Abstract
Objective:
The present study was designed to investigate the ameliorative potential and possible mechanism of hydroalcoholic extract of flowers of P. granatum in glycerol-induced acute renal failure (ARF) in rats.
Materials and Methods:
The rats were subjected to rhabdomyolytic ARF by single intramuscular injection of hypertonic glycerol (50% v/v; 8 ml/kg) and the animals were sacrificed after 24 hours of glycerol injection. The plasma creatinine, blood urea nitrogen, creatinine clearance, and histopathological studies were performed to assess the degree of renal injury.
Results:
Pretreatment with hydroalcoholic extract of flowers of P. granatum (125 and 250 mg/kg p.o. twice daily for 3 days) significantly attenuated hypertonic glycerol-induced renal dysfunction in a dose-dependent manner. BADGE (Bisphenol-A-diglycidyl ether) (30 mg/kg), a peroxisome proliferator-activated receptor (PPAR)-γ antagonist, and N(omega)-nitro-l-arginine-methyl ester (L-NAME) (10, 20, and 40 mg/kg), nitric oxide synthase inhibitor, were employed to explore the mechanism of renoprotective effects of Punica granatum. Administration of BADGE (30 mg/kg) and L-NAME (40 mg/kg) abolished the beneficial effects of P. granatum in glycerol-induced renal dysfunction.
Conclusion:
Hydroalcoholic extract of flowers of P. granatum has ameliorative potential in attenuating myoglobinuric renal failure and its renoprotective effects involve activation of PPAR-γ and nitric oxide-dependent signaling pathway.
Keywords: Acute renal failure, glycerol, nitric oxide, peroxisome proliferator-activated receptor-γ, Punica granatum, rhabdomyolysis
Introduction
Acute renal failure (ARF) is characterized by a rapid decline in glomerular filtration rate (GFR) over hours to days. The mortality rate of patients with ARF is very high (25-70%) despite the use of various pharmacologic agents.[1] Glycerol is used for the induction of ARF in vivo. Intramuscular administration of hypertonic glycerol is the most common used animal model of myoglobinuric ARF, characterized by a considerable reduction in renal blood flow and GFR.[2] It is reported that the acute volume depletion models of glycerol-induced ARF are more closely related to the syndrome of ARF in human beings than the chronic dehydration model. Rhabdomyolysis is one of the important causes of ARF and about 33% of rhabdomyolytic patients undergo severe degree of ARF. Rhabdomyolysis is a potentially life-threatening syndrome characterized by the breakdown of skeletal muscle resulting in the subsequent release of intracellular contents into the circulatory system. The factors known to contribute to rhabdomyolysis-induced ARF include hypovolemia, acidosis, tubular obstruction, and the nephrotoxic effects of myoglobin.[3]
Punica granatum Linn. (Punicaceae), commonly known as pomegranate, is a shrub or a small tree, extending throughout the Mediterranean region, eastward to China and India, and on to the American Southwest, California, and Mexico in the New World. In Ayurvedic medicine, the pomegranate is considered “a pharmacy unto itself,” and its different parts have been used as antihelmintic, vermifuge, antidiarrheal, blood tonic, antiabortifacient, and antidiabetic.[4] Furthermore, it has been proposed to produce beneficial effect in the treatment of acquired immune deficiency syndrome, allergies, cardiovascular diseases, cancer, inflammatory and ophthalmic diseases.[5] In our own study, hydroalcoholic extract of P. granatum was documented to attenuate dextran sulfate sodium-induced ulcerative colitis.[6] Recent studies have shown the hepatoprotective properties of P. granatum in a mice model of ferric nitrilotriacetate[7] and also the juice of P. granatum has been documented to produce protective effect in ethylene glycol-induced nephrolithiasis in rats.[8]
