Abstract
Interest in alternative medicine and plant-derived medications that affect the “mind” is growing. The aim of present study was to explore the anti-anxiety activity of hydroalcoholic extract of Coriandrum sativum (Linn.) using different animal models (elevated plus maze, open field test, light and dark test and social interaction test) of anxiety in mice. Diazepam (0.5 mg/kg) was used as the standard and dose of hydroalcoholic extract of C. sativum fruit (50, 100 and 200 mg/kg) was selected as per OECD guidelines. Results suggested that extract of C. sativum at 100 and 200 mg/kg dose produced anti-anxiety effects almost similar to diazepam, and at 50 mg/kg dose did not produce anti-anxiety activity on any of the paradigm used. Further studies are needed to identify the anxiolytic mechanism(s) and the phytoconstituents responsible for the observed central effects of the hydroalcoholic extract of C. sativum.
Keywords: Coriandrum sativum (Linn.), elevated plus maze, light and dark test, open field test, social interaction test
Introduction
Anxiety affects one-eighth of the total population of the world and has become a very important area of research interest in psychopharmacology during this decade. Interest in alternative medicine and plant-derived medications that affect the “mind” is growing. Anxiety, a state of excessive fear, is characterized by motor tension, sympathetic hyperactivity, apprehension and vigilance syndromes. Benzodiazepines are the major class of compounds used in anxiety and they have remained the most commonly prescribed treatment for anxiety, despite the important unwanted side effects that they produce such as sedation, muscle relaxation, ataxia, amnesia, ethanol and barbiturate potentiation and tolerance.[1] Various types of herbal medicines have been used as anxiolytic drugs in different parts of the world. Self-administration of herbal medicines is among the most popular of the alternative therapies that also include massage therapy, megavitamins, and homeopathy. Plants have long been used to treat central nervous system (CNS) disorders. Folk medicines have particular values, for example, plants that “calm down”, tranquilize, and raise mood, such as Passiflora coerulea, Valeriana officinalis, Matricaria recutita, Jatropa cilliata, Salvia guaranitica, Tilia tormentosa, and Tilia europeae.
Coriandrum sativum L. Apiaceae (Umbelliferae) is an annual herb commonly used in Middle Eastern, Mediterranean, Indian, Latin American, African and Southeast Asian cuisine. In the Indian traditional medicine, coriander is used in the disorders of digestive, respiratory and urinary systems, as it has diaphoretic and diuretic effects. Pharmacological studies have demonstrated the following actions: Hypoglycemic,[2] anti-inflammatory, hypolipidemic,[3–5] antimutagenic,[6] antihypertensive,[7] antioxidant,[8] anxiolytic,[9] antimicrobial,[10] post-coital antifertility,[11] sedative,[12] diuretic,[13] carminative, antispasmodic and relaxant.[14]
In acute toxicity studies, no mortality was observed up to a dose level of 750 mg/kg[15] and LD50 of coriander oil was reported as 4.13 g/kg (2.48–6.14 g/kg) in rodents. The data available on the toxicity of coriander oil are limited. However, coriander and its oil have a long history of dietary use, with no record of harm caused by consumption of these ingredients.[16] Emamghoreishi et al. in 2005 evaluated its anxiolytic activity using the aqueous extract of C. sativum fruit in mice using elevated plus maze (EPM) model. They also assessed the spontaneous activity and neuromuscular coordination. Other than this, no model(s) for anxiety (except EPM) has been used for further evaluation of anxiolytic activity of C. sativum fruit extract, to our knowledge. The aim of the present study was to explore the anti-anxiety activity of hydroalcoholic extract of C. sativum using different animal models [EPM, open field (OF) test, light and dark test and social interaction test] of anxiety in mice.
Materials and Methods
Animals
Swiss albino mice (males; 20–25 g) were used in the present study. The animals were procured from Disease Free Small Animal House, Suresh Gyan Vihar University, Jaipur, Rajasthan, India. They were provided normal diet and tap water ad libitum and were exposed to 12-h light and 12-h dark cycle. The animals were acclimatized to the laboratory conditions before experiments. Experimental protocol was approved by Institutional Animal Ethics Committee. Care of the animals was taken as per guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forests, Government of India. Experiment protocol was approved by Institutional Animal Ethics Committee.
Plant Material
Fruits of C. sativum were collected from Jaipur, Rajasthan, India, and were authenticated at the Department of Botany, Rajasthan University (voucher specimen no. RUBL20875).
