Abstract
Aim:
The aim of the present study was to investigate whether Tribulus terrestris Linn (TT) could protect the cadmium (Cd)-induced testicular tissue peroxidation in rats and to explore the underlying mechanism of the same.
Materials and Methods:
In vitro and in vivo studies were conducted to know the protective effect of ethanolic extract of TT (eTT) in Cd toxicity. In in vitro studies, total antioxidant and ferrous metal ion chelating activity of TT was studied. In vivo studies were conducted in rats. A total of 40 Wistar strain adult male rats were divided into four groups. Group 1 served as control, while group 2 to 4 received CdCl2 (3 mg/kg b. wt. s/c once a week). In addition to Cd, group 3 and 4 rats also received eTT (5 mg/kg b.wt. daily as oral gavage) and α-tocopherol (75 mg/kg daily by oral gavage), respectively. At the end of 6th week, all the rats were sacrificed and the separated testes were weighted and processed for estimation of tissue peroxidation markers, antioxidant markers, functional markers, and Cd concentration. The testes were also subjected to histopathological screening.
Results:
In in vitro studies, the percentage of metal ion chelating activity of 50 μg/ml of eTT and α-tocopherol were 2.76 and 9.39, respectively, and the antioxidant capacity of eTT was equivalent to 0.063 μg of α-tocopherol/μg of eTT. In in vivo studies, administration of Cd significantly reduced the absolute and relative testicular weight, antioxidant markers such as superoxide dismutase and glutathione, and functional markers such as LDH and ALP, along with significant increase in peroxidation markers such as malondialdehyde and protein carbonyls in testicular tissue. Testes of Cd only-treated group showed histological insults like necrotic changes in seminiferous tubules and interstitium, shrunken tubules with desquamated basal lamina, vacuolization and destruction of sertoli cells, and degenerating Leydig cells. This group also had higher Cd levels in testicular tissue. Co-treatment with eTT and α-tocopherol significantly reduced the Cd burden in the testes along with reversal of the Cd-induced changes.
Conclusions:
eTT exhibited protective effect against Cd-induced testicular damage. The protective effect appears to be mediated through inhibition of testicular tissue peroxidation by antioxidant and metal chelator activity and also, may be indirectly by stimulating the testosterone production from Leydig cells.
Keywords: Cadmium, rats, Tribulus terrestris, α-tocopherol
Introduction
Tribulus terrestris(TT) is an indigenous medicinal plant used in Indian and Chinese system of medicine for treating various male reproductive disorders. Protodioscin, a steroidal glycoside found in the TT,[1] increased the levels of testosterone, dihydrotestosterone, and dihydroepiandrosterone, and thereby improved libido, erectile dysfunction,[2] and low seminological indices.[3] The whole plant extract of TT showed antioxidant activity and exerted protective effect on streptozotocin-induced diabetic rats by inhibiting oxidative stress.[4] In cardiomyopathy, TT extract administration restored endogenous myocardial antioxidant status or free radical scavenging activity.[5]
Cadmium (Cd) is one of the main environmental pollutants of metallic origin.[6] Exposure to Cd reduces male fertility in both human beings and rodents[7] and as low as 1 to 2 mg/kg body weight exposure can cause testicular damage without pathological changes to other organs.[8] Cd disrupts sertoli cell's tight junctions (Tj) by reducing occludin (Tj protein) expression in sertoli cells.[9] Cd also decreases the plasma levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone by affecting the hypothalamic-pituitary-testicular axis.[10]
Considering the substantial antioxidant and steroidogenic potential of TT, we sought to investigate whether TT could protect the testicular damage induced by Cd in rats; and to explore the underlying mechanisms of the same in comparison with αtocopherol, a potent chain-breaking antioxidant.[11]
Materials and Methods
Whole herb of TT collected from the college campus and farm was identified by a botanist and a copy of herbarium was deposited to Department of Pharmacology and Toxicology for record.
