Abstract
Objective:
To evaluate the concomitant administration of methotrexate and curcumin for antiarthiritic activity in rats.
Materials and Methods:
Arthritis was induced in rats following a single subplantar injection of Freund's complete adjuvant (0.1 ml). Rats were divided into six groups of six animals each. Group I and II were control injected with saline and Freund's complete adjuvant (0.1 ml), respectively. Group III arthritic rats were treated with curcumin (100 mg/kg, i.p.) on alternate days. Group IV received methotrexate (MTX) (2 mg/kg, i.p.) once in a week. Group-V and VI were treated with MTX (1 mg/kg, i.p.) once in a week and after 30 min received curcumin (30 mg/kg and 100 mg/kg, thrice a week, i.p.) from 10th to 45th days, respectively. Body weight and the paw volume was measured on 9th, 16th, 23rd, 30th, 37th, and 45th days. Determination of complete blood cell counts, hemoglobin concentration, hematocrit, mean corpuscular volume, and mean corpuscular hemoglobin concentration was determined on the 46th day.
Results:
An improvement in body weight and a significant (P < 0.05) reduction in arthritis was observed with the combination treatment as compared to the positive control. A significant improvement in the hematological profile was also observed in rats treated with curcumin and methotrexate.
Conclusion:
The study showed a significant anti-arthritic action and protection from hematological toxicity with the combination treatment of methotrexate and curcumin.
Keywords: Curcumin, Freund's complete adjuvant, hematological toxicity, methotrexate, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is characterized by non-specific inflammation of the peripheral joints, morning stiffness, destruction of articular tissues and joint deformities. Conventional treatment strategies include the use of steroids and nonsteroidal anti-inflammatory drugs (NSAIDS). Biological response modifiers like tumor necrosis factor alpha (TNF-α) and interleukin-1beta (IL-B) antagonists are also used in the management of RA. In addition, methotrexate (MTX) an antimetabolite and immunosuppressant is regarded as a disease modifying antirheumatic drug (DMARD).[1] Low dose methotrexate is widely used in the treatment of RA. However, low dose MTX can result in adverse effects like gastrointestinal, hepatic, hematological and rarely respiratory disturbances.[2]
Turmeric is obtained from the rhizomes of Curcuma longa, a member of the family Zingiberaceae. Major curcuminoids isolated from Curcuma longa includes curcumin (diferuloyl methane), demethoxycurcumin and bisdemethoxycurcumin. Curcumin is the important fraction that is responsible for the biological activities of turmeric[3] and has been extensively utilized for skin allergy, viral hepatitis, inflammatory conditions, rheumatism, urticaria, sore throat, and wounds. Research on curcumin revealed potential anti-inflammatory, anti-hyperlipidemic, anti-oxidant, anti-viral, and anti-microbial activity. Curcumin is claimed to possess anti-neoplastic, anti-apoptotic, anti-angiogenic, immunomodulatory, anti-thrombotic, anti-diabetogenic, and anti-lithogenic actions.[4,5]
Experimentally curcumin is reported to possess anti-arthritic action.[6] Hence, it was hypothesized that a combination of curcumin and MTX would be beneficial to hematological toxicity produced by MTX.
Materials and Methods
Drugs and Chemicals
Curcumin, MTX, Freund's complete adjuvant purchased from Sigma Aldrich, USA. All other reagents and chemicals used were of analytical grade.
Dose Selection
The dose of curcumin (30 and 100 mg/kg) was selected based on previous report.[7] Similarly the dose of MTX used was 2 mg/kg body weight.[8]
Animals
Female Albino Wistar rats weighing 150–230 g were procured from National Institute of Nutrition, Hyderabad and acclimatized in our own animal house for one week prior to the experiment. The animals were maintained at 22 ± 3°C under natural light dark conditions and fed on standard diet with free access to water. The experimental protocol was approved by the institutional animal ethical committee and the experiments were conducted as per guidelines of CPCSEA.
Induction of Arthritis
Following anesthesia with ketamine (120 mg/kg), 0.1 ml of Freund's complete adjuvant (FCA) (a suspension of heat-killed Mycobacterium tuberculosis in mineral oil; Sigma Aldrich, USA.) was injected into the subplantar tissue of the right posterior paw.[8] In positive control animals, subplantar injection of Freund's complete adjuvant produced a definite edema within a few hours with progressive arthritis by the 9th day after inoculation. All the treatments were initiated from the 10th day and continued till the 45th day.
Experimental Design
Animals were divided into six groups of six rats each.
Group-I: Rats were injected with saline daily by i.p route for 45 days.