Peroxisome proliferator-activated receptors (PPARs) form a subfamily of the nuclear receptor superfamily and three isoforms encoded by separate genes have been identified: PPAR-α, PPAR-β/δ, and PPAR-γ. PPARs are ligand-dependent transcription factors that regulate target gene expression by binding to specific peroxisome proliferator response elements (PPREs) in enhancer sites of regulated genes. PPAR-γ receptors have been localized in urinary system including glomerulus, collecting ducts, proximal tubules, and renal microvasculature. There have been reports suggesting that activation of PPAR-γ triggers protection in different models of renal failure like chronic renal allograft damage[9] and renal ischemia-reperfusion injury.[10] The studies have suggested that activation of PPAR-γ receptors directly attenuate glomerular diseases, possibly by inhibiting mesangial growth, which occurs early in the process of nephropathy. Furthermore, in glycerol-induced renal failure model, the generation of oxidative stress and proinflammatory mediators are reported to downregulate PPAR-γ expression.[11] The studies have shown that the extract of P. granatum activates the PPAR-γ receptors[12] and therefore, the plant extract has been successfully used to treat type II diabetes mellitus,[13] to inhibit postprandial hyperglycemia, and to diminish cardiac fibrosis.[14]
Nitric oxide (NO), identified as an endothelium-derived relaxing factor, is a major regulator of systemic and renal hemodynamics, as it is crucial in the maintenance of a state of basal vasodilatation. NO is formed during the oxidation of L-arginine to L-citrulline that is catalyzed by NO synthases (NOS). Three NOS isoforms have been characterized and cloned: Neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS). The three isoforms are differentially expressed throughout the kidney with eNOS expression in renal vascular endothelial cells; nNOS in epithelial cells of the macula densa and the principal cells of the collecting duct and iNOS in tubule epithelia, including the proximal tubule, thick ascending limb, and distal convoluted tubule.[15] The NO generated from the expression of the different NOS isoforms has been shown to play an important role in various physiological processes in the kidney, including salt and fluid reabsorption, renin secretion, and tubuloglomerular feedback.[16] The specific effect produced by NO may be related to the location of the particular isoforms. The protective role of NO in different models of renal failure has been documented,[17] including glycerol-induced renal failure.[9,18] The studies have shown that extract of P. granatum restores the shear stress-induced decreased eNOS expression and reverses the stress-induced proatherogenic effects.[19] It has also been shown to increase the eNOS expression in obese Zucker rats.[20] Therefore, the present study was designed to investigate the potential of hydroalcoholic extract of flowers of P. granatum in glycerol-induced ARF and to explore the role of PPAR-γ and NO in P. granatum-mediated biological actions.
Materials and Methods
Animals
Wistar rats (Haryana Agriculture University, Hisar, India) of either sex weighing 200 ± 50 g maintained on the standard laboratory diet and water ad libitum were employed in the present study. They were housed in the departmental animal house and were exposed to natural cycles of light and dark. The experimental protocol was duly approved by the Institutional Animal Ethics Committee (IAEC) and the care of the animals was done as per the guidelines of the Committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India (Reg. No-107/1999/CPCSEA).
Drugs and Chemicals
Glycerol (Loba Chemie Pvt. Ltd., India), BADGE (Bisphenol-A-diglycidyl ether) (Sigma-Aldrich, St. Louis, U.S.A.), and N(omega)-nitro-l-arginine-methyl ester (L-NAME) (Caymann Chemicals, USA) were used in the present study. Creatinine diagnostic kit was purchased from Medsource Ozone Biomedicals Pvt. Ltd, Haryana, India. Blood urea nitrogen kit was purchased from Erba Diagnostics Mannheim, Germany. All the other chemicals and reagents were purchased from S.D Fine Chemical Ltd. Mumbai, India, and were of analar quality. L-NAME was dissolved in 0.14 M NaCl, while BADGE was suspended in 0.5% carboxymethyl cellulose (CMC). All drug solutions were freshly prepared.
Plant Material and Preparation of Hydroalcoholic Extract
P. granatum flowers were collected locally and a sample has been kept as a voucher specimen (PuP-030/2009-2010) at Punjabi University, Patiala. The flowers of P. granatum were dried in shade and ground to make a coarse powder. The powder was then extracted thrice with methanol–water mixture (3 : 1) by stirring at room temperature for 1 hour each time. The extract was filtered and the collected solvent was completely removed at 50°C under reduced pressure. The yield of the extract was 15% (w/w) in terms of dried starting material.