Preparation of Tribulus Terrestris Extract
The ethanolic extract of TT (eTT) was prepared by adding coarse powder of shade-dried whole plant to ethanol at the rate of 1: 20 in conical flask. The flask was closed airtight with nonabsorbent cotton and subjected to constant shaking for overnight on orbital shaker at room temperature. The following day, filtrate was subjected to slow evaporation by keeping in water bath at 60°C temperature until a consistent solid material mass was formed.
Chemicals
All the chemicals were of analytical grade and obtained from commercial sources.
In Vitro Studies
Total antioxidant capacity
The quantitative antioxidant capacity of the eTT and α-tocopherol was measured by the phosphomolybdenum method,[12] which is based on the reduction of Mo (VI) to Mo (V) by the sample analyte and the subsequent formation of green phosphate/Mo (V) complex with a maximum absorption at 695 nm.
Ferrous metal ions chelating activity
Chelation of ferrous ions by the eTT and αtocopherol was estimated by the method described by Dinis et al.[13] Briefly, the samples (25, 50, and 100 μg/ml) were added to a solution of 2 mM FeCl2 (0.05 ml). The reaction was initiated by the addition of 5 mM ferrozine (0.2 ml) and the mixture was shaken vigorously and left standing at room temperature for 10 minutes. After the mixture had reached equilibrium, the absorbance of the solution was measured spectrophotometrically at 562 nm. All test and analyses were run in triplicate and averaged. The percentage of inhibition of ferrozine-Fe2+ complex formation was calculated as: % inhibition = [{An – A1}/An] × 100, where An was the absorbance of the control and A1 was the absorbance in the presence of the samples.
Animals
Sixty-day-old Wistar strain male rats with average body weight of 147 g were obtained from M/s Mahaveer Enterprises (Regn. No. 146/1999/CPCSEA), Hyderabad. The animals kept in polypropylene cages were maintained under standard conditions prescribed by the committee for the purpose of control and supervision on experiments on animals (CPCSEA).
Experimental Design
A total of 40 rats were randomly divided into four groups with ten rats in each. Group 1 was maintained as normal, while group 2 rats acted as Cd toxicity control. These rats were given Cd @3 mg/kg b.wt. sc, once a week as CdCl2 dissolved in distilled water. Group 3 and 4 also received Cd as above, but along with eTT and αtocopherol, respectively, daily by oral gavage. The doses of eTT and α-tocopherol were 5 and 75 mg/kg body wt, respectively. The study was approved by Institutional Animal Ethics Committee.
In this experiment, the dose of Cd to induce oxidative stress was based on a report by Adaikpoh et al.[14] The oral dose of TT whole plant extract was selected as per Gauthaman and Ganesan,[2] who reported that administration of eTT to rats @ 2.5, 5.0, and 10.0 mg/kg b.wt. once daily for 8 weeks resulted in increase of some sex hormone levels in blood without showing adverse effects. The selected dose of αtocopherol was as per Adaikpoh and Obi,[15] who stated that αtocopherol at a dose of 75 mg/kg body weight orally for 4 weeks effectively protected the testes and prostrate against Cd-induced alteration in lipid patterns.
All the forty rats were sacrificed at the end of 6th week by cervical dislocation and testes were collected immediately. They were separated from epididymis and other tissues, mopped with tissue paper, and weighed. Soon after weighing, eight right testes were homogenized individually to make 10% homogenate in 50 mM phosphate buffer (pH 7.0) with tissue homogenizer. This homogenate was used to study the tissue peroxidation markers such as thiobarbituric acid reacting substances (TBARS) and protein carbonyls, tissue antioxidants such as glutathione (GSH) and superoxide dismutase (SOD), and testicular functional markers such as lactate dehydrogenase (LDH) and alkaline phosphatase (ALP). From the remaining 12 testes, three testes were kept in Bouin's fixative and three were kept in 2.5% glutaraldehyde in 0.05 M phosphate buffer (pH 7.2) to study histopathology under light microscope and electron microscope, respectively. The remaining six testes were kept in hot air oven for 24 hours for further processing to estimate their Cd concentration. Feed and drinking water were also analyzed for Cd levels.