Group-II: Arthritis was induced by injecting FCA (0.1 ml) in a single dose into the subplantar region of right hind paw.
Group-III: Arthritic rats were injected with curcumin in a dose of 100 mg/kg bodyweight (bw) for 5 weeks on alternate days by i.p route.
Group-IV: Arthritic rats were injected with 2 mg/kg MTX by i.p route once in a week for 5 weeks.
Group-V: Arthritic rats were injected with 1 mg/kg MTX by i.p route once in a week for 5 weeks. After 30 min of MTX treatment, same rats were injected with 30 mg/kg/thrice a week curcumin by i.p route for 5 weeks.
Group-VI: Arthritic rats were injected with 1 mg/kg MTX by i.p route once in a week for 5 weeks. Curcumin (100 mg/kg/thrice a week) was administered 30 min later by i.p. route for 5 weeks.
Body weights of animals were monitored regularly during the course of the experiment.
Arthritic Assessment
To follow the course of the disease, swelling of the adjuvant-injected hind paw was determined with a plethysmograph. The paw volume was measured on 3rd, 5th, 7th, and 9th day to confirm the development of RA. The paw volume was periodically measured on 16th, 23rd, 30th, 37th, and 45th day thereafter to confirm the reduction of RA.
Hematological Assessment
On the 46th day, animals were anesthetized with enflurane and blood was withdrawn from the retro orbital plexus into a test tube containing anticoagulant (5% EDTA) and was subjected to complete blood cell assessment. The total erythrocyte count (RBC) was determined by the method of Huxtable,[9] white blood cell count (WBC) was determined using Raghuramulu,[10] platelet was determined using the method of Brecher and Cronkite.[11] Hemoglobin concentration was estimated by the cyanmethemoglobin method of Drabkin and Austin,[12] differential leukocyte count , hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) were determined using Haemstar-21 by kinetic method.
Statistical Analysis
Data was analyzed by One-Way ANOVA followed by Dunnett comparison test using GraphPad InStat version 3.10 for Windows 2009 (GraphPad Software). P < 0.05 was considered statistically significant.
Results
Positive control animals exhibited a sharp reduction in body weight. On the other hand, treatment of arthritic rats with curcumin showed restoration in body weight; however treatment with methotrexate showed a reduction in body weight. Curcumin in higher doses (100 mg/kg) along with methotrexate in arthritic animals showed a significant (P < 0.05) increase in body weight as compared with the positive control [Figure 1].
Figure 1.
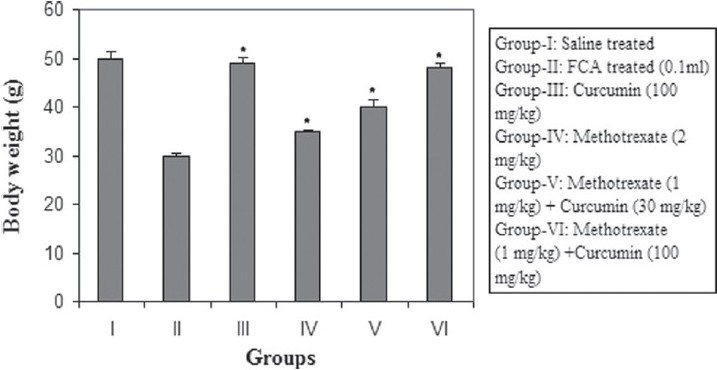
Effect of the combination of methotrexate and curcumin on mean body weight in arthritic rats. Values are mean ± SEM, n = 6, FCA-Freund's complete adjuvant, *P < 0.05 as compared with positive control
The time course of arthritis after the administration of FCA showed swelling of hind paw from the 9th day and persisted up to the end of the treatment. Treatment with MTX alone and curcumin produced significant (P < 0.05) reduction in paw volume as to the positive control [Table 1]. In addition, arthritis induced by FCA was significantly (P < 0.05) reduced with both doses of curcumin in combination with MTX when compared to positive control.
Table 1.
Effect of methotrexate and curcumin treatment on paw volume in arthritic rats (n = 6)
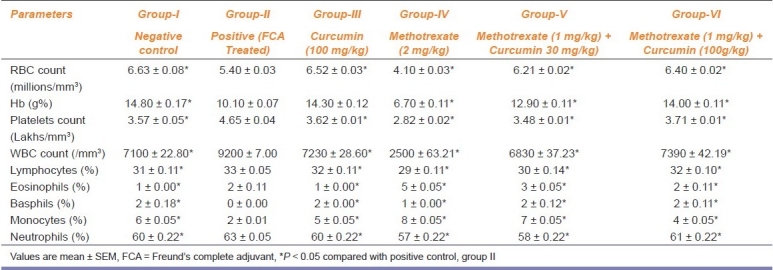
Hematological Changes
Treatment with MTX in combination with curcumin exhibited a significant (P < 0.05) elevation in the RBC count and hemoglobin levels as compared to the positive control [Table 2]. Arthritis-induced animals (group-II) showed a sharp decline in hemoglobin concentration. Mean WBC count and platelet count significantly (P < 0.05) declined in animals treated with MTX (2 mg/kg) as compared with the positive control. FCA produced alterations in platelet count to 4.65 ± 0.042 lakhs/ml. A significant (P < 0.05) improvement in platelet count occurred in rats treated with MTX and curcumin compared to the positive control [Table 2].