Induction of Myoglobinuric Acute Renal Failure
Myoglobinuric ARF was induced in rats by intramuscular injection of glycerol dissolved in saline (50% v/v, 8 ml/kg) in divided dose in both the hind limbs, after a 24-hour water deprivation period.[18,21] The urine was collected by placing the animals in the metabolic cages.[18] After 24 hours of the glycerol injection, the animals were sacrificed with a high dose of anesthesia (diethyl ether) and the blood was collected in centrifuged tubes coated with ethylenediaminetetraacetic acid (EDTA). The plasma was separated by centrifuging blood samples at 4 000 r.p.m. for 30 minutes. The kidneys were removed through a midline incision and were stored in 10% formalin for the histological examination.
Biochemical Parameters
Plasma creatinine
The plasma creatinine levels were evaluated by diagnostic kit based on principle of Jaffe's Method.
Blood urea nitrogen
The blood urea nitrogen (BUN) levels were evaluated with the diagnostic kit based on principle of glutamate dehydrogenase-urease method.
Creatinine clearance
The total urine was collected in a period of 24 hours and creatinine clearance was calculated by the formula
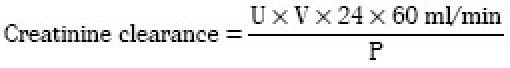
U = Creatinine concentration in urine; V = Total urine volume collected in period of 24 hours; P = Creatinine concentration in plasma.
Renal Histopathology
The right kidney was isolated immediately after sacrificing the animal and washed with ice-cold saline. It was then fixed in a 10% neutral buffered formalin solution, embedded in paraffin, and used for histopathological examination. Five-micrometer thick sections were cut, deparaffinized, hydrated, and stained with hematoxylin and eosin. The renal sections were examined in blind fashion for intact glomeruli, hemorrhage and rupture, and vacuolization of the medullary tubular cells.
Experimental Protocol
Twelve groups, each comprising six Wistar albino rats, were employed in the present study. The animals were allowed free access to food but deprived of drinking water for 24 hours before glycerol injection.
Group I: Normal control
Rats received equivalent volume of saline corresponding to hypertonic glycerol in group II. All the animals were sacrificed after 24 hours of receiving saline and plasma creatinine, BUN, and creatinine clearance were assayed along with histopathological examination.
Group II: Glycerol-treated (8 ml/kg)
The rats received an intramuscular injection of 8 ml/kg hypertonic glycerol (50% v/v) as divided doses in both the hind limbs. All the animals were sacrificed 24 hours after treatment and different parameters were assessed, as described in group I.
Group III and IV: Punica granatum (125 and 250 mg/kg) in glycerol-treated
Hydroalcoholic extract of P. granatum (125 and 250 mg/kg p.o) was suspended in 0.5% CMC and was administered twice daily at an interval of 12 hours, starting two days before the induction of ARF by glycerol. On the third day, P. granatum was administered 30 minutes prior to glycerol injection and after 12 hours, same dose of P. granatum was administrated in rats without administration of hypertonic glycerol. All the animals were sacrificed 24 hours after glycerol treatment and different parameters were assessed, as described in group I.
Group V: Punica granatum (250 mg/kg) per se
P. granatum was suspended in 0.5% CMC and was administered twice daily at an interval of 12 hours for three days. All the animals were sacrificed 12 hours after the last injection of P. granatum and different parameters were assessed, as described in group I.
Group VI: Punica granatum (250 mg/kg) and bisphenol-A-diglycidyl ether (30 mg/kg) in glycerol-treated
Rats received P. granatum (250 mg/kg p.o.) and BADGE (30 mg/kg p.o.) twice daily at an interval of 12 hours, starting two days before the induction of ARF by glycerol. On the third day, P. granatum and BADGE was administered 30 minutes prior to glycerol injection and after 12 hours, same doses of P. granatum and BADGE were administered in rats without administration of glycerol. All the animals were sacrificed 24 hours after glycerol treatment and different parameters were assessed, as described in group I.
Group VII: Bisphenol-A-diglycidyl ether (30 mg/kg) per se
Rats received BADGE (30 mg/kg p.o.) twice daily at an interval of 12 hours for 3 days. All the animals were sacrificed 12 hours after the last administration of BADGE. Different parameters were assessed as described in group I.