Biochemical Analysis of Testicular Tissue
Antioxidant markers
SOD was estimated by the method that involved inhibition of superoxide-dependant reduction of tetrazolium dye methyl thiazolyl tetrazolium (MTT) to its formazan.[16] GSH was estimated based on a reaction of reduced GSH with 5-5’ dithiobis-2-nitrobenzoic acid.[17]
Peroxidation markers
Malondialdehyde, the product of lipid peroxidation, was estimated by reaction with thiobarbituric acid as per the method prescribed by Subramanian et al.[18] Protein carbonyls were estimated based on the reaction of amino carbonyls with 2, 4-dinitrophenyl hydrazine to form hydrazones, which were detected by spectrophotometer at 372 nm.[19]
Functional marker enzymes
Testicular functional marker enzymes such as LDH and ALP were estimated by using standard kits from Diagnosticum Zrt, Hungary and Transasia Bio-medicals Ltd, HP, respectively.
Total protein
Total protein in testicular tissue homogenate was quantified as per Lowry et al.'s[20] method.
Histological studies
For light microscopy examination, the Bouin's fixed tissues were dehydrated through ascending grades of alcohol, cleared in three changes of xylene, and were embedded in paraffin. Serial sections, each of 4-micron thickness, were cut and stained with H and E as per standard protocols.[21] For transmission electron microscopy (TEM), the glutaraldehyde-fixed tissues were used. Specimen preparation, staining, and the observations were done at the designated RUSKA Lab, SV Veterinary University, Hyderabad.
Estimation of cadmium concentration
Dry ashing procedure was used for the Cd analysis in organs. Wet tissue was dried at 100°C for 24 hours. Dried samples were then transferred to a cool muffle furnace and the temperature was slowly raised to 450°C and ashed overnight. The ash was dissolved in 2 ml HNO3 plus double distilled water (DDW). After filtering, the digesta was made up to 25 ml with DDW and concentration of Cd was determined with atomic absorption spectrophotometer (NOVA 300).[22]
Statistical Analysis
The data were subjected to statistical analysis by applying one way ANOVA using Statistical Package for Social Sciences (SPSS) 12th version. Differences between means were tested using Duncan's multiple comparison test and significance was set at P < 0.05.
Results
Antioxidant capacity of eTT was equivalent to 0.063 μg of αtocopherol/μg of eTT [Figure 1]. The percentage of metal chelating capacity of 50 μg/ml of eTT and αtocopherol were 2.76 and 9.39, respectively. The metal scavenging effect of eTT was 3.4 times less than αtocopherol [Figure 2].
Figure 1.
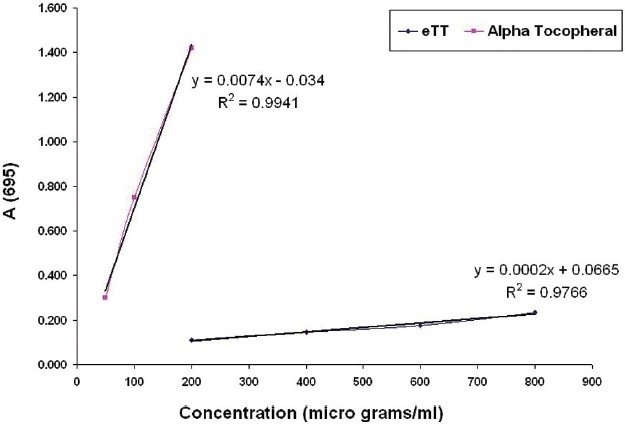
In vitro antioxidant capacity of eTT and α-tocopherol
Figure 2.