Preparation of Hydroalcoholic Extract
Fruits of C. sativum were collected, washed thoroughly in water, chopped and air dried at 35–40°C for a week. Dried fruits were pulverized in an electric grinder to obtain a fine powder. The powder obtained was defatted with petroleum ether and successively extracted with 70% ethanol using a soxhlet apparatus. Extract was filtered, concentrated under reduce pressure and dried.
Drugs
Diazepam hydrochloride (Calmpose injection, Ranbaxy Laboratories, Gurgaon, India) was used as a reference drug. It was diluted with saline to the required strength before use. Different concentrations of the C. sativum extract were prepared by serial dilution from a stock solution of 100 mg/ml of the extract in sterile water. All the solutions were prepared freshly on test days and administered intraperitoneally (i.p.) in a volume of 0.1 ml/10 g body weight of mice.
Elevated Plus Maze
The plus maze apparatus consisted of two open arms, measuring 16 × 5 cm, and two closed arms, measuring 16 × 5 × 12 cm, connected to a central platform (5 × 5 cm). The maze was elevated to a height of 25 cm above the floor. Each mouse was placed individually at the center of elevated plus maze with its head facing toward an open arm and observed for 5 min to record the number of entries into open arm, closed arm and time spent in each arm.[17] In EPM test, the percent time spent on the open arms was determined as follows:
% = 100 × Number of seconds spent on open arms/300 total seconds (5 min observation time).
Light and Dark Box Test
The apparatus consisted a rectangular box (45 × 27 × 27 cm), partitioned into two compartments connected by a 7.5 × 7.5 cm opening in the wall between compartments. An animal was placed in the center of the light compartment and was observed for 5 min for the time spent in open (white/light) compartment.[18] Percent time spent in the light compartment was determined as follows:
% = 100 × Number of seconds spent in light compartment/300 total seconds (5 min observation time).
Open Field Test
The OF test, which provides simultaneous measures of locomotion, exploration and anxiety, was used for this study. The open field is a 400 × 400 × 300 mm arena with thin black stripes painted across the floor, dividing it into 16 quadratic blocks. Mouse was placed in the center of arena and an observer quantified the spontaneous ambulatory locomotion of each mouse for 5 min. During this period, the number of squares crossed and number of rearing were measured.[19]
Social Interaction Test
The social interaction arena was an open topped box (22 × 15 × 12 cm). Mice were isolated for 1 h before the test. After introduction to the test arena, the mice were observed for cumulative time spent in genital investigation, sniffing a partner, following, grooming, kicking, biting, wrestling, climbing over and under, neck licking and boxing.[20]
Experimental Protocol
Experimental animal groups used in the present study consisted of six mice in each group. All the instruments used in present study were fabricated from local market as per the standard dimensions available from scientific research. Mice were exposed to EPM and light–dark test for normal duration (5 min), sufficient to assess the anxiety levels in rodents.[21] Behavioral tests were performed in independent groups of mice. Drugs were administered 30 min before the evaluations in the apparatus. Doses of ethanolic extract of C. sativum (50, 100 and 200 mg/kg) were selected on the basis of acute toxicity study according to OECD guidelines (up and down method) and the dose of diazepam was 0.5 mg/kg. The apparatus was thoroughly cleaned using 5% ethanol before placing each mouse in the cage.
Statistical Analysis
All the results were expressed as Mean ± SEM. Data were analyzed by analysis of variance (ANOVA) in Graph Pad Instat (GPIS) package, version 3.05. P < 0.05 was considered as significant.
Results
Elevated Plus Maze
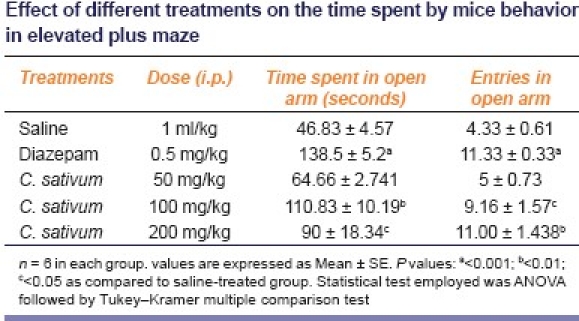
Administration of diazepam (0.5 mg/kg) significantly increased the amount of time spent in the open arms and the percentage of open arm entries (P < 0.001) compared to saline-treated group [Table 1]. Hydroalcoholic extract of C. sativum fruit at 100 mg/kg (P < 0.01) and 200 mg/kg (P < 0.05) significantly increased the time spent in the open arms. Entries in the open arms increased significantly at 100 mg/kg (P < 0.05) and 200 mg/kg (P < 0.01). Plant extract at 50 mg/kg had no significant effects on any of the parameters that were measured on the EPM.