Group VIII, IX, and X: Punica granatum (250 mg/kg) and N(omega)-nitro-l-arginine-methyl ester (10, 20, and 40 mg/kg) in glycerol-treated
Rats received P. granatum (250 mg/kg p.o.) and L-NAME (10, 20, and 40 mg/kg i.p.) twice daily at an interval of 12 hours, starting two days before the induction of ARF by glycerol. On the third day, P. granatum and L-NAME were administered 30 minutes prior to glycerol injection and after 12 hours, same doses of P. granatum and L-NAME were administered in rats without administration of glycerol. All the animals were sacrificed after 24 hours and different parameters were assessed, as described in group I.
Group XI: N(omega)-nitro-l-arginine-methyl ester (40 mg/kg) per se
Rats received an intraperitoneal injection of L-NAME (40 mg/kg) twice daily at an interval of 12 hours for 3 days. All the animals were sacrificed 12 hours after the last L-NAME injection and different parameters were assessed, as described in group I.
Group XII: Carboxymethyl cellulose per se
0.5% CMC (1 ml/kg p.o.) was administered twice daily at an interval of 12 hours, starting two days before the induction of ARF by glycerol. On the third day, CMC was administered 30 minutes prior to glycerol injection and after 12 hours, same dose of CMC was administrated in rats without administration of hypertonic glycerol. All the animals were sacrificed 24 hours after glycerol treatment and different parameters were assessed, as described in group I.
Statistical Analysis
The data were presented as mean ± S.E.M. One way analysis of variance (ANOVA) followed by Tukey's multiple range test was applied to calculate the statistical significance between different various groups. A value of P < 0.05 was considered to be statistically significant.
Results
Effect of Punica granatum, Bisphenol-A-diglycidyl Ether, and N(omega)-nitro-l-arginine-methyl Ester on Plasma Creatinine in Glycerol-induced Acute Renal Failure
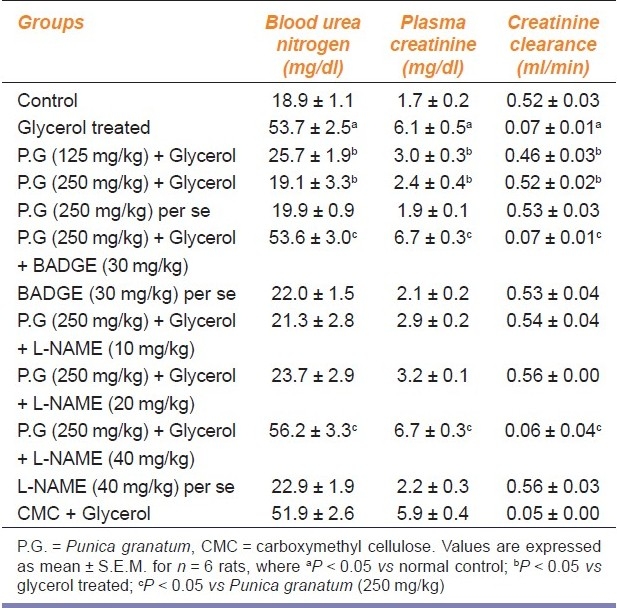
Single intramuscular injection of glycerol (50% v/v, 8 ml/kg) in divided dose in both hind limbs led to significant elevation of plasma creatinine levels after 24 hours in glycerol-treated rats, as compared with corresponding saline-treated normal rats. Pretreatment with hydroalcoholic extract of P. granatum (125 and 250 mg/kg p.o.) significantly attenuated glycerol-induced rise in plasma creatinine level in a dose-dependent manner. Administration of BADGE (30 mg/kg p.o.) and L-NAME (40 mg/kg i.p.) abolished the attenuating effects of P. granatum (250 mg/kg p.o.) on glycerol-induced increase in plasma creatinine levels. Per se administration of L-NAME (40 mg/kg i.p.) and BADGE (30 mg/kg p.o.) did not alter the plasma creatinine levels in normal rats in a significant manner. Administration of CMC (1 ml/kg p.o) did not modulate plasma creatinine levels in glycerol-treated rats [F (11, 60) = 42.67, P < 0.001] [Table 1].
Table 1.