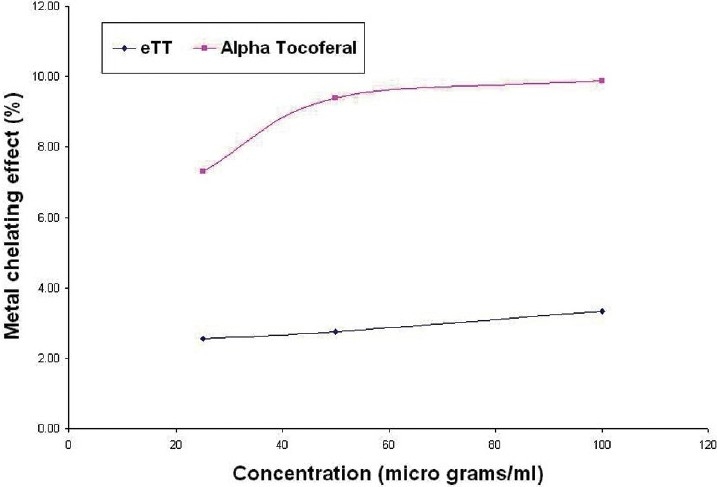
In vitro metal ion chelating effect of eTT and α-tocopherol
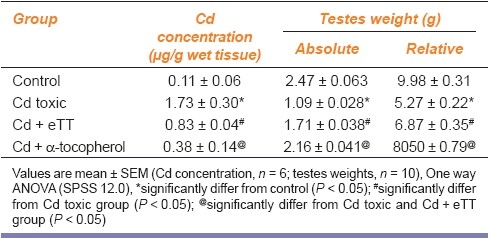
Table 1 shows a significant decrease in absolute and relative weights of testis of Cd-treated group 2 rats compared with control rats of group 1. eTT and α-tocopherol co-treatment in group 3 and 4 showed a significant increase in testicular weight compared with group 2.
Table 1.
Effect of ethanolic extract of Tribulus terrestris on testes cadmium concentration and weights in cadmium administered rats
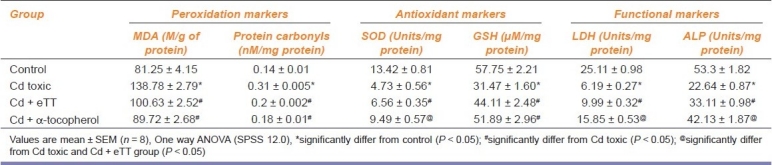
In Cd toxic group, the peroxidation markers such as malondialdehyde (MDA) and protein carbonyls were significantly increased and the levels of antioxidants such as SOD and reduced GSH were reduced significantly compared with control. Administration of eTT and α-tocopherol along with Cd significantly reversed the above alterations. The testicular tissue functional markers such as LDH and ALP were found significantly lowered (against control group) in Cd toxic group, but these were significantly elevated (against Cd toxic group) in eTT and α-tocopherol coadministered groups [Table 2].
Table 2.
Effect of ethanolic extract of Tribulus terrestris on peroxidation, antioxidant, and functional markers of testicular tissue of cadmium treated rats
Testes of Cd toxic group showed alteration in normal histoarchitecture, with necrotic changes both in seminiferous tubules and the interstitial tissue. In light microscopy, most of the tubules were shrunken with desquamation or loss of basal lamina, pyknosis, destruction of nuclei of germ cells and generalized germ cell depletion with multinucleated giant cells, vascular congestion, and interstitial edema were observed. Spermatids losing their characteristic adluminal location and being oriented in different directions between the spermatogenetic cells were also seen [Figure 3d]. In TEM, the supporting sertoli cells were destroyed and some of them showed cytoplasmic vacuolization. In the interstitial tissue, the degenerating Leydig cells appeared dark and edematous [Figure 3c]. Recovery from histological injury was observed in eTT coadministered rats with few shrunken tubules with spermatogenic and sertoli cells in their normal location [Figure 3e]. Vacuolization was observed in few sertoli cells. Leydig cells were normal and blood vessels were intact with slight edema in the interstitial space [Figure 3f]. In α-tocopherol coadministered rats, testicular histoarchitecture appeared near normal and the tubules and interstitial tissue showed no signs of histological injury [Figure 3g].
Figure 3d.