Table 2.
Effect of methotrexate and curcumin treatment on hematological indices in arthritic rats (n = 6)
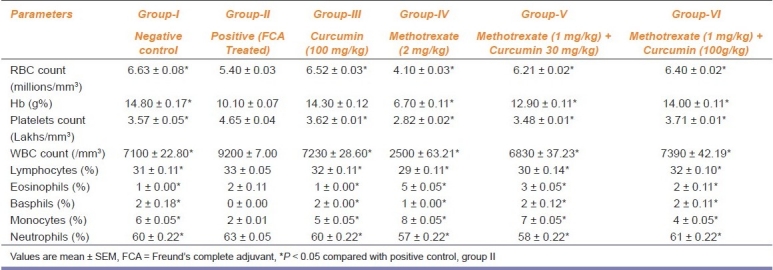
MTX treatment provoked a decline in the percentage of lymphocyte and neutrophils. MTX combined with low dose of curcumin reversed the differential count; however, MTX with curcumin (100 mg/kg) produced a significant (P < 0.05) alteration in DLC compared with positive control [Table 2].
A significant (P < 0.05) decrease in hematocrit was observed in the FCA-treated animals; however, treatment with 100 mg/kg of curcumin along with MTX produced a significant (P < 0.05) increase in the hematocrit compared to the positive control. A significant (P < 0.05) decrease in mean corpuscular volume was observed in the FCA-treated animals compared with the negative control. A significant (P < 0.05) increase in MCV was observed following methotrexate treatment as compared to positive control. The mean MCV of rats in MTX (1 mg/kg) + curcumin (100 mg/kg) was restored compared with the positive control (P < 0.05). Treatment with FCA and MTX alone produced a decrease in the MCHC values. The mean corpuscular hemoglobin and the MCHC values were significantly (P < 0.05) elevated in groups V and VI as compared to the positive control [Figure 2].
Figure 2.
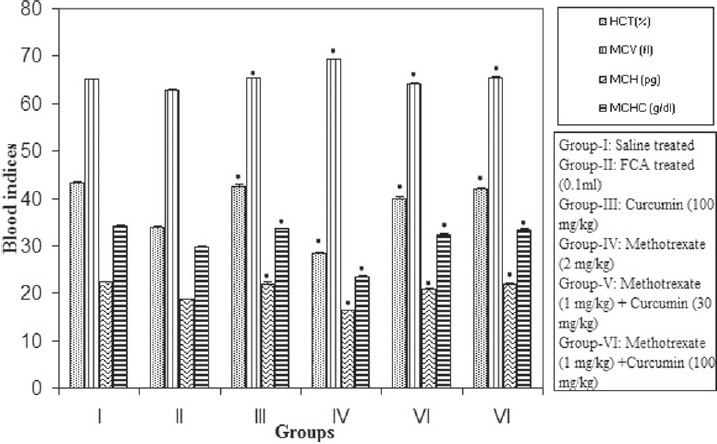
Effect of methotrexate and curcumin treatment on blood indices in arthritic rats (n = 6). Values are mean ± SEM, FCA-Freund's complete adjuvant, *P < 0.05 compared with positive control
Discussion
Freund's complete adjuvant is used to induce arthritis in animals and shows several clinical and histological similarities to human RA. In the present study, a single injection of Freund's complete adjuvant at the subplantar surface was capable of producing pronounced arthritis in the paws and ankles of animals by the 9th day. As the incidence and severity of arthritis increases, loss of body weight might be due to alterations in the metabolic activities of diseased rats.[13] Rise in the levels of TNF-a in the arthritic state has been reported to be responsible for loss of body weight.[14] A significant improvement in body weight was observed with the combination treatment could be attributed to the ability of curcumin to diminish the levels of TNF.