Effect of Punica granatum on blood urea nitrogen levels, plasma creatinine levels, and creatinine clearance in glycerol-induced renal failure in rats
Effect of Punica granatum, Bisphenol-A-diglycidyl Ether, and N(omega)-nitro-l-arginine-methyl Ester on Blood Urea Nitrogen Levels in Glycerol-induced Acute Renal Failure
Single intramuscular injection of glycerol (50% v/v, 8 ml/kg) in divided dose in both hind limbs led to significant elevation of BUN levels after 24 hours in glycerol-treated rats as compared with corresponding saline-treated normal rats. Pretreatment with hydroalcoholic extract of P. granatum (125 and 250 mg/kg p.o.) attenuated glycerol-induced rise in BUN levels in a significant manner. Administration of BADGE (30 mg/kg p.o.) and L-NAME (40 mg/kg i.p.) abolished the attenuating effects of P. granatum (250 mg/kg p.o.) on glycerol-induced increase in BUN levels. Per se administration of L-NAME (40 mg/kg i.p.) and BADGE (30 mg/kg p.o.) did not alter the BUN levels in normal rats in a significant manner. Administration of CMC (1 ml/kg p.o.) did not modulate BUN levels in glycerol-treated rats [F (11, 60) = 44.64, P < 0.001] [Table 1].
Effect of Punica granatum, Bisphenol-A-diglycidyl Ether, and N(omega)-nitro-l-arginine-methyl Ester on Creatinine Clearance in Glycerol-induced Acute Renal Failure
Single dose of intramuscular glycerol (50% v/v, 8 ml/kg) in divided dose in both hind limbs led to significant decrease in creatinine clearance after 24 hours in glycerol-treated rats as compared with corresponding saline-treated normal rats. Pretreatment with hydroalcoholic extract of P. granatum (125 and 250 mg/kg p.o.) improved glycerol-induced decrease in creatinine clearance in a significant manner. Administration of BADGE (30 mg/kg p.o.) and L-NAME (40 mg/kg i.p.) abolished the attenuating effects of P. granatum (250 mg/kg) on glycerol-induced decrease in creatinine clearance. Per se administration of L-NAME (40 mg/kg i.p.) and BADGE (30 mg/kg p.o.) did not alter the creatinine clearance in normal rats in a significant manner. Administration of CMC (1 ml/kg p.o.) did not modulate creatinine clearance in glycerol-treated rats [F (11, 60) = 51.75, P < 0.001] [Table 1].
Renal Histopathology
Administration of glycerol resulted in histopathological alterations in the form of ruptured glomeruli, hemorrhage and vacuolization of medullary tubular cells. However, pretreatment with P. granatum (125 and 250 mg/kg) attenuated glycerol-induced histopathological alterations [Figure 1]. However, administration of BADGE (30 mg/kg p.o.) and L-NAME (40 mg/kg i.p.) abolished the attenuating effects of P. granatum (250 mg/kg) on glycerol-induced histopathological alterations.
Figure 1.
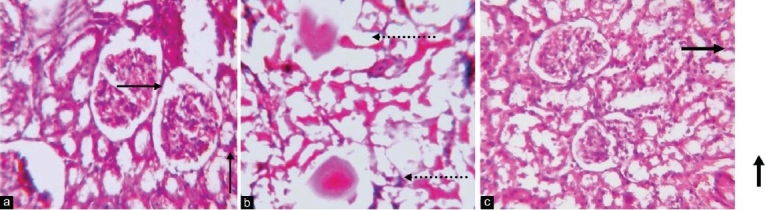
Hematoxylin and eosin-stained sections of rat kidneys in light microscopy (Original magnifi cation ×100): Kidney section of saline-treated rats, showing intact glomeruli represented by normal arrow in addition shows normal medullary tubular cells (a). Kidney section of glycerol-treated rats shows rupture glomeruli shown by broken arrow, also rupture and vacuolization of medullary tubular cells represented by broken arrow (b). Kidney section of rats pretreated with Punica granatum is shown by bold arrow, before glycerol shows almost near morphology to saline-treated normal rats (c)
Discussion
In the present study, single intramuscular injection of hypertonic glycerol (8 ml/kg) in divided dose in both the hind limbs led to ARF within 24 hours, assessed in terms of decrease in creatinine clearance, an increase in plasma creatinine and BUN levels, and histopathological alterations. Glycerol-induced renal failure is one of the most commonly used animal model of ARF.[2,21] Intramuscular injection of hypertonic glycerol has been documented to induce rhabdomyolysis which in turn leads to ARF. Rhabdomyolysis is a potentially life-threatening syndrome characterized by the breakdown of skeletal muscle, resulting in the subsequent release of intracellular contents from myocytes into the circulatory system.[3]
In the present investigation, pretreatment with the hydroalcoholic extract of flowers of P. granatum (125 and 250 mg/kg p.o.) significantly attenuated glycerol-induced renal dysfunction. The juice of P. granatum has also been documented to produce protective effect in ethylene glycol-induced nephrolithiasis in rats.[8] To the best of our knowledge, this is the first report suggesting the protective effects of hydroalcoholic extract of flowers of P. granatum in myoglobinuric ARF in rats. To explore the possible mechanism of renoprotective effect of P. granatum, BADGE, a PPAR-γ antagonist, was employed in the present investigation.[22] Administration of BADGE (30 mg/kg) significantly attenuated P. granatum-mediated nephroprotective effects in glycerol-induced renal failure, suggesting that the noted beneficial effects of P. granatum in the present study are mediated through activation of PPAR-γ receptors.