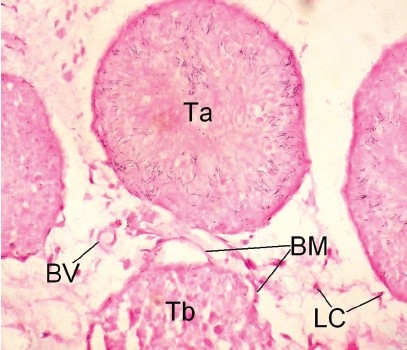
Photomicrograph of the testes of LM (×400) of Cd control rats showing shrunken tubule without lumen and desquamated basement membrane and dead spermatozoa oriented in different directions. Ta: Tubules with dead spermatozoa dispersed all directions, Tb: Shrunken tubule with desquamated basement membrane, BV: Blood vessel, LC: Leydig cell
Figure 3c.
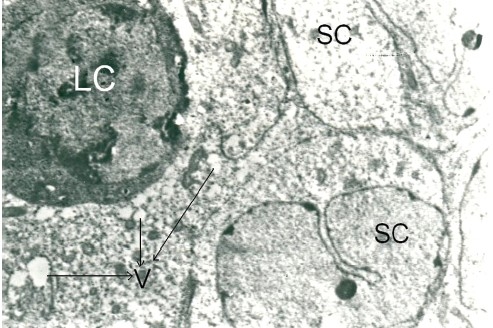
Photomicrograph of the testes of TEM (×4000) of Cd toxic rats showing sertoli cells with vacuoles in the cytoplasm, loss of basement membrane, and degenerating Leydig cell which are dark. SC: Sertoli cells, LC: Leydig cell
Figure 3e.
Photomicrograph of the testes of LM (×100)
Figure 3f.
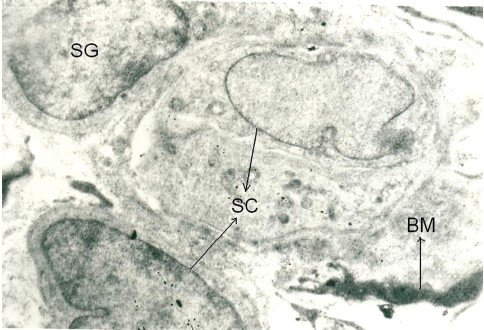
Photomicrograph of the testes of TEM (×5000) of eTT-treated Cd toxic rats showing recovery form toxic effects showing basement membrane and without vacuoles. SC: Sertoli cells, SG: Spermatogonium
Figure 3g.
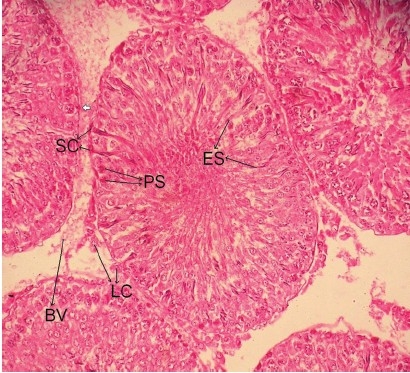
Photomicrograph of the testes of LM (×400) of α-tocopheroltreated Cd toxic rats showing near normal architecture LC: Leydig cell, SC: Sertoli cells, PS: Pachytene spermatocytes, ES: Elongated spermatids, BV: Blood vessel
Figure 3a.
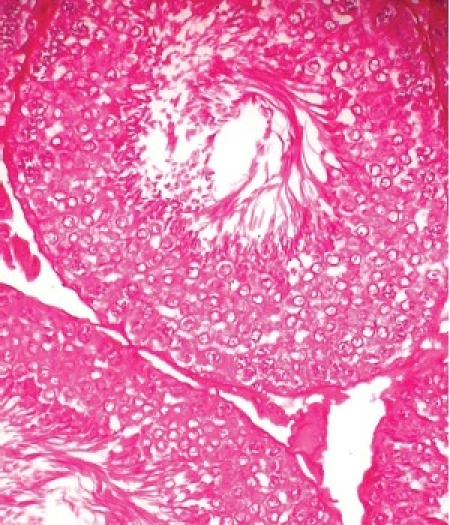
Photomicrograph of the testes of LM (×400) of normal control rats showing organized distribution of various types of cells in the seminiferous epithelium of the tubules and interstitium
Figure 3b.
