and OVX-LN groups. Data are presented as mean ± SEM (n = 10 in each group). There was no significant difference between OVX-LA group and OVX group; however, in OVX-LN group, the three base reaction latency times were nonsignificantly lower than those of OVX group (P = 0.077)
The Chronic Effects of l-Arginine and l-NAME on MPE(%) After the Injection of Morphine
After the injection of morphine, there was no significant difference between sham and OVX groups in MPE [Figure 4]. MPE in sham-LN group was higher than that of sham group (P < 0.05); however, there was no significant difference between sham-LA and sham groups [Figure 5].
Figure 4.
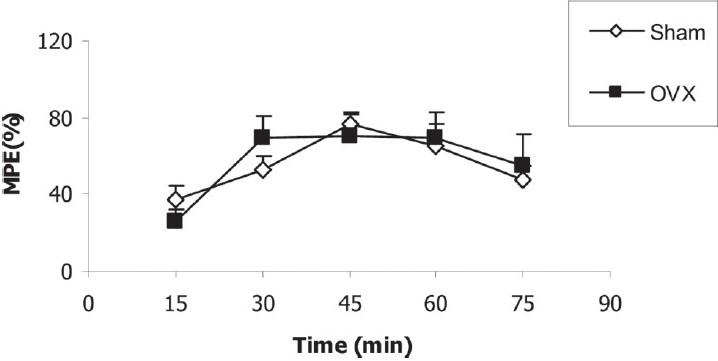
Comparison of MPE between sham and OVX groups. Data are presented as mean ± SEM (n = 10 in each group). There was no significant difference between the two groups when repeated-measure ANOVA was used
Figure 5.
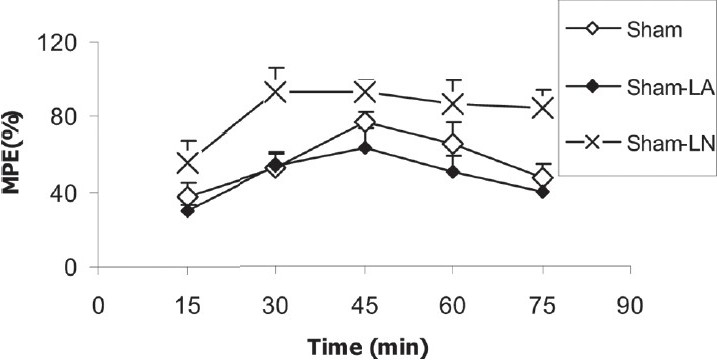
Comparison of MPE between sham, sham-LA and sham-LN groups. Data are presented as mean ± SEM (n = 10 in each group). Analysis with repeated-measure ANOVA showed that MPE in sham-LN group was higher than that of sham group (P < 0.05)
There was no significant difference between OVX, OVX-LN and OVX-LA groups in MPE [Figure 6].
Figure 6.
