Abstract
Objectives:
The antioxidant activities of two Indian mangrove plants, Bruguiera cylindrica and Ceriops decandra, were investigated.
Materials and Methods:
Total phenolics and total flavonoid contents of the mangroves were determined using folin-ciocalteu reagent method and aluminium chloride method, respectively. Antioxidant capacity was assessed by the following methods: 1,1-diphenyl-2-picryl hydroxyl (DPPH.) quenching assay; 2,2’- azinobis-3-ethylbenzothiozoline-6-sulfonic acid (ABTS.+) cation decolorization test; scavenging capacity towards hydroxyl ion radicals (.OH); reductive capacity; and antihemolytic activity.
Results:
The mangroves yielded 233.3 ± 0.062 and 283.31 ± 0.04 mg gallic acid equivalent/g phenolic contents and 11.6 ± 0.12 and 15.1 ± 0.02 mg quercetin equivalent/g flavonoid contents. The methanol extracts of both mangroves exhibited high antiradical activity against DPPH., ABTS.+, and .OH radicals. The reductive capacity of the extracts increased with increasing concentration of samples. The extracts also inhibited H2O2 induced hemolysis in cow blood erythrocytes. The antioxidant activities were found stronger than that of the reference standard, butylated hydroxy toluene (BHT). The antioxidant activity of mangrove plants was correlated with total phenolics and flavonoid contents.
Conclusion:
Both plants can be considered as good sources of natural antioxidants for medicinal uses. Further studies are necessary to isolate active principles responsible for the overall antioxidant activity of the extracts.
Keywords: ABTS.+ assay, Bruguiera cylindrica, Ceriops decandra, DPPH., H22 induced mangroves, red blood cell hemolysis, total flavonoids, total phenolics
Introduction
Free radicals, such as reactive oxygen species (ROS) and reactive nitrogen species (RNS), induce oxidative damage to biomolecules, such as deoxyribonucleic acid (DNA), lipids, proteins, and carbohydrates. They have been implicated with variety of pathological processes including cancer, diabetes, atherosclerosis, neurodegenerative disorders and arthritis. Antioxidants of plant origin are believed to help protect the cells from free radical damage. Therefore, several studies in recent years showed that polyphenols in plants scavenge active oxygen species and effectively prevent oxidative cell damage.[1] Hence, considerable attention has already been focused on the isolation, characterization, and utilization of natural antioxidants as potential disease preventing agents. However, little is known about the antioxidant potentials of Indian mangroves.
Bruguiera cylindrica (L.) Blume, (family: Rhizhophoraceae) is a rare tree mangrove found along the western coast of India. The bark of B.cylindrica is used to stop hemorrhage and applied to malignant ulcers.[2] Pentacyclic triterpenoid esters from the fruits[3] and brugine alkaloid from stem and bark[4] have been isolated. Ceriops decandra (Griff.) Ding Hou (family: Rhizhophoraceae) is reported to cure hepatitis.[5] Earlier, antiviral activity of B. cylindrica and C. decandra leaves was studied.[6] A study was carried out to evaluate phenolic content, DPPH (1,1 - diphenyl - 2-picryl hydroxyl) reduction activity and reducing power of leaves on B. cylindrica and C. decandra leaves.[7] Leaf extract of C. decandra exhibited antidiabetic activity in alloxan induced diabetic rats.[8] Leaf of C. decandra was found to have radical scavenging activity against superoxide anion.[9] However, there has been no detailed in vitro studies on antioxidant properties of stem bark of these mangrove medicinal plants from Pichavaram forest, Tamil Nadu, India. Hence, in the present study, we aimed to evaluate the total phenolic content and total flavonoid content and to examine the antioxidant activities. In the present study, the antioxidant effects of B. cylindrica and C. decandra were evaluated using in vitro assays including DPPH· radical quenching test, ABTS.+ radical decolorization test, ˙ OH scavenging assay, reducing power capacity, and antihemolytic activity.