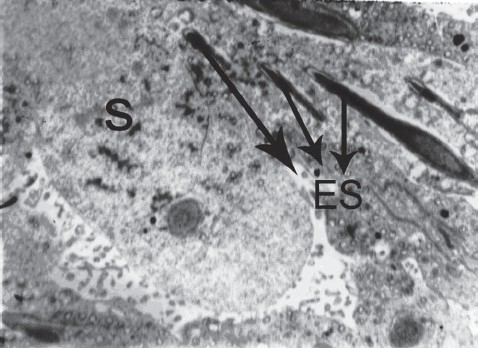
Photomicrograph of the testes of TEM (×2000) of normal control showing normal spermatocytes and elongating spermatids; S: Spermatocyte, ES: Elongated spermatids
The concentration of Cd in testicular tissue of Cd toxic group was 17 times more than control, whereas with concurrent administration of eTT and α-tocopherol, the Cd levels were significantly lowered [Table 1]. The concentration of Cd in the feed was 0.44 μg/g and in drinking water was 0.008 μg/ml.
Discussion
Of all the organs of the body, testis is highly susceptible to the Cd toxicity.[8] Most of the toxic effects of Cd are related to its ability to generate free radicals at a rate high enough to overwhelm the natural antioxidant defense system of the body.[23] In addition to the antioxidant activity,[4] Gouthaman and Ganesan[2] found that administration of eTT to male rats improved libido and erectile dysfunction. In this study, we demonstrated the relative protective effect of TT and α-tocopherol against Cd-induced testicular damage and rational behind that protective effect.
Administration of Cd induced the testicular damage that was revealed by altered histoarchitecture, with necrotic changes both in seminiferous tubules and the interstitial tissue. Cd induces free radical generation by multifractional mechanisms[24] and causes peroxidation, which in the present study was revealed by significant increase in peroxidation markers of testicular tissue such as MDA and protein carbonyls and decrease in antioxidant markers such as SOD and GSH. Peroxidative damage causes reduction in testicular function, which was reflected by significant reduction in functional markers such as LDH and ALP. These findings are in consistent with earlier report by Sadik[25] who observed a decrease in body weight, paired testicular weight and relative testicular weight, along with lowered antioxidant capacity and functional enzymes, viz. LDH and ALP in testicular tissue.
Cd reduces the expression of occludin in sertoli cells.[9] Occludins are Tj integral membrane proteins of sertoli cells and their expression is induced by testosterone.[26] Hence, testosterone counters the Cd-induced damage to sertoli cells Tj barrier function.
In Cd-treated rats, the sertoli cells of testes were either destroyed or contained cytoplasmic vacuolization, which can be ascribed to oxygen free radical-induced peroxidation and also may indirectly through damage to endothelial cells of capillaries and Leydig cells, which was revealed histologically by damaged blood vessels and degenerating Leydig cells. Once Leydig cells are damaged, the testosterone production will be reduced and thereby protective effect of testosterone on sertoli cells is inhibited. Hampered sertoli cell's structural support, nutrient supply, and regulatory paracrine factors together caused shrunken tubules with desquamation or loss of basal lamina, pyknosis, generalized germ cell depletion with multinucleated giant cells.
α-tocopherol is a chain-breaking antioxidant that exists in cell membrane[27] and plasma.[28] It eliminates lipid peroxyl and alkoxyl radicals, suppresses the chain reaction of LPO and promotes the production of scavenger antioxidant enzymes.[29] In our in vivo study, α-tocopherol exhibited significant protective effect in all aspects as evident from reversal of histological alterations, lowered tissue peroxidation, and Cd accumulation along with elevated antioxidant capacity and functional enzymes activity. The antioxidant and protective effects of α-tocopherol against Cd-induced toxicity were well reported previously.[11,30]
In our in vitro study, even though the eTT was significantly less potent antioxidant and metal chelating agent compared with α-tocopherol in our in vivo study, eTT administration to Cd-treated rats showed a remarkable protective effect in all aspects as evident from significant recovery from histological alterations, tissue peroxidation, and Cd accumulation along with elevated antioxidant capacity and functional enzymes activity.