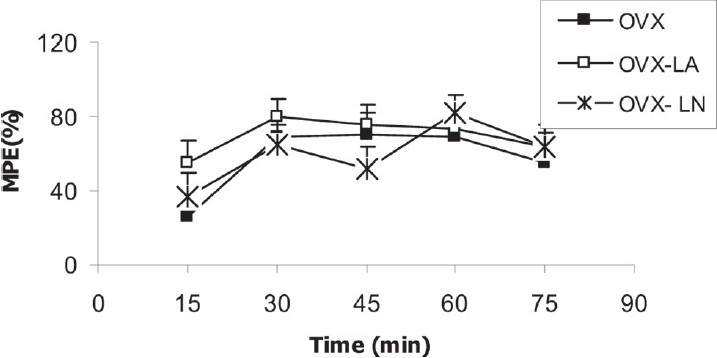
Comparison of MPE between OVX, OVX-LA and OVX-LN groups. Data are presented as mean ± SEM (n = 10 in each group). There was no significant difference between the three groups when repeated-measure ANOVA was used
Discussion
It has been hypothesized that sex differences in opioid antinociception and pain perception are a consequence of actions of female or male gonadal hormones.[2] Interaction of both sex hormones and NO with neurotransmitters which are involved in pain modulation has been widely documented.[11] On the other hand, the role of NO in sex hormone dependent changes of behavioral responses of female rats has also been reported.[14] In the present study, the chronic effect of LA and LN on nociception and morphine-induced antinociception in OVX and naive female rats was investigated. Hot plate test used in the present study is a well-known standard method for pain threshold evaluation after administering morphine or other analgesic drugs.[12]
The results of this study show no significant differences in base reaction latency times between sham and OVX rats. This implies that there is no difference in pain threshold in the presence and absence of ovarian hormones while using hot plate test set at 52 ± 0.2°C. These results are consistent with the result of Ali et al. who showed using hot plate test that baseline reaction in OVX rats was not significantly different from that of sham animals.[15] In a previous study, we also showed that there was no significant difference between male and female rats in base reaction latency time.[12] In contrast, the result of another study demonstrated that hot plate latencies in sham animals were longer than those of OVX females.[4] The discrepancy in the results may be due to the kind of tested animal or the temperature of hot plate test. It has been shown that treatment of OVX Sprague-Dawley rats with estrogen or estrogen/progesteron significantly prolonged the reaction time latency on the 50°C hot plate test, but there were no significant differences on the 54°C hot plate test.[16] It has been also reported that baseline latencies in F344 OVX rats were longer than those of sham animals, whereas they were similar in the Sprague-Dawley sham and OVX rats.[17]
NO is a gaseous messenger which is produced in different cell types.[6] It acts as a neuronal messenger and a modulator of neurotransmission in the nervous system.[5] NO appears to act as a pronociceptive[6,8] as well as an antinociceptive agent. In the present study, three recorded base reaction latency times were compared between sham, sham-LA and sham-LN groups. The results show that chronic administration of the precursor of NO, LA, attenuates the reaction time in hot plate test. It seems that LA directly or by increasing the production of NO acts as a pronociceptive agent in the presence of ovarian hormones. This result is in agreement with the result of Ferreira et al. who showed that LN inhibits, but LA potentiates the glutamate-induced hyperalgesia.[6] It was also shown that LN had an antinociceptive effect in the capsaicin-induced paw licking procedure which was reversed by LA.[18] Although the reports are very controversial, it seems that NO may have a role as a pronociception agent. It has been demonstrated that NO donors enhance the release of neuropeptides such as substance P and calcitonin gene-related peptide(CGRP) which have a role in pain transmission.[19] Also, it has been suggested that LA acts as a pronociceptive via the NO-cycle GMP pathway in the brain and spinal cord.[8] Considering the results of the present study, it might be suggested that the effect of LA or even the role of NO in nociception is different in the presence or absence of ovarian hormones; there was no significant difference in base reaction latency times between OVX-LA and OVX groups.