Materials and Methods
General
The ultraviolet (UV) spectra were recorded on Elico SL 177, India UV-visible (UV-VIS) spectrophotometer. All chemicals used including the solvents were of analytical grade; 1, 1-diphenyl-2-picryl hydrazyl (DPPH), 2, 2’- azinobis-3-ethylbenzothiozoline-6-sulfonic acid (ABTS), quercetin, butylated hydroxy toluene (BHT) were purchased from Merck Chemical Co., Mumbai, India. Gallic was procured from Riedel-de-Hahn, Germany.
Plant Material
The stem barks of B. cylindrica and C. decandra were collected from Pichavaram mangrove forest (area 1400 ha), Tamil Nadu, India in June 2008. The taxonomic identification was done in Botanical Survey of India (BSI), Southern circle, Coimbatore. A voucher specimen was deposited in the Department of Plant Biotechnology, IFGTB, Coimbatore, India.
Preparation of Extract
The stem barks of B. cylindrica and C. decandra were washed in tap water, shade dried, and powdered. The powder (100 gm) was exhaustively extracted with methanol in the ratio of 1:7 (w/v) for 24 h by using soxhlet apparatus. The extract was evaporated to dryness using rotary flash evaporator (Buchi type, Switzerland). Different concentrations of extracts were prepared from the resultant crude methanol to determine in vitro antioxidant assays and polyphenolic contents.
Total Phenolic Content
The content of phenolics was determined UV spectrophotometrically using folin-ciocalteu method.[10] Aliquots (0.1 ml) of the extracts were mixed with 0.5 ml of folin-ciocalteu reagent and made up to 3 ml with distilled water. After 3 min, 2 ml of sodium carbonate (20%) was added and mixed thoroughly. The sample was then incubated for 5 min at 50°C and cooled. The absorbance was measured at 650 nm against the reagent blank. The total phenolic content was expressed as mg gallic acid equivalent per gm of extract. The coefficient of determination was r2 = 0.9968.
Total Flavonoid Content
The determination of total flavonoid content in B. cylindrica and C. decandra was based on aluminium chlroride method.[11] A volume of 0.5 ml of AlCl3 ethanol solution (2 %) was added to 0.5 ml of sample solution. After 1 h incubation at room temperature, the absorbance was measured at 420 nm. Extract samples were evaluated at a final concentration of 0.1 mg/ml. The total flavonoid content was calculated as mg quercetin equivalent per gm of extract. The coefficient of determination was r2 = 0.9965.
DPPH· Quenching Activity
DPPH· radical scavenging activity was determined using the method described previously[12] with minor changes. Briefly, 5 ml of methanol solution, DPPH· (0.1 mM) was added to 1 ml of the sample solution at different concentrations (5-1000 μ/ml) and vortexed. The mixtures were still stood for 20 min at room temperature. After incubation, the absorbance was spectrophotometrically measured at 517 nm against a blank. BHT was used as reference compound. The inhibition percentage was calculated using the following formula:
Inhibition of DPPH· (%) = 1 – Sample517 nm/Control517 nm × 100, where, Sample517 nm was the absorbance of the sample and Control517 nm was the absorbance of the control. The results were expressed as EC50, which means the concentration at which DPPH· radicals were quenched by 50%.
2, 2’- Azinobis (3-Ethylbenzothiazoline Sulphonic Acid) ABTS.+ Cation Decolorization Test
ABTS.+ cation decolorization activity of the mangrove extracts was determined by the method described previously.[13] The test was based on the relative activity of antioxidants to quench the radical cation ABTS.+. The stock solution included ABTS.+ solution (7 mM) and potassium persulfate solution (2.4 mM). The working solution was then prepared by mixing the two stock solutions in equal quantities and allowing them to react for 12 h at room temperature in the dark. The solution was then diluted by mixing 1 ml ABTS.+ solution with 60 ml methanol to obtain an absorbance of 0.706 ± 0.001 units at 734 nm using the spectrophotometer. Bark extracts and BHT of various concentrations (5-1000 mg/ml) were allowed to react with 1 ml of the ABTS.+ solution and the absorbance was taken at 734 nm after 7 min using the spectrophotometer. The ABTS.+ scavenging capacity of the extract was compared with that of BHT. Percentage inhibition was calculated as ABTS.+ radical scavenging activity (%) = 1 – Sample734 nm/Control734 nm × 100, where, Sample734 nm was the absorbance of the sample and Control734 nm was the absorbance of the control.