PPARs are transcription factors belonging to the nuclear receptor superfamily and are activated by fatty acids, eicosanoids, and various synthetic ligands. PPAR-γ isoform has been shown to control numerous physiological functions including glucose metabolism, adipocyte differentiation, lipid synthesis and its uptake. PPAR-γ agonists are presently employed clinically for the management of type II diabetes mellitus. It is becoming increasingly clear that PPAR-γ ligands represent a promising therapeutic strategy for other diseases as well, including atherosclerosis, cancer, cardiovascular complications, neuropathic pain, spinal cord injury, and neurodegenerative disorders such as Alzheimer's disease.[23]
The role of PPAR-γ has also been investigated in regulating renal functions. There have been reports suggesting that activation of PPAR-γ triggers protection in different models of renal failure like chronic renal allograft damage[9] and renal ischemia-reperfusion injury.[10] Furthermore, it has also been shown that reduced expression and activity of PPAR-γ is involved in the pathogenesis of glycerol-induced ARF and reversal of renal damage with ciglitazone, a PPAR-γ activator, has also been documented.[24] There have been a number of studies suggesting that methanolic extract of flowers of P. granatum activates PPAR-γ receptors.[13] Furthermore, the beneficial effects of P. granatum in improving insulin receptor sensitivity in Zucker diabetic fatty rats,[13] inhibiting postprandial hyperglycemia and in diminishing cardiac fibrosis,[14] have been shown to be mediated through activation of PPAR-γ receptors.
There have been reports suggesting that ethanolic extract of flowers of P. granatum modulate different functions through NO signaling pathway.[7] It has been reported that P. granatum reverses shear stress-induced proatherogenic effects by increasing the eNOS expression.[19] Furthermore, it has also been documented to improve the arterial functions in obese Zucker rats by increasing eNOS expression.[20] Therefore, to investigate the contributory role of NO in P. granatum-mediated renoprotection, L-NAME, NOS inhibitor, was employed in three-dose schedule, i.e., 10, 20, and 40 mg/kg. Administration of L-NAME (40 mg/kg i.p.) significantly attenuated P. granatum-mediated renoprotection in glycerol-induced renal failure, suggesting the key role of NO in renoprotective effects of P. granatum. Per se administration of L-NAME for 2 days (40 mg/kg) did not modulate renal functions in the normal rats. The protective role of NO in different models of renal failure has been documented,[17] including glycerol-induced renal failure.[18,25] These studies have demonstrated that levels of NO are decreased in glycerol-induced renal failure and different agents have shown to produce renoprotection by increasing the NO production. In the present study, single dose of BADGE was employed, while L-NAME was administered at three-dose levels. The dose of BADGE (30 mg/kg) was selected on the basis of our own previous study, suggesting that the beneficial effect of curcumin in experimental dementia involves activation of PPAR-γ receptors.[22] However, for L-NAME, three doses with the range of 10, 20, and 40 mg/kg were used so as to select the proper dose and in the present study, 40 mg/kg was shown to attenuate the protective effects of P. granatum.
The recent studies have reported that endothelial PPAR-γ regulates vascular NO production. Activation of PPAR-γ induced increased NO synthesis has been linked to Akt-eNOS signaling pathway.[26] On the other hand, NO has also been shown to activate PPAR-γ and increase its binding to PPRE (PPAR response element).[27,28] From these studies, it may be proposed that these two signaling pathways are interlinked and constitute an integrated mechanism to produce protective effects including renoprotection in glycerol-induced ARF in rats.