Table 1.
Effect of different treatments on the time spent by mice behavior in elevated plus maze
Light and Dark Box Test
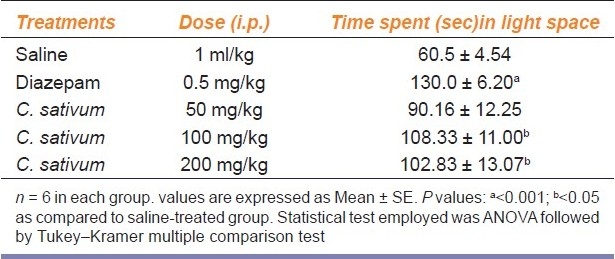
Diazepam (0.5 mg/kg) significantly increased the time spent in light compartment (P < 0.001) compared to saline-treated group [Table 2]. Significant increase in the time spent in the light compartment (P < 0.05) was seen with administration of 100 and 200 mg/kg of hydroalcoholic extract of C. sativum fruit compared to saline-treated group [Table 2]. Plant extract at 50 mg/kg did not produce any significant effects that were measured by light and dark box test.
Table 2.
Effect of different treatments on the time spent by mice behavior in light and dark test
Open Field Test
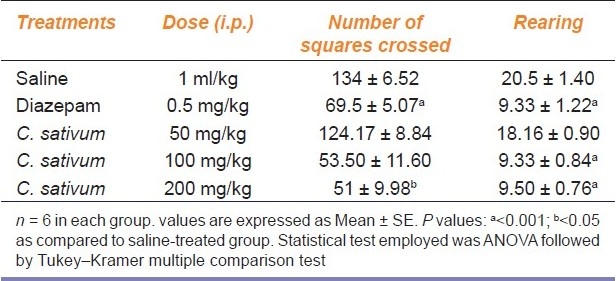
Treatment with the plant extract at 200 mg/kg showed anxiolytic activity in this paradigm, as there were significant differences (P < 0.05) in saline-treated as well as plant extract treated groups [Table 3]. There was decrease in locomotion activity in extract-treated (200 mg/kg) groups as the number of squares crossed in the perimeter was decreased between extract-treated groups and differed significantly (P < 0.05) from the control groups. The frequency of rearing also decreased significantly (P < 0.001) after administration of hydroalcoholic extract of C. sativum fruit at 100 and 200 mg/kg compared to saline-treated group [Table 3]. No significant effects were produced at 50 mg/kg of the plant extract of C. sativum.
Table 3.
Effect of different treatments on the time spent by mice behavior in open field test
Social Interaction Test
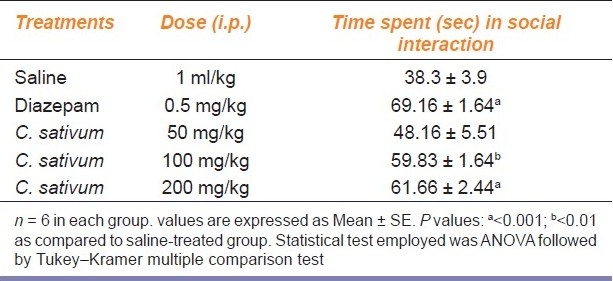
Diazepam (0.5 mg/kg) significantly (P < 0.001) increased the time spent in social interaction among mice as compared to its effect in the saline-treated group [Table 4]. C. sativum hydroalcoholic extract at 100 mg/kg (P < 0.01) and 200 mg/kg (P < 0.001) significantly increased the time spent in social interaction as compared to saline-treated group [Table 4]. No significant effects were produced at 50 mg/kg dose of C. sativum hydroalcoholic extract.
Table 4.