Hydroxyl Radical Scavenging Activity
The hydroxyl radical (˙ OH) scavenging activity of the B. cylindrica and C. decandra extracts was measured based on the method described previously.[14] Various concentrations of extracts (5-1000 μg/ml) were added with 1.0 ml of iron-ethylenediaminetetraacetic acid (EDTA) solution (0.13% ferrous ammonium sulfate and 0.26 % EDTA), 0.5 ml of EDTA solution (0.018%), and 1.0 ml of dimethyl sulfoxide (DMSO) (0.85% v/v in 0.1 M phosphate buffer, pH 7.4). The reaction was initiated by adding 0.5 ml of ascorbic acid (0.22%) and incubated at 80-90°C for 15 min in a water bath. After incubation, the reaction was terminated by the addition of 1.0 ml of ice-cold tricholoro acetic acid (TCA) (17.5% w/v). Three ml of Nash reagent (75.0 g of ammonium acetate, 3.0 ml of glacial acetic acid, and 2 ml of acetyl acetone were mixed and raised to 1 l with distilled water) was added and left at room temperature for 15 min. The reaction mixture without sample was used as control. The intensity of the color formed was measured spectrophotometrically at 412 nm against reagent blank. BHT was used as positive control. The ˙ OH scavenging activity was calculated by the following formula: ˙ OH scavenging activity = 1 – (difference in absorbance of sample/difference in absorbance of blank) ×100.
Reducing Capacity
The reducing power of the extracts of B. cylindrica and C. decandra was measured using the potassium ferricyanide reduction method.[15] Various concentrations of the extracts and standards (50, 100, 250, 500, 750, 1000 mg/ml) were added to 2.5 ml of (0.2 M) sodium phosphate buffer (pH 6.6) and 2.5 ml of potassium ferricyanide [K3Fe3(CN)6] (1%) solution and vortexed. After incubation at 50°C for 20 min, 2.5 ml of TCA (10%, w/v) was added to all the tubes and centrifuged at ×3000 g for 10 min. Afterwards, upper layer of the solution (5 ml) was mixed with deionized water (5 ml). To this, 1 ml of FeCl3 (1%) was added to each test tube and incubated at 35°C for 10 min. The formation of Perl's Prussian color was measured at 700 nm in a UV-VIS spectrophotometer. Increased absorbance of the reaction mixture indicated increasing reducing power. Here, the EC50 value is the effective concentration at which absorbance was 0.5 % for the reducing capacity. BHT was used for comparison.
Antihemolytic Activity
Inhibition of H202 induced red blood cell (RBC) hemolysis of methanolic extract was examined by the in vitro method described previously.[16] The erythrocytes from cow blood were separated by centrifugation and washed with saline or isotonic sodium phosphate buffer (pH 7.4) until the supernatant was colorless. The erythrocytes were then diluted with saline or phosphate buffer to give a 4% suspension. Varying amounts of sample (200-1000 μg/ml) with saline or buffer were added to 2 ml of the suspension of erythrocytes and the volume was made up to 3.5 ml with saline or buffer. This mixture was preincubated for 5 min and then 0.5 ml H202 solutions of appropriate concentration in saline or buffer were added. The concentration of H202 in the reaction mixture was adjusted so as to bring 90% hemolysis of blood cells after 120 min incubation. About 80-90% hemolysis of rat erythrocytes was obtained after 4-6 h. Incubation was concluded after these time intervals by centrifugation during 5 min at ×l000 g and the extent of hemolysis was determined by measurement of the absorbance at 540 nm corresponding to hemoglobin liberation. Antihemolytic activity was expressed as the inhibition percentage and was calculated using the following formula: Antihemolytic activity (%) = 1 – Sample540 nm/Control540 nm × 100, where, Sample540 nm was the absorbance of the sample and Control540 nm was the absorbance of the control.
Statistical Analysis
The experimental results concerning the study were mean ± standard deviation of three parallel measurements. Linear regression analysis was used to calculate the EC50 values. One-way analysis of variance (ANOVA) and Duncan's Multiple Range Test (DMRT) were carried out to determine significant differences between the parameters by Statistical Package for the Social Sciences (SPSS) (version 10 for Windows 98, SPSS Inc.).