MTX is effective in RA as it interferes with the production of potent pro-inflammatory agents such as prostaglandins.[15,16] MTX polyglutamates affects 5, 10-methylene-THF reductase leading to inhibition of purine and pyrimidine metabolism causing inhibition of DNA and RNA synthesis.[17] It is toxic due to its action on the S-phase of the cell-cycle, affecting tissues with high turnover such as bone marrow.[18] Therefore, the risk involved with the use of MTX on the hematological system is high.
Curcumin inhibits the synthesis of proinflammatory prostaglandins, leukotrienes and hinders the conversion of linoleic acid to arachidonic acid. It suppresses the activation of nuclear factor kappa B (NFkB) leading to decreased production of cyclooxygenase-2. Decreased generation of prostanoids definitely contributes to the anti-arthritic action of curcumin.[19] Curcumin down regulates the expression of cytokines like TNF-a and IL which play a pivotal role in arthritis. Although the anti-arthritic action of curcumin has been established, our focus was to ascertain the impact of concomitant administration of natural products with available therapeutic agents on the progression of RA as information on herb-drug interactions is almost in its infancy. We observed a pronounced, significant and synergistic anti-arthritic action with the combination of methotrexate and curcumin particularly at a dose of 100 mg/kg, therefore the dose of MTX has been reduced to half.
Cell population and size could be altered on exposure to drugs like methotrexate, cyclosporine, indomethacin as also as with impending diseases like RA. Hemoglobin concentration, hematocrit and RBC counts are cardinal measurements to ascertain hematological toxicity. MTX treatment produced a significant decline in these values; however, our studies reveal that the combination produces an improvement in these indices. Corpuscular values such as MCV, MCH, and MCHC are good indicators of erythrocytic characteristics and can be obtained by computation. An elevation in MCV is a good predictor of hematologic toxicity. As a folate depletory, MTX produced an evident rise in the MCV values, which was restored by the combination. The MCH and MCHC values were also improved with MTX and curcumin. A mild to moderate rise in the WBC count was observed in arthritis-induced animals, which can be related to the release of interleukin-Iβ. This in turn increases the production of both granulocyte and macrophage colony stimulating factor. It must be emphasized that T-lymphocytes also play a central role in the pathogenesis of RA. These cells comprise the majority of the lymphoid cells found in the rheumatoid synovium.
MTX can induce oxidative stress even when administered in low doses.[20] As blood cell membrane is rich in polyunsaturated fatty acids, the susceptibility to oxidative damage is high. The protective mechanism of curcumin largely revolves around its ability to quench reactive oxygen species, which could salvage blood cells from MTX-induced damage. Further, the exact role of curcumin in minimizing hematological toxicity is difficult to ascertain as it acts on several signaling pathways, second messenger systems, transcription factors, and enzymes.[21] However, we propose that curcumin might exert its protective action possibly by enhancing erythropoiesis. It has been reported that erythroid progenitor cells are adversely affected by a rise in the levels of TNF, which occurs in RA leading to decline in erythropoiesis.[22,23] MTX inhibits TNF only in vitro with minimal effects on TNF in vivo. Curcumin is a potent inhibitor of NF-kB, favouring the transcription of TNF gene thereby facilitating erythropoiesis. Curcumin stabilizes the lysosomal membrane causing enhanced blood cell survival. The restoration of platelet, WBC, and the differential count suggest that curcumin could mask the hematological abnormalities induced by methotrexate in RA possibly by suppressing the release of interleukins and leukotriens. This implies that reduction in the dose of methotrexate and incorporation of curcumin protects against the risk of hematological toxicity.