The results of the present study also show that injection of morphine (10 mg/kg) had analgesic effect in both sham and OVX rats; reaction latency time was increased in both sham and OVX groups in comparison with base reaction latency. However, there was no significant difference in MPE between sham and OVX rats. Banerjee et al. reported that response to morphine was attenuated in OVX rats, while 17 beta-estradiol replacement therapy did not alter this attenuation,[20] although Kepler et al. reported that analgesic effects of mu and delta selective agonists were not affected by gonadectomy in rats.[21] It has also been shown that in OVX rats, the percentage of increase in reaction time after injection of 5 mg/kg morphine was significantly higher than that of control group.[15] In another study, morphine antinociceptive potency in castrated female increased, only when compared to sham tested during estrus.[16] However, the results of another study showed that hot plate response to hydromorphone in female intact rats in any stage of ovarian cycle was not different from that of gonadectomized females.[4] Other researchers suggested that morphine was more potent in OVX rats when tested in a warm-water, tail-withdrawal procedure.[2] All of these findings confirm that sex hormones might have an important role in opioidergic systems and may modulate the antinociception effects of opioids. The results of our previous study also showed that 10 mg/kg morphine had more analgesic effects in male rats in comparison with the effects on females.[12] The presence of high opioid receptor density in hypothalamus during proestrous phase, when estrogen levels are elevated,[2] may affect the analgesic functions of opioids. The association of high levels of estrogen with increased levels of proenkephalin gene expression has also been reported.[22] The change of kappa opioid receptor density in lumbosacral spinal cord during the estrous cycle has also been shown.[2] There are evidences showing that estrogen decreases the functional coupling of the μ-opioid and GABA receptors and suppresses mu-opioid and GABA-mediated hyperpolarization of hypothalamic arcuate neurons.[23]
NO is a gaseous messenger which appears to act as a pronociceptive[6,8] as well as an antinociceptive agent.[2] Ferreira et al. reported that injection of LN inhibited glutamate-induced hyperalgesia, but LA potentiated it significantly.[6] Sakurada also demonstrated that in the capsaicin-induced paw licking procedure, administration of LN produced a significant antinociceptive effect, while it was reversed by LA administration.[18] Although the reports are very controversial, it seems that NO may have a role as a pronociception agent. It has been demonstrated that NO donors enhance the release of the neuropeptides such as substance P and CGRP which have a role in pain transmission.[19] It has been also suggested that LA (the precursor of NO) acts as a pronociceptive via the NO-cycle GMP pathway in the brain and spinal cord.[8] On the other hand, it has been documented that NO has a modulatory function on antinociceptive effects of opioids.[9] In the present study, chronic administration of LN increased the antinociceptive effects of morphine in sham-operated rats. Xu and Tseng (1995) also reported that the antinociceptive effect of morphine was increased by injection of N(omega)-nitro-L-argnine (L-NNA) (NOS inhibitor), but was attenuated by administration of LA.[5] In contrast, Pataki and Telegdy (1998) reported that L-NNA diminished the morphine-induced analgesia. However, pretreatment with LA potentiated the analgesic effect of morphine.[24] It might be suggested that chronic treatment with LN decreases brain NOS activity and probably increases the morphine-induced antinociception by modification of the transport of morphine across the blood–brain barrier or its effect may be related to alterations in the distribution of morphine in the central nervous system sites involved in the nociceptive response.[9] In the present study, chronic administration of LA had no significant effects on the analgesic effects of morphine in both sham and OVX rats. It has been shown that orally or itrathecal(i.t.) administration of LA—reduced the analgesic effect of morphine in hot plate, tail flick and acetic acid tests, while i.c.v. injection of LA had no effect on morphine-induced antinociception in hot plate test.[25] It may be suggested that the route of drug administration and the duration may affect the result.
The interaction of gonadal hormones and NO system in both peripheral and nervous tissues has been well documented.[10] It has been shown that estrogen affects eNOS and nNOS activities and expression and NO levels in the nervous system.[10] The results of the present study show that the role of NO in the analgesic effect of morphine is different in the presence or absence of female sex hormones; chronic treatment of sham-operated animals by LN increased the analgesic effects of morphine; however, it had no significant effect in OVX rats. In the previous study, we also showed that chronic administration of LN (10 mg/kg) enhanced the analgesic effects of morphine in female, but not male rats.[12] It has been reported that pretreatment with LN causes a significant increase in the latency time in hot plate test only in male, but not in female mice.[5] It has been suggested that ovariectomy decreases serum estrogen level and also reduces nNOS and eNOS expression.[10] It has been suggested that estrogen replacement therapy in postmenopausal women increases the plasma levels of nitrite and nitrate.[10] Based on the results of the present study, it might be suggested that LN decreases the NOS activity in the presence but not in the absence of estrogen and it may affect the morphine-induced analgesia, with this mechanism.