Results
Total Phenolic and Flavonoid Contents
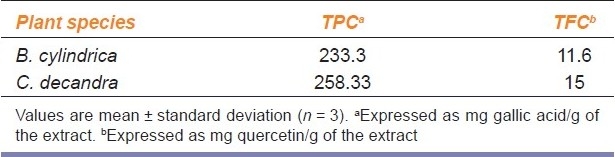
The phenolic and flavonoid contents of the bark of B. cylindrica and C. decandra are reported in Table 1. The amount of phenolic contents was 233.3 and 258.33 mg of gallic acid equivalents/g dry plant material in B. cylindrica and C. decandra, respectively. The flavonoid content was 11.6 and 15 mg of quercetin equivalents (QE)/g of dry material in B. cylindrica and C. decandra.
Table 1.
Total phenolic and flavonoid contents of B. cylindrica and C. decandra
DPPH· Quenching Capacity
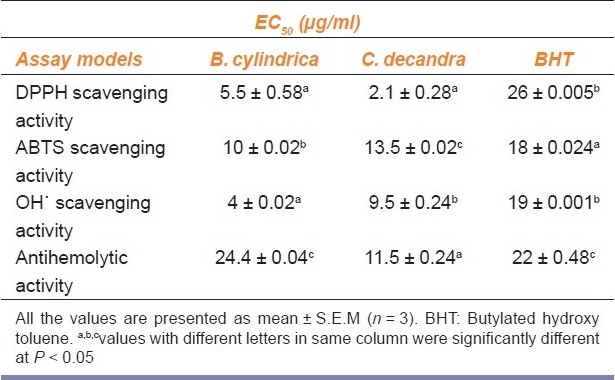
The extracts of B. cylindrica and C. decandra exhibited a concentration dependent antiradical activity [Figure 1a] by quenching DPPH radical with values EC 50 of 5.5 ± 0.58 and 2.1 ± 0.28, respectively. The DPPH scavenging activity of the extracts is higher than that of the BHT (EC50 26 ± 0.005) (P < 0.05) [Table 2].
Figure 1a.
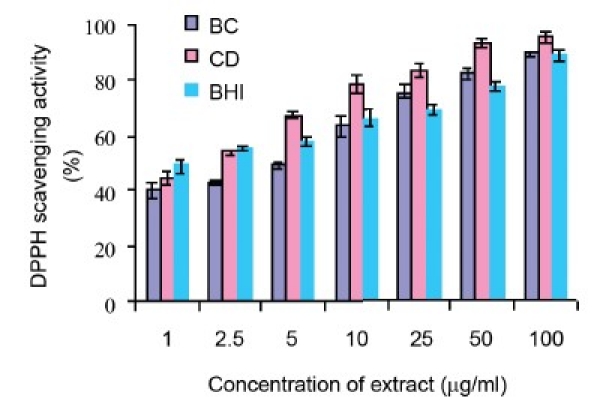
DPPH· quenching activity of methanol extracts of B. cylindrica (BC), C. decandra (CD) and butylated hydroxy toluene (BHT). All the values are presented as mean ± S.E.M (n = 3)
Table 2.
EC50 values for the methanol extracts of B. cylindrica and C. decandra and standards in various free radical scavenging assay models
ABTS+ Scavenging Capacity
Stable free radical ABTS.+ was scavenged by bark extracts of both the mangrove species produced by the oxidation of 2, 2-azinobis-3-ethylbenzothiazoline-6-sulphate is illustrated in [Figure 1b]. The methanol extracts of the selected mangroves mopped up more than percentage of radicals in vitro. ABTS.+ scavenging activity of the B. cylindrica bark extract was higher than that of C. decandra and comparable to that of BHT (P < 0.05) [Table 2].
Figure 1b.