The concomitant administration of curcumin with MTX is better as compared to monotherapy in FCA-induced arthritis. Curcumin could support the action of MTX possibly by interfering with the formation of arachidonic acid, reducing the synthesis of thromboxanes, modulating the activity of neutrophils and lymphocytes. Curcumin is established as a powerful quencher of reactive oxygen species and this might prevent oxidative damage to various blood cells. It can be concluded that the combination therapy is effective in inhibiting arthritis and circumventing hematological toxicity produced by MTX in arthritic animals.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kumar A, Marwaha V. New therapies for rheumatoid arthritis. MJAFI. 2003;59:90–2. doi: 10.1016/S0377-1237(03)80045-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stamp L, Roberts R, Kennedy M, Barclay M, Donnell JO’, Chapman P. The use of low dose methotrexate in rheumatoid arthritis-are we entering a new era of therapeutic drug monitoring and pharmacogenomics? Biomed Pharmacother. 2006;60:678–87. doi: 10.1016/j.biopha.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Cikrikci S, Mozioglu E, Yilmaz H. Biological activity of curcuminoids isolated from curcuma longa L. Rec Nat Prod. 2008;2:19–24. [Google Scholar]
- 4.Araujo CA, Leon LL. Biological Activities of Curcuma longa L. Mem Inst Oswaldo Cruz. 2001;96:723–8. doi: 10.1590/s0074-02762001000500026. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: The Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) J Biol Chem. 1995;270:24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 7.Hemeida RA, Mohafez OM. Curcumin attenuates methotrexate-induced hepatic oxidative damage in rats. J Egypt Natl Canc Inst. 2008;20:141–8. [PubMed] [Google Scholar]
- 8.Laima L, Eiva B, Ruta B, Dalia V, Elvyra R, Vytautas A. Antiarthritic and hepatoprotective effect of derinat on adjuvant arthritis in rats. Acta Med Lituanica. 2006;13:236–44. [Google Scholar]
- 9.Huxtable RJ. Activation and pulmonary toxicity of Pyrrolizidine alkaloids. Pharmacol Ther. 1990;47:371–89. doi: 10.1016/0163-7258(90)90063-8. [DOI] [PubMed] [Google Scholar]
- 10.Raghuramulu N, Madhavan K, Kalyana SS. India: Silver Prints; 1983. Hematological Techniques. A Manual of Laboratory Techniques; pp. 254–8. [Google Scholar]
- 11.Brecher G, Cronkite EP, Tocantins LM, Kazal LA. New York: Grune, Strallon Inc Ed; 1964. Estimation of the Number of platelets by phase Microscopy. In blood coagulation, Hemorrhage and Thrombosis. [Google Scholar]
- 12.Drabkin DL, Austin JM. Spectrophotometric constants for common hemoglobin derivatives in human, dog and rabbit blood. J Biol Chem. 1932;98:719–33. [Google Scholar]
- 13.Roubenoff R, Freeman LM, Smith DE, Abad LW, Dinarello CA, Kehayias JJ. Adjuvant arthritis as a model of inflammatory cachexia. Arthritis Rheum. 1997;40:534–9. doi: 10.1002/art.1780400320. [DOI] [PubMed] [Google Scholar]
- 14.Ibanez de Caceres I, Villanua M, Soto L, Martin I, Lopez-Calderon A. IGF-I and IGF-I-binding proteins in rats with adjuvant-induced arthritis given recombinant human growth hormone. J Endocrinol. 2000;165:537–4. doi: 10.1677/joe.0.1650537. [DOI] [PubMed] [Google Scholar]
- 15.Chan ES, Cronstein BN. Molecular action of methotrexate in inflammatory diseases. Arthritis Res. 2002;4:266–73. doi: 10.1186/ar419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cutolo M, Sulli A, Pizzorni C, Seriolo B. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann Rheum Dis. 2001;60:729–35. doi: 10.1136/ard.60.8.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Ede AE, Laan RF, Blom HJ, De Abreu RA, Van de Putte LB. Methotrexate in rheumatoid arthritis: An update with focus on mechanisms involved in toxicity. Semin Arthritis Rheum. 1998;27:277–92. doi: 10.1016/s0049-0172(98)80049-8. [DOI] [PubMed] [Google Scholar]
- 18.Ajit KS, Saxena AK, Divya S, Gajendra S. Structural interaction between drug - DNA and protein- A novel approach for Bioinformatics in medicine. Biomed Res. 2009;20:28–34. [Google Scholar]
- 19.Rajendran R, Krishnakumar E. Anti-arthritic activity of Premna serratifolia Linn. wood against adjuvant induced arthritis. Avicenna J Med Biotech. 2010;2:101–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Herman S, Zurgil N, Deutsch M. Low dose methotrexate induces apoptosis with reactive oxygen species involvement in T lymphocytic cell lines to a greater extent than in monocytic lines. Inflamm Res. 2005;54:273–80. doi: 10.1007/s00011-005-1355-8. [DOI] [PubMed] [Google Scholar]
- 21.Chathoth S, Thayyullathil F, Galadari S. Curcumin cell signaling: A possible target for chemotherapy. Curr Trends Biotechnol Pharm. 2008;2:226–38. [Google Scholar]
- 22.Roodman GD, Bird A, Hatzler D, Montgomery W. Tumor necrosis factor alpha and hematopoietic progenitors: Effect of tumor necrosis factor on the growth of erythroid progenitors CFU-E and BFU-E and the hematopoietic cell lines K562, HL60, and HEL cells. Exp Hematol. 1987;15:928–35. [PubMed] [Google Scholar]
- 23.Johnson RA, Waddelow TA, Caro J, Oliff A, Roodman GD. Chronic exposure to tumor necrosis factor in vivo preferentially inhibits erythropoiesis in nude mice. Blood. 1989;74:130–8. [PubMed] [Google Scholar]