Conclusion
It may be concluded that hydroalcoholic extract of flowers of P. granatum has ameliorative potential in attenuating the state of myoglobinuric renal failure and its renoprotective effects may involve activation of PPAR-γ and NO-dependent signaling pathway.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
Disclosure Statement: No competing financial interests exist in the conduct of this study
References
- 1.Parikh CR, McSweeney P, Schrier RW. Acute renal failure independently predicts mortality after myeloablative allogeneic hematopoietic cell transplant. Kidney Int. 2005;67:1999–2005. doi: 10.1111/j.1523-1755.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 2.Zurovsky Y. Models of glycerol-induced acute renal failure in rats. J Basic Clin Physiol Pharmacol. 1993;4:213–28. doi: 10.1515/jbcpp.1993.4.3.213. [DOI] [PubMed] [Google Scholar]
- 3.Khan FY. Rhabdomyolysis: A review of the literature. Neth J Med. 2009;67:272–83. [PubMed] [Google Scholar]
- 4.Naovi SA, Khan MS, Vohora SB. Antibacterial, anti-fungal and anthelmintic investigations on Indian medicinal plants. Fitoterapia. 1991;62:221–8. [Google Scholar]
- 5.Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Singh K, Jaggi AS, Singh N. Exploring the Ameliorative Potential of Punica granatum in Dextran Sulfate Sodium Induced Ulcerative Colitis in Mice. Phytother Res. 2009;23:1565–74. doi: 10.1002/ptr.2822. [DOI] [PubMed] [Google Scholar]
- 7.Kaur G, Jabbar Z, Athar M, Alam MS. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Cheml Toxicol. 2006;44:984–93. doi: 10.1016/j.fct.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Tugcu V, Kemahli E, Ozbek E, Arinci YV, Uhri M, Erturkuner P, et al. Protective effect of a potent antioxidant, pomegranate juice, in the kidney of rats with nephrolithiasis induced by ethylene glycol. J Endourol. 2008;22:2723–31. doi: 10.1089/end.2008.0357. [DOI] [PubMed] [Google Scholar]
- 9.Kiss E, Popovic ZV, Bedke J, Adams J, Bonrouhi M, Babelova A, et al. Peroxisome proliferator-activated receptor (PPAR) gamma can inhibit chronic renal allograft damage. Am J Pathol. 2010;176:2150–62. doi: 10.2353/ajpath.2010.090370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doi S, Masaki T, Arakawa T, Takahashi S, Kawai T, Nakashima A, et al. Protective effects of peroxisome proliferator-activated receptor gamma ligand on apoptosis and hepatocyte growth factor induction in renal ischemia-reperfusion injury. Transplantation. 2007;84:207–13. doi: 10.1097/01.tp.0000269614.21367.3f. [DOI] [PubMed] [Google Scholar]
- 11.Itoh H, Doi K, Tanaka T, Fukunaga Y, Hosoda K, Inoue G, et al. Hypertension and insulin resistance: Role of peroxisome proliferator activates receptor gamma. Clin Exp Pharmacol Physiol. 1999;26:558–60. doi: 10.1046/j.1440-1681.1999.03082.x. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Qi Y, Huang TH, Yamahara J, Roufogalis BD. Pomegranate flower: A unique traditional antidiabeteic medicine with dual PPAR-α/γ activator properties. Diabetes Obes Metab. 2007;10:1326–463. doi: 10.1111/j.1463-1326.2007.00708.x. [DOI] [PubMed] [Google Scholar]
- 13.Huang TH, Peng G, Kota BP, Li GQ, Yamahara J, Roufogalis BD, et al. Anti-diabetic action of Punica granatum flower extract: Activation of PPAR-gamma and identification of an active component. Toxicol Appl Pharmacol. 2005;207:160–9. doi: 10.1016/j.taap.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Huang TH, Yang Q, Harada M, Li GQ, Yamahara J, Roufogalis BD, et al. Pomegranate flower extract diminishes cardiac fibrosis in Zucker diabetic fatty rats: Modulation of cardiac endothelin-1 and nuclear factor-kappaB pathways. J Cardiovasc Pharmacol. 2005;46:856–62. doi: 10.1097/01.fjc.0000190489.85058.7e. [DOI] [PubMed] [Google Scholar]