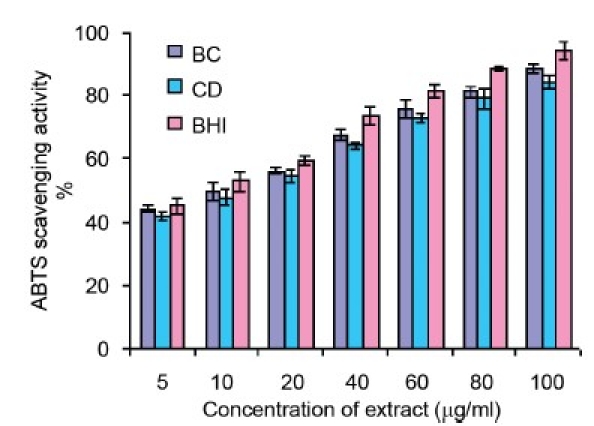
ABTS.+ scavenging activity of methanol extracts of B. cylindrica (BC), C. decandra (CD) and butylated hydroxy toluene (BHT). All the values are presented as mean ± S.E.M (n = 3)
OH Scavenging Capacity
Figure 1c depicts a concentration-dependent inhibition by the extracts of B.cylindrica and C. decandra against ˙ OH as evident by low EC50 value 41 ± 0.002 mg/ml [Table 2]. The extracts showed significantly better scavenging activity than BHT, the reference standard [Table 2].
Figure 1c.
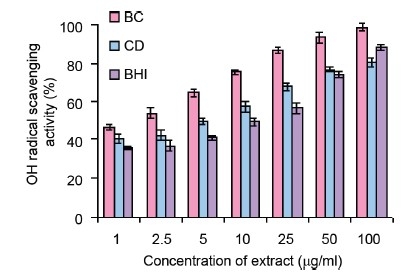
(OH˙) scavenging activity of methanol extracts of B. cylindrica (BC), C. decandra (CD) and butylated hydroxy toluene (BHT). All the values are presented as mean ± S.E.M (n = 3)
Reducing Capacity
The reduction of ferrous ion (Fe3+) to ferric ion (Fe2+) is measured by the intensity of the resultant blue-green solution which absorbs at 700 nm. The methanol extracts exhibited a concentration dependent increase in reducing power [Figure 1d]. They caused significant elevation of reducing power potential with optical density (OD) value of 1.56 ± 0.021 in B. cylindrica and 1.927 ± 0.56 in C. decandra at 1000 mg/ml.
Figure 1d.
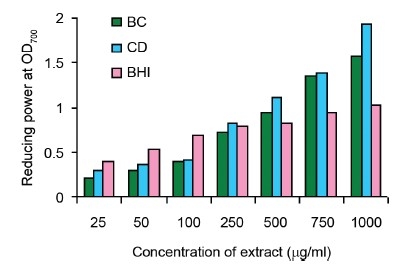
Reducing capacity of methanol extracts of B. cylindrica (BC), C. decandra (CD) and butylated hydroxy toluene (BHT). All the values are presented as mean ± S.E.M (n = 3)
Antihemolytic Capacity
The methanolic extracts of B. cylindrica and C. decandra bark inhibited the hemolysis of RBC by H202 induced peroxy radicals in a concentration dependent manner [Figure 1e] with the values of EC50 11.5 ± 0.24 and EC50 24.4 ± 0.04 mg/ml, respectively [Table 2].
Figure 1e.
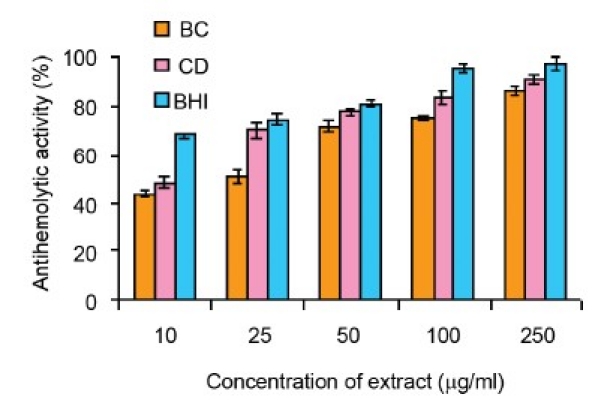
Antihemolytic of methanol extracts of B. cylindrica (BC), C. decandra (CD) and butylated hydroxy toluene (BHT). All the values are presented as mean ± S.E.M (n = 3)
Correlation Between Phytoconstituents and Antioxidant Assays
In this present study, the association between antioxidant activities and total phenolic content and total flavonoid content was observed based on regression analysis. A positive linear correlation was established between total phenolic content and corresponding inhibitory efficacy on DPPH radical by the extract (r2 = 0.833) of B. cylindrica. Positive correlations were observed between total phenolic content and ABTS.+ cation scavenging (r2 = 0.788) and reducing power capacity (r2 = 0.8). Lower correlations were found between total phenolic content and hydroxyl ion radical scavenging (r2 = 0.524) and antihemolytic activity (r2 = 0.461). In the methanol extract of C. decandra, positive correlations were established between total phenolic content and DPPH (r2 = 0.96), hydroxyl ion (r2 = 0.93), ABTS.+ cation (r2 = 0.733) radical scavenging and antihemolytic activity (r2 = 0.646).