Effect of different treatments on the time spent by mice behavior in social interaction test
Discussion
Benzodiazepines have been extensively used for the last 40 years to treat several forms of anxiety, but due to their unwanted side effects, alternative treatment strategies with favorable side-effect profiles, credible benefits and moderate costs are of interest, especially in primary care settings. Medicinal plants are a good source to find new remedies for these disorders. In the search for an alternative, more specific, and perhaps cost-free therapy, research has been conducted to investigate natural anxiolytic drugs as well as new antidepressant principles.[22] The effects of 100 and 200 mg/kg of hydroalcoholic extract of C. sativum on the EPM, light–dark test, OF test (200 mg/kg only) and social interaction test were almost equivalent to that of 0.5 mg/kg diazepam. Emamghoreishi et al. in 2005 showed that C. sativum has an active anxiolytic activity at 100 mg/kg dose. In the present study, the anxiolytic activity of the C. sativum fruit extract was observed at doses of 100 and 200 mg/kg in mice. These observations clearly indicate that C. sativum exerts an anxiolytic activity.
Anxiolytic activity of C. sativum is likely to be associated with its essential oil content and flavonoids. It is reported that coriander seed oil contains linalool (60–70%) as the major essential oil component.[23] Linalool has marked effects at the CNS, including hypnotic and anticonvulsant properties, and anxiolytic and sedative effects are also shown by linalool in human subjects. Other components of the essential oil which are not found to be active for anti-anxiety effects are: α-thujene, sabinene, β-pinene, myrcene, p-vymene, limonene, z-β-ocimene, y-terpenin, terpinolene, camphor, citronellal, trpinene-4-ol, and decanal.[24] Mechanism of action by which C. sativum shows anxiolytic activity may be similar to that of diazepam [that acts via the gamma-aminobutyric acid (GABA)A receptor complex], as flavonoids and diazepam are structurally similar. Effects of flavonoids as anxiolytics have been observed in many plant species used as folk medicine, such as P. coerulea.
C. sativum is also used for the management of CNS disorders. C. sativum may be a useful in the management of neurodegenerative diseases, such as Alzheimer's disease, on account of its multifarious effects, that is, memory-improving property, cholesterol-lowering property and anticholinesterase activity.[25] C. sativum has also shown potential as an antihypertensive[7] and a sedative.[12] In summary, the hydroalcoholic extract of C. sativum fruit has an anxiolytic activity. Further pharmacological and chemical investigations are required to elucidate the exact mechanism of action of this extract and to isolate the active principles responsible for such effects.
Acknowledgments
The authors are grateful to Bhupesh Sharma (Patiala, India) and Ramesh Nitharwal (Nagpur, India) for their precious advice and helpful discussion.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Lader M, Morton S. Benzodiazepine problems. Br J Addict. 1991;83:823–8. doi: 10.1111/j.1360-0443.1991.tb01831.x. [DOI] [PubMed] [Google Scholar]
- 2.Gray AM, Flatt PR. Insulin-releasing and insulin-like activity of the traditional anti-diabetic plant C. sativum (coriander) Br J Nutr. 1999;81:203–9. doi: 10.1017/s0007114599000392. [DOI] [PubMed] [Google Scholar]
- 3.Chithra V, Leelamma S. Hypolipidemic effect of coriander seeds (C. sativum): Mechanism of action. Plant Foods Hum Nutr. 1997;51:167–72. doi: 10.1023/a:1007975430328. [DOI] [PubMed] [Google Scholar]
- 4.Chithra V, Leelamma S. Coriandrum sativum changes the levels of lipid peroxides and activity of antioxidant enzymes in experimental animals. Indian J Biochem Biophys. 1999;36:59–61. [PubMed] [Google Scholar]
- 5.Lal AA, Kumar T, Murthy PB, Pillai KS. Hypolipidemic effect of C. sativum L. in triton-induced hyperlipidemic rats. Indian J Exp Biol. 2004;42:909–12. [PubMed] [Google Scholar]