Whole plant extract of TT was reported to exhibit antioxidant activity[4] and aphrodisiac property that was attributed to increased androgen production.[31] The significant in vivo protective effect of eTT can be attributed to inhibition of oxygen free radicals generation, thereby preventing testicular tissue peroxidation and in addition, it might be possible that eTT protected the sertoli cells by stimulating Leydig cells to produce testosterone[2] that plays a crucial role in the regulation of sertoli cells Tj-permeability barrier.
In conclusion, it is observed in the present study that eTT offered a protective effect against Cd-induced testicular damage in rats. The beneficial effect of TT on testes can be attributed to antioxidant and metal chelating effect of TT and also possibly to enhanced testosterone production from Leydig cells. Further studies are needed to confirm the protective effect through testosterone production.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Dikova N, Ognyanova V. Sofia: Chemical Pharmaceutical Research Institute; 1983. Pharmacokinetic studies of Tribestan Anniversary Scientific Session-35; pp. 1–7. [Google Scholar]
- 2.Gauthaman K, Ganesan AP. The hormonal effects of Tribulus terrestris and its role in the management of male erectile dysfunction - An evaluation using primates, rabbit and rat. Phytomedicine. 2008;15:44–54. doi: 10.1016/j.phymed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Balanathan K, Omar MH, Zainul Rashid MR, Ong FB, Nurshaireen A, Jamil MA. A clinical study on the effect of Tribulus terrestris (Tribestan) on the semen profile in males with low sperm count and low motility. Malay. J Obstet Gynaecol. 2001;7:69–78. [Google Scholar]
- 4.Amin A, Lotfy M, Shafiullah M, Adeghate E. The protective effect of Tribulus terrestris in diabetes. Ann N Y Acad Sci. 2006;1084:391–401. doi: 10.1196/annals.1372.005. [DOI] [PubMed] [Google Scholar]
- 5.Ojha SK, Nandave M, Arora S, Narang R, Dinda AK, Arya DS. Chronic administration of Tribulus terrestris Linn. extract improves cardiac function and attenuates myocardial infarction in rats. IJP - Int J Pharmacol. 2008;4:1–10. [Google Scholar]
- 6.International Programme on Chemical Safety (IPCS) Geneva: WHO; 1992. World Health Organization. Environmental Health Criteria; p. 134. [Google Scholar]
- 7.Benoff S, Jacob A, Hurley IR. Male infertility and Environmental Exposure to Lead and Cadmium. Hum Reprod Update. 2000;6:107–21. doi: 10.1093/humupd/6.2.107. [DOI] [PubMed] [Google Scholar]
- 8.Prozialeck WC, Edwards JR, Woods JM. The Vascular Endothelium as a Target of Cadmium Toxicity. Life Sci. 2006;79:1493–506. doi: 10.1016/j.lfs.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Chung NP, Cheng CY. Is Cadmium chloride-induced inter-sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology. 2001;142:1878–88. doi: 10.1210/endo.142.5.8145. [DOI] [PubMed] [Google Scholar]
- 10.Lafuente A, Cano P, Esquifino A. Are cadmium effects on plasma gonodotropins, prolactin, ACTH, GH and TSH levels, dose-dependent? Biometals. 2003;16:243–50. doi: 10.1023/a:1020658128413. [DOI] [PubMed] [Google Scholar]
- 11.Kaczmarski M, Wojicicki J, Samochowiee L, Dutkiewicz T, Sych Z. The influence of exogenous antioxidants and physical exercise on some parameters associated with production of free radicals. Pharmazie. 1999;54:303–6. [PubMed] [Google Scholar]
- 12.Prieto P, Manuel P, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific applications to the determination of vitamin E. Anal Biochem. 1999;269:337–41. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 13.Dinis TC, Madeira VM, Almeida ML. Action of phenolic derivates (acetaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–9. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 14.Adaikpoh MA, Orhue NE, Igbe I. The protective role of Scoparia dulcis on tissue antioxidant defense system of rats exposed to Cadmium. Afr J Biotechnol. 2007;6:1192–6. [Google Scholar]