Discussion
Recent years have seen an exponential increase in research on the antioxidant properties of medicinal plants. The identification and investigation on antioxidants from medicinal plants is a fast expanding field of research and several antioxidants have been investigated such as flavonoids and other phenolic compounds. In this study, we examined total phenolics and flavonoids and evaluated the antioxidant effects of crude methanolic extracts of B. cylindrica and C. decandra bark. Methanolic extracts of both the plants exhibited promising antioxidant activity in all the experimental models adopted.
In this study, the barks of the mangroves, B. cylindrica and C. decandra, collected from Pichavaram, South India were found to contain appreciable content of polyphenolics. The total phenolic content in C. decandra is higher when compared to the species (94.4 mg/g) found in Sundarbans, India.[17] Mangroves in Sundarbans, North India were found to contain high content of polyphenolics like tannins.[18] Previous reports revealed that mangroves are rich in polyphenols and tannins.[19,20] Phenolics widely encountered in the plants tested as the most active radical scavengers. They are present in a variety of plants utilized as important components of both human and animal diets.[21,22] There is a strong evidence on the preventive effects of phenolics on age related chronic diseases.[23]
The DPPH· assay constitutes a quick and low cost method which has frequently been used for the evaluation of the antioxidative potential of various natural products.[24] In this study, the extracts of B. cylindrica and C. decandra exhibited a concentration dependent antiradical activity. The DPPH scavenging activity of the extracts are higher than that of mangrove studied from Sundarbans, India (IC50 = 0.65 mg/ml).[17] It was evident that the bark extracts of B. cylindrica and C. decandra hydrogen donating ability to act as antioxidants.[25] In this study, C. decandra showed higher potential among the mangroves hitherto investigated.
The decolorization of ABTS.+ cation reflects the capacity of an antioxidant species to donate electrons or hydrogen atoms to deactivate these radical species.[26] In the present study, the methanol extracts of the investigated mangrove plants showed strong decolorizing effect towards ABTS .+ cation in a dose dependent manner. It is noteworthy that ABTS.+ scavenging activity of the B. cylindrica bark extract was higher than that of C. decandra and comparable to that of BHT.
Hydroxyl radical (OH.), the most reactive free radical, has the capacity to conjugate with nucleotides in DNA, cause strand breakage, and lead to carcinogenesis, mutagenesis and cytotoxicity.[27] The.OH ion scavenging results indicate that extracts scavenged .OH radicals and percentage inhibition was proportional to the concentration of the extract. Earlier, Babu et al.,[28] observed a strong . OH radical scavenging activity of a mangrove, Acanthus ilicifolius leaf extract. In our study, the scavenging effect of methanolic bark extracts of B. cylindrica and C. decandra was found stronger than that of A. ilicifoilius.
The methanol extracts caused significant elevation of reducing power. The reducing powers of the extracts were higher when compared with BHT (1.022 at 1000 mg/ml). The reduction of ferrous ion (Fe3+) to ferric ion (Fe2+) is measured by the intensity of the resultant blue-green solution which absorbs at 700 nm. The reducing properties are generally associated with the presence of reductones. Gordan[29] reported that the antioxidant action of reductones is based on the breaking of the free radical chain by donating a hydrogen atom. The result presented here indicates that the marked antioxidant activity of bark extracts of B. cylindrica and C. decandra seems to be due to the presence of polyphenols which may act as reductones to convert free radicals into more stable products and terminate free radical chain reaction.