- 15.Yousefipour Z, Oyekan A, Newaz M. Interaction of oxidative stress, nitric oxide and peroxisome proliferator activated receptor gamma in acute renal failure. Pharmacol Ther. 2010;125:436–45. doi: 10.1016/j.pharmthera.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Kone BC, Baylis C. Biosynthesis and homeostatic roles of nitric oxide in the normal kidney. Am J Physiol. 1997;272:F561–78. doi: 10.1152/ajprenal.1997.272.5.F561. [DOI] [PubMed] [Google Scholar]
- 17.Valdivielso JM, López-Novoa JM, Eleno N, Barriocanal PF. Role of glomerular nitric oxide in glycerol-induced acute renal failure. Can J Physiol Pharmacol. 2000;78:476–82. [PubMed] [Google Scholar]
- 18.Chander V, Chopra K. Molsidomine, a nitric oxide donor and l-arginine protects against rhabdomyolysis-induced myoglobinuric acute renal failure. Biochim Biophys Acta. 2005;1723:208–14. doi: 10.1016/j.bbagen.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 19.De Nigris F, Williams-Ignarro S, Sica V, Lerman LO, D’Armiento FP, Byrns RE, et al. Effects of a pomegranate fruit extract rich in punicalagin on oxidation-sensitive genes and eNOS activity at sites of perturbed shear stress and atherogenesis. Cardiovasc Res. 2007;73:414–23. doi: 10.1016/j.cardiores.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 20.De Nigris F, Balestrieri ML, Williams-Ignarro S, D’Armiento FP, Fiorito C, Ignarro LJ, et al. The influence of pomegranate fruit extract in comparison to regular pomegranate juice and seed oil on nitric oxide and arterial function in obese Zucker rats. Nitric Oxide. 2007;17:50–4. doi: 10.1016/j.niox.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim M, Luchese C, Pinton S, Roman SS, Hassan W, Nogueira CW, et al. Involvement of catalase in the protective effect of binaphthyl diselenide against renal damage induced by glycerol. Exp Toxicol Pathol. 2010;63:331–5. doi: 10.1016/j.etp.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Rinwa P, Kaur B, Jaggi AS, Singh N. Involvement of PPAR-gamma in curcumin-mediated beneficial effects in experimental dementia. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:529–39. doi: 10.1007/s00210-010-0511-z. [DOI] [PubMed] [Google Scholar]
- 23.Jain V, Jaggi AS, Singh N. Ameliorative potential of rosiglitazone in tibial and sural nerve transection-induced painful neuropathy in rats. Pharmacol Res. 2009;59:385–92. doi: 10.1016/j.phrs.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Newaz M, Yousefipour Z, Oyekan A. Role of PPAR-γ on the pathogenesis and vascular changes in glycerol-induced acute renal failure. Pharmacol Res. 2006;54:234–40. doi: 10.1016/j.phrs.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Aydogdu N, Atmaca G, Yalcin O, Taskiran R, Tastekin E, Kaymak K. Protective effects of L-carnitine on myoglobinuric acute renal failure in rats. Clin Exp Pharmacol Physiol. 2006;33:119–24. doi: 10.1111/j.1440-1681.2006.04336.x. [DOI] [PubMed] [Google Scholar]
- 26.Liang C, Ren Y, Tan H, He Z, Jiang Q, Wu J, et al. Rosiglitazone via upregulation of Akt/eNOS pathways attenuates dysfunction of endothelial progenitor cells, induced by advanced glycation end products. Br J Pharmacol. 2009;158:1865–73. doi: 10.1111/j.1476-5381.2009.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ptasinska A, Wang S, Zhang J, Wesley RA, Danner RL. Nitric oxide activation of peroxisome proliferator activated receptor gamma through a p38 MAPK signaling pathway. FASEB J. 2007;21:952–61. doi: 10.1096/fj.06-6822com. [DOI] [PubMed] [Google Scholar]
- 28.Borniquel S, Jansson EA, Cole MP, Freeman BA, Lundberg JO. Nitrated oleic acid up-regulates PPARγ and attenuates experimental inflammatory bowel disease. Free Radic Biol Med. 2010;48:499–505. doi: 10.1016/j.freeradbiomed.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