- 6.Cortes-Eslava J, Gomez-Arroyo S, Villalobos-Pietrini R, Espinosa-Aguirre JJ. Antimutagenicity of coriander (Coriandrum sativum) juice on the mutagenesis produced by plant metabolites of aromatic amines. Toxicol Lett. 2004;153:283–92. doi: 10.1016/j.toxlet.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Medhin DG, Hadhazy, Bakos P, Verzar-Petri G. Hypotensive effects of Lupinus termis and C. sativum in anaesthetized rats. A preliminary study. Acta Pharm Hung. 1986;56:59–63. [PubMed] [Google Scholar]
- 8.Melo EA, Bion FM, Filho JM, Guerra NB. In vivo antioxidant effect of aqueous and etheric coriander (C. sativum L) extracts. Eur J Lipid Sci Technol. 2003;105:483–7. [Google Scholar]
- 9.Emamghoreishi M, Khasaki M, Aazam MF. C. sativum: Evaluation of its anxiolytic effect in the elevated plus-maze. J Ethnopharmacol. 2005;96:365–70. doi: 10.1016/j.jep.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Kubo I, Fujita K, Kubo A, Nihei K, Ogura T. Antibacterial activity of coriander volatile compounds against Salmonella choleraesuis. J Agric Food Chem. 2004;52:3329–32. doi: 10.1021/jf0354186. [DOI] [PubMed] [Google Scholar]
- 11.Al-Said MS, Al-Khamis KI, Islam MW, Parmar NS, Tariq M, Ageel AM. Post-coital antifertility activity of the seeds of Coriandrum sativum in rats. J Ethnopharmacol. 1987;21:165–73. doi: 10.1016/0378-8741(87)90126-7. [DOI] [PubMed] [Google Scholar]
- 12.Emamghoreishi M, Heidari-Hamedani G. Sedative-hypnotic activity of extracts and essential oil of coriander seeds. Iran J Med Sci. 2006;31:22–7. [Google Scholar]
- 13.Benjumea D, Abdala S, Hernandez-Luiz F, Pérez-Paz P, Martin-Herrera D. Diuretic activity of Artemisia thuscula, an endemic canary species. J Ethnopharmacol. 2005;100:205–9. doi: 10.1016/j.jep.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Vejdani R, Shalmani HR, Mir-Fattahi M, Sajed-Nia F, Abdollahi M, Zali MR, et al. The efficacy of an herbal medicine, carmint, on the relief of abdominal pain and bloating in patients with irritable bowel syndrome: A pilot study. Dig Dis Sci. 2006;51:1501–7. doi: 10.1007/s10620-006-9079-3. [DOI] [PubMed] [Google Scholar]
- 15.Sreelatha S, Padma PR, Umadevi M. Protective effects of Coriandrum sativum extracts on carbon tetrachloride-induced hepatotoxicity in rats. Food Chem Toxicol. 2009;47:702–8. doi: 10.1016/j.fct.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Burdock GA, Carabin IG. Safety assessment of coriander (Coriandrum sativum L.) essential oil as a food ingredient. Food Chem Toxicol. 2009;47:22–34. doi: 10.1016/j.fct.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni SK. Delhi: Vallabh Prakashan; 1999. Handbook of Experimental Pharmacology; pp. 135–37. [Google Scholar]
- 18.Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–70. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 19.Toriizuka K, Kamiki H, Ohmura N, Fuji M, Hori Y, Fukumura M, et al. Anxiolytic effect of Gardeniae Fructus-extract containing active ingredient from Kamishoyosan (KSS), a Japanese traditional Kampo medicine. Life Sci. 2005;77:3010–20. doi: 10.1016/j.lfs.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 20.File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1996;2:219–38. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- 21.Calatayud F, Belzung C, Aubert A. Ethological validation and the assessment of anxiety-like behaviours: Methodological comparison of classical analyses and structural approaches. Behav Processes. 2004;67:195–206. doi: 10.1016/j.beproc.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen M, Frokjaer S, Braestrup C. High affinity of the naturally-occurring biflavonoid, amentoflavon, to brain benzodiazepine receptors in vitro. Biochem Pharmacol. 1988;37:3285–7. doi: 10.1016/0006-2952(88)90640-5. [DOI] [PubMed] [Google Scholar]
- 23.Guenther E. Vol. 4. Florida, USA: REK Publishing Company; 1950. The essential oil; pp. 602–15. [Google Scholar]
- 24.Do Vale TG, Furtado EC, Santos JG, Jr, Viana GS. Central effects of citral, myrcene and limonene, constituents of essential oil chemotypes from Lippia alba (Mill) n.e. Brown. Phytomedicine. 2002;9:709–14. doi: 10.1078/094471102321621304. [DOI] [PubMed] [Google Scholar]
- 25.Mani V, Parle M, Ramasamy K, Abdul Majeed AB. Reversal of memory deficits by Coriandrum sativum leaves in mice. J Sci Food Agric. 2011;1591:186–92. doi: 10.1002/jsfa.4171. [DOI] [PubMed] [Google Scholar]