H2O2 mediated lipid oxidation in cow blood erythrocyte membrane induce membrane damage and hemolysis. In the present study, the extracts of the mangroves were reported to possess dose dependent inhibitory effects towards hemolysis of cow blood erythrocyte. The antihemolytic effect of the C. decandra was found stronger than that of B. cylindrica and BHT (EC50 22 ± 0.48 mg/ml).
In this study, a linear correlation was found between phenolics contents and antioxidant activity. Many supportive reports emphasize the positive correlation between phenolic content and antioxidant efficacy.[30–33] A positive correlation between antioxidant activity and polyphenol content was found suggesting that the antioxidant capacity of the plant extracts is due to a great extent to their polyphenols.[34,35]
In summary, the results indicate the potential of bark extracts as dietary supplement and in the treatment of chronic diseases caused by overproduction of free radicals. Additional work is therefore necessary to fractionate the extract further to elicit a better understanding of how each chemical fraction contributes to the overall antioxidant activity and to determine whether the unique mixture of plant phenolics contributes to a synergistic antioxidant activity.
Acknowledgments
The authors are grateful to the management of Karpagam Educational Institutions for its generous support and encouragement.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Vagi E, Rapavi E, Hadolin M, Vasarhelyine Peredi K, Balazs A, Blazovics A, et al. Phenolic and triterpenoid antioxidants from Origanum majorana L. herb and extracts obtained with different solvents. J Agric Food Chem. 2005;53:17–21. doi: 10.1021/jf048777p. [DOI] [PubMed] [Google Scholar]
- 2.Kirtikar KR, Basu BD. 2nd ed. Dehradun: International Book Distributors; 1999. Indian Medicinal plants; p. 1012. [Google Scholar]
- 3.Laphookhieo S, Karalai C, Ponglimanont C, Chantrapromma K. Pentacyclic triterpenoid esters from the fruits of Bruguiera cylindrica. J Nat Prod. 2004;67:886–8. doi: 10.1021/np0305122. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi KA. Brugine from Bruguiera cylindrica. Phytochemistry. 1975;14:1458. [Google Scholar]
- 5.Magwa ML, Gundidza M, Gweru N, Humphrey G. Chemical composition and biological activities of essential oil from the leaves of Sesuvium portulacastrum. J Ethnopharmacol. 2005;103:85. doi: 10.1016/j.jep.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Premnathan M, Chandra K, Bajpai SK, Kathiresan K. A survey of some Indian marine plants for antiviral activity. Bot Mar. 1992;35:321–4. [Google Scholar]
- 7.Agoramoorthy G, Chen F, Venkatesalu V, Kuo DH, Shea PC. Evaluation of antioxidant polyphenols from selected mangrove plants of India. Asian J Chem. 2008;20:1311–22. [Google Scholar]
- 8.Nabeel MA, Kathiresan K, Manivannan S. Antidiabetic activity of mangrove species Ceriops decandra in alloxan-induced diabetic rats. J Diabetes. 2010;2:97–103. doi: 10.1111/j.1753-0407.2010.00068.x. [DOI] [PubMed] [Google Scholar]
- 9.Sakagami H, Kashimata M, Togishi M, Satoh K, Odanaka Y, Ida Y, et al. Radical modulation activity of lignins from a mangrove plant, Ceriops decandra (Griff.) Ding Hou. In vivo. 1998;12:327–32. [PubMed] [Google Scholar]
- 10.Sadasivam S, Manickam A. New Delhi: New Age International (P) Limited; 1996. Biochemical methods. [Google Scholar]
- 11.Ordonez AA, Gomez JD, Vattuone MA, Isla MI. Antioxidant activities of Sechium edule (Jacq.) Swart extracts. Food Chem. 2006;97:452–8. [Google Scholar]
- 12.Singh RP, Chidambara Murthy KN, Jayaprakash GK. Studies on antioxidant polyphenol content of aqueous extracts from pomegranate peel and seed extracts using in vitro models. J Agric Food Chem. 2002;50:86–9. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- 13.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 14.Klein SM, Cohen G, Cederbaum AI. Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical generating systems. Biochem J. 1981;20:6006–12. doi: 10.1021/bi00524a013. [DOI] [PubMed] [Google Scholar]