In conclusion, NO has a role in pain perception and also in analgesic properties of morphine. This role of NO is different in the absence and presence of ovarian hormones. All of these data confirm that there are potential levels of interaction between sex hormones and the NO signaling systems in morphine-induced antinociception, but further investigations need to be done.
Acknowledgments
The authors would like to thank the Vice Presidency of Research of Mashhad University of Medical Sciences for financial support.
Footnotes
Source of Support: Vice Presidency of Research of Mashhad University of Medical Sciences.
Conflict of Interest: None declared.
References
- 1.Craft RM. Modulation of pain by estrogens. Pain. 2007;132(Suppl 1):S3–12. doi: 10.1016/j.pain.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 2.Terner JM, Lomas LM, Picker MJ. Influence of estrous cycle and gonadal hormone depletion on nociception and opioid antinociception in female rats of four strains. J Pain. 2005;6:372–83. doi: 10.1016/j.jpain.2005.01.354. [DOI] [PubMed] [Google Scholar]
- 3.Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: The role of gonadal hormones. Eur J Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Stoffel EC, Ulibarri CM, Folk JE, Rice KC, Craft RM. Gonadal hormone modulation of mu, kappa, and delta opioid antinociception in male and female rats. J Pain. 2005;6:261–74. doi: 10.1016/j.jpain.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu JY, Tseng LF. Nitric oxide/cyclic guanosine monophosphate system in the spinal cord differentially modulates intracerebroventricularly administered morphine-and beta-endorphin-induced antinociception in the mouse. J Pharmacol Exp Ther. 1995;274:8–16. [PubMed] [Google Scholar]
- 6.Ferreira J, Santos AR, Calixto JB. The role of systemic, spinal and supraspinal L-arginine-nitric oxide-cGMP pathway in thermal hyperalgesia caused by intrathecal injection of glutamate in mice. Neuropharmacology. 1999;38:835–42. doi: 10.1016/s0028-3908(99)00006-4. [DOI] [PubMed] [Google Scholar]
- 7.Budzinski M, Misterek K, Gumulka W, Dorociak A. Inhibition of inducible nitric oxide synthase in persistent pain. Life Sci. 2000;66:301–5. doi: 10.1016/s0024-3205(99)00421-x. [DOI] [PubMed] [Google Scholar]
- 8.Meller ST, Dykstra C, Gebhart GF. Production of endogenous nitric oxide and activation of soluble guanylatecyclase are required for N-methyl-D-aspartate-produced facilitation of the nociceptive tail-flick reflex. Eur J Pharmacol. 1992;214:93–6. doi: 10.1016/0014-2999(92)90102-a. [DOI] [PubMed] [Google Scholar]
- 9.Machelska H, Labuz D, Przewlocki R, Przewlocka B. Inhibition of nitric oxide synthase enhances antinociception mediated by mu, delta and kappa opioid receptors in acute and prolonged pain in the rat spinal cord. J Pharmacol Exp Ther. 1997;282:977–84. [PubMed] [Google Scholar]
- 10.Grohe C, Kann S, Fink L, Djoufack PC, Paehr M, van Eickels M, et al. 17 Beta-estradiol regulates nNOS and eNOS activity in the hippocampus. Neuroreport. 2004;15:89–93. doi: 10.1097/00001756-200401190-00018. [DOI] [PubMed] [Google Scholar]
- 11.Guevara-Guzman R, Emson PC, Kendrick KM. Modulation of in vivo striatal transmitter release by nitric oxide and cyclic GMP. J Neurochem. 1994;62:807–10. doi: 10.1046/j.1471-4159.1994.62020807.x. [DOI] [PubMed] [Google Scholar]