- 15.Adaikpoh MA, Obi FO. Prevention of Cadmium-induced alteration in rat testes and prostate lipid patterns by α-tocopherol. Afr J Biochem Res. 2009;3:321–5. [Google Scholar]
- 16.Madesh M, Balasubramanian KA. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J Biochem Biophys. 1998;35:184–8. [PubMed] [Google Scholar]
- 17.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S transferase in rat lung and liver. Biochim Biophys Acta. 1979;582:67–8. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 18.Subramanian KA, Manohar M, Mathan VI. An unidentified inhibitor of lipid peroxidation in intestinal mucosa. Biochim Biophys Acta. 1988;962:51–8. doi: 10.1016/0005-2760(88)90094-x. [DOI] [PubMed] [Google Scholar]
- 19.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–78. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 20.Lowry OH, Rosenbroufgh MJ, Farr AL, Rawdell RA. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 21.Bancroft JD, Gamble M. 5th Ed. New York: Churchill Livingstone; 2003. Theory and Practice of histological techniques; pp. 593–620. [Google Scholar]
- 22.Uyanik F, Even M, Atasever A, Tuncoku G, Kolsuz AH. Changes in some Biochemical Parameters and organs of broilers exposed to cadmium and effect of zinc on cadmium induced alterations. Isr J Vet Med. 2001;56:128–34. [Google Scholar]
- 23.Bagchi D, Bagchi M, Hassoun EA, Stohs SJ. Cadmium induced excretion of urinary lipid metabolites, DNA damage, glutathione depletion and hepatic lipid peroxidation in Sprage–Dawley rats. Biol Trace Elem Res. 1996;52:143–54. doi: 10.1007/BF02789456. [DOI] [PubMed] [Google Scholar]
- 24.Bharavi K, Reddy AG, Rao GS, Kumar PR, Kumar DS, Prasadini PP. Prevention of cadmium bioaccumulation by herbal adaptogens. Indian J Pharmacol. 2011;43:45–9. doi: 10.4103/0253-7613.75669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadik NA. Effects of diallyl sulfide and zinc on testicular steroidogenesis in Cadmium-treated male rats. J Biochem Mol Toxicol. 2008;22:345–53. doi: 10.1002/jbt.20247. [DOI] [PubMed] [Google Scholar]
- 26.Sui ER, Mruk DD, Porto CS, Cheng CY. Cadmium induced testicular injury. Toxicol Appl Pharmacol. 2009;238:240–9. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palamanda JR, Kehrer JP. Involvement of vitamin E and protein thiols in the inhibition of microsomal lipid peroxidation by glutathione. Lipids. 1993;28:427–31. doi: 10.1007/BF02535941. [DOI] [PubMed] [Google Scholar]
- 28.Bursell SE, King GL. Can protein kinase C inhition and vitamin E prevent the development of diabetic vascular complications? Dabetes Res Clin Pract. 1999;45:169–82. doi: 10.1016/s0168-8227(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 29.Ernster L, Forsmark P, Nordenbrand K. The mode of action of lipid soluble antioxidants in biological membranes: Relationship between the effects of ubiquinol and vitamin E as inhibitors of lipid peroxidation in submitochondrial particles. Biofactors. 1992;3:241–8. [PubMed] [Google Scholar]
- 30.Yang HS, Han DK, Kim JR, Sim JC. Effects of α-tocopherol on cadmium induced toxicity in rat testis and spermatogenesis. J Korean Med Sci. 2006;21:445–51. doi: 10.3346/jkms.2006.21.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gauthaman K, Ganesan AP, Prasad RN. Sexual effects of puncturevine (Tribulus terrestris) extract (protodioscin): An evaluation using a rat model. J Altern Complement Med. 2003;9:257–65. doi: 10.1089/10755530360623374. [DOI] [PubMed] [Google Scholar]