- 15.Oyaizu M. Studies on products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–15. [Google Scholar]
- 16.Naim M, Gestetner B, Bondi A, Birk Y. Antioxidative and antihemolytic activities of soybean isoflavones. J Agric Food Chem. 1976;24:1174–7. doi: 10.1021/jf60208a029. [DOI] [PubMed] [Google Scholar]
- 17.Banerjee D, Chakrabarti S, Hazra AK, Banerjee S, Ray J, Mukherjee B. Antioxidant activity and total phenolics of some mangroves in Sundarbans. Afr J Biotechnol. 2008;7:805–10. [Google Scholar]
- 18.Kathiresan K, Ravi V. Seasonal changes in tannin content of mangrove leaves. Indian Forester. 1990;116:390–2. [Google Scholar]
- 19.Ravi AV, Kathiresan K. Seasonal variation in gallotannin from mangroves. Indian J Marine Sci. 1990;19:224–5. [Google Scholar]
- 20.Bravo L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56:317–33. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 21.Crozier A, Lean ME, McDonald MS, Black C. J Agric Food Chem. 1997;45:590–5. [Google Scholar]
- 22.Boyer J, Rui HL. Apple phytochemicals and their health benefits. Nutr J. 2004;3:5. doi: 10.1186/1475-2891-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroon P, Williamson G. Polyphenols: Dietary components with established health benefits. J Sci Food Agric. 2005;85:1239–40. [Google Scholar]
- 24.Molyneux P. The use of the stable free radical diphenyl picryl hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 2004;26:211–9. [Google Scholar]
- 25.Sanchez-Mareno C. Review: Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci Technol Inter. 2002;8:121–37. [Google Scholar]
- 26.Pellegrini N, Re R, Yang M, Rice-Evans C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2, 2’- azinobis-3-ethylbenzothiazoline-6- sulfonic acid radical cation decolorization assay. Met Enzymol. 1999;299:379–89. [Google Scholar]
- 27.Naidu MM, Sulochanamma G, Sampathu SR, Srinivas P. Studies on extraction and antioxidant potential of green coffee. Food Chem. 2008;107:377–84. [Google Scholar]
- 28.Babu BH, Shylesh BS, Padikkala L. Antioxidant and hepatoprotective effect of Acanthus ilicifolius. Fitoterapia. 2001;72:272–7. doi: 10.1016/s0367-326x(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 29.Gordan MH. Food antioxidants. London/New York: Elsevier; 1990. [Google Scholar]
- 30.Kukic J, Petrovic S, Niketic M. Antioxidant activity of four endemic Stachys taxa. Biol Pharmaceut Bull. 2006;29:725–9. doi: 10.1248/bpb.29.725. [DOI] [PubMed] [Google Scholar]
- 31.Buřičová L, Réblová Z. Czech medicinal plants as possible sources of antioxidants. Czech J Food Sci. 2008;26:132–8. [Google Scholar]
- 32.Canadanovic-Brunet J, Cetkovic G, Djilas S, Tumbas V, Bogdanovic G, Mandic A, et al. Radical scavenging, antibacterial, and antiproliferative activities of Melissa officinalis L.extracts. J Med Food. 2008;11:133–43. doi: 10.1089/jmf.2007.580. [DOI] [PubMed] [Google Scholar]
- 33.Suaib L, Ritesh K, Kaushik S, Suchitra S, Mahendra PD, Suman PS. Antioxidant potential of root of Vetiveria zizaniodes (L.) Nash. Indian J Biochem Biophy. 2009;46:122–5. [PubMed] [Google Scholar]
- 34.Ng TB, Liu F, Wang ZT. Antioxidative activity of natural products from plants. Life Sci. 2000;66:709–23. doi: 10.1016/s0024-3205(99)00642-6. [DOI] [PubMed] [Google Scholar]
- 35.Kiselova Y, Ivanova D, Chervenkov T, Gerova D, Galunska B, Yankova T. Correlation between the in vitro antioxidant activity and polyphenol content of aqueous extracts from Bulgarian herbs. Phythother Res. 2006;11:961–5. doi: 10.1002/ptr.1985. [DOI] [PubMed] [Google Scholar]