- 12.Hosseini M, Taiarani Z, Hadjzadeh MA, Salehabadi S, Tehranipour M, Alaei HA. Different responses of nitric oxide synthase inhibition on morphine-induced antinociception in male and female rats. Pathophysiology. 2011;18:143–9. doi: 10.1016/j.pathophys.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Hosseini M, Sadeghnia HR, Salehabadi S, Alavi H, Gorji A. The effect of L-arginine and L-NAME on pentylenetetrazole induced seizures in ovariectomized rats, an in vivo study. Seizure. 2009;18:695–8. doi: 10.1016/j.seizure.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Sadeghipour HR, Ghasemi M, Sadeghipour H, Riazi K, Soufiabadi M, Fallahi N, et al. Nitric oxide involvement in estrous cycle-dependent changes of the behavioral responses of female rats in the elevated plus-maze test. Behav Brain Res. 2007;178:10–7. doi: 10.1016/j.bbr.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 15.Ali BH, Sharif SI, Elkadi A. Sex differences and the effect of gonadectomy on morphine-induced antinociception and dependence in rats and mice. Clin Exp Pharmacol Physiol. 1995;22:342–4. doi: 10.1111/j.1440-1681.1995.tb02012.x. [DOI] [PubMed] [Google Scholar]
- 16.Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103:285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terner JM, Barrett AC, Grossell E, Picker MJ. Influence of gonadectomy on the antinociceptive effects of opioids in male and female rats. Psychopharmacology (Berl) 2002;163:183–93. doi: 10.1007/s00213-002-1143-x. [DOI] [PubMed] [Google Scholar]
- 18.Sakurada T, Sugiyama A, Sakurada C, Tan-No K, Yonezawa A, Sakurada S, et al. Effect of spinal nitric oxide inhibition on capsaicin-induced nociceptive response. Life Sci. 1996;59:921–30. doi: 10.1016/0024-3205(96)00390-6. [DOI] [PubMed] [Google Scholar]
- 19.Garry MG, Richardson JD, Hargreaves KM. Sodium nitroprusside evokes the release of immunoreactive calcitonin gene-related peptide and substance P from dorsal horn slices via nitric oxide-dependent and nitric oxide-independent mechanisms. J Neurosci. 1994;14:4329–37. doi: 10.1523/JNEUROSCI.14-07-04329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee P, Chatterjee TK, Ghosh JJ. Ovarian steroids and modulation of morphine-induced analgesia and catalepsy in female rats. Eur J Pharmacol. 1983;96:291–4. doi: 10.1016/0014-2999(83)90319-9. [DOI] [PubMed] [Google Scholar]
- 21.Kepler KL, Standifer KM, Paul D, Kest B, Pasternak GW, Bodnar RJ. Gender effects and central opioid analgesia. Pain. 1991;45:87–94. doi: 10.1016/0304-3959(91)90168-W. [DOI] [PubMed] [Google Scholar]
- 22.Romano GJ, Harlan RE, Shivers BD, Howells RD, Pfaff DW. Estrogen increases proenkephalin messenger ribonucleic acid levels in the ventromedial hypothalamus of the rat. Mol Endocrinol. 1988;2:1320–8. doi: 10.1210/mend-2-12-1320. [DOI] [PubMed] [Google Scholar]
- 23.Kelly MJ, Loose MD, Ronnekleiv OK. Estrogen suppresses mu-opioid-and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci. 1992;12:2745–50. doi: 10.1523/JNEUROSCI.12-07-02745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pataki I, Telegdy G. Further evidence that nitric oxide modifies acute and chronic morphine actions in mice. Eur J Pharmacol. 1998;357:157–62. doi: 10.1016/s0014-2999(98)00561-5. [DOI] [PubMed] [Google Scholar]
- 25.Brignola G, Calignano A, Di Rosa M. Modulation of morphine antinociception in the mouse by endogenous nitric oxide. Br J Pharmacol. 1994;113:1372–6. doi: 10.1111/j.1476-5381.1994.tb17149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


