Abstract
A-24 year-old male was prescribed prednisolone (60 mg/day) for left sided facial palsy. After three days of therapy, the patient complained of black spots in his vision in right eye. Fluorescein angiography of right eye showed evidence of central serous retinopathy (CSR). Prednisolone dose was withdrawn gradually and the patient improved within a week. There were no other systemic or ophthalmic diseases reported by the patient, which could have caused this condition. An improvement after dechallenge confirmed steroid-induced CSR. Recurrent CSR is known to cause permanent loss of vision. Hence, awareness regarding this adverse drug reaction (ADR) with steroids and its reporting can minimize this complication and help in better patient management.
Keywords: Adverse drug reaction, central serous retinopathy, corticosteroids
Introduction
Central serous chorioretinopathy (CSR) is a well-established clinical entity known to occur in individuals with type A personality. Its incidence is 9.9 per 100000 for men and 1.7 for women.[1] Systemic corticosteroid therapy triggers this condition.[2] The prevalence of steroid-induced CSR is less than 10%.[3] We report here a case of CSR due to oral prednisolone therapy.
Case Report
A 24-year-old man with left sided facial palsy was prescribed tablet prednisolone 60 mg per day for five days. Three days after initiation of prednisolone, the patient reported seeing black spots in his vision in the right eye. Primary investigations (perimetry and fundus examination) revealed central scotoma in the right eye. Fluorescein angiography showed an early spot of hyperfluorescence at the site of leakage with expansion into the area of serous detachment [Figure 1]. Based on these observations, the patient was diagnosed as a case of central serous retinopathy. The dose of prednisolone was gradually reduced after the appearance of ADR. It was reduced to 40 mg for seven days and then it was stopped. Ketorolac eye drops (0.3%) and antioxidant capsules containing L-arginine and vitamin E were prescribed. The visual disturbances abated gradually within one week after prednisolone was withdrawn.
Figure 1.
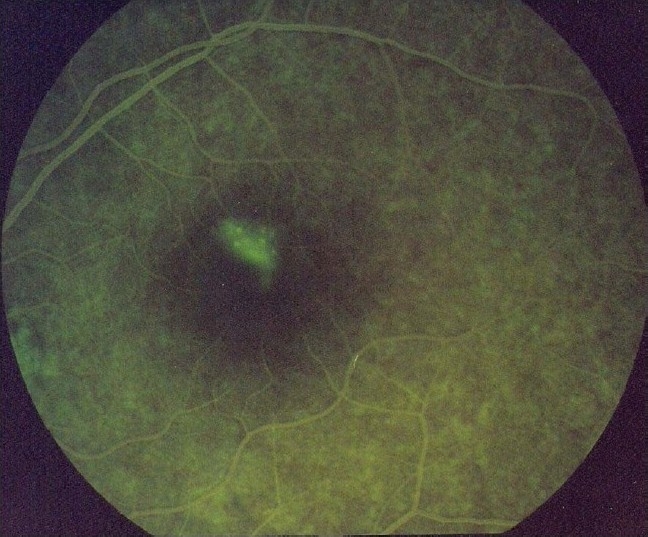
Hyperfluorescence at site of the leakage
Discussion
There are various causes of CSR, including stress, type A personality and treatment with corticosteroids. However, it may also be idiopathic. In the present case, the patient was prescribed systemic steroids for five days. Symptom of black spots in vision and fluorescein angiography confirmed the diagnosis of central serous retinopathy. Symptoms abated after prednisolone was withdrawn. Other possible causes of CSR were ruled out. There was no history of systemic diseases or other ophthalmological causes that could have contributed to this ADR. The time course of the onset of ADR and response to dechallenge suggests the probability of prednisolone as the causal drug. Causality assessment using WHO-UMC scale[4] and Naranjo's algorithm[5] categorized “probable” for prednisolone. Rechallenge was not carried out for ethical reasons. An analysis of the ADR on the Hartwig scale, showed that the ADR was moderate in nature.[6] Further, the preventability assessment carried out using modified Schumock and Thornton criteria showed that the ADR was not preventable.[7]
Central serous retinopathy is attributed to the disruption of the ionic pump of the retinal pigmented epithelial cells (RPE) or hyperpermeability of the choroidal vasculature.[8] Glucocorticoids are known to cause CSR probably by increasing cAMP in RPE cells, and hence changing the ionic pump function or by altering the permeability of blood aqueous barrier and disrupting the outer blood retinal barrier.[9] Central serous retinopathy attributed to steroids has also been reported by Tandon et al.,[6] where a patient had posterior globe rupture and was prescribed analgesics, intravenous antibacterials and oral prednisolone (1 mg/day). The patient developed CSR after 15 days of therapy. Prednisolone was gradually tapered and the patient improved within five days.
Facial palsy is a common condition in young adults caused by viral infections like herpes zoster, mycoplasma infection or idiopathic.[10] Central serous retinopathy usually presents with loss of visual acuity, color vision and depth of perception. In 97% of cases, visual acuity returns to normal but in some cases it may affect the vision permanently.[11] However, the condition is reversible if detected early and aggravating factors are withdrawn in time. Therefore, early detection, reporting and dissemination of this rare yet important and disabling ADR can minimize its occurrence and help in better patient management.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathyin Olmsted County, Minnesota 1980-2002. Ophthalmology. 2008;115:169–73. doi: 10.1016/j.ophtha.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Gregory DL, Jones DC, Denton ER, Harnett AN. Acute visual loss induced by dexamethasone during neoadjuvant docetaxol. Clin Med Oncol. 2008;2:37–42. doi: 10.4137/cmo.s339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardwig PW, Silva AO, Pulido JS. Forgotten exogenous corticosteroid as a cause of central serous chorioretinopathy. Clin Ophthalmol. 2008;2:199–201. doi: 10.2147/opth.s2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Who-umc.org. The Uppsala Monitoring Centre. [Last accessed on 2010 Sep 24]. Available from: http://www.who-umc.org/DynPage.aspx?id = 22682 .
- 5.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 6.Hartwing SC, Siegel J, Schnelder PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49:2229–32. [PubMed] [Google Scholar]
- 7.Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27:538. [PubMed] [Google Scholar]
- 8.Tandon R, Vanathi M, Verma L, Bharadwaj A. Central serous retinopathy masquerading as sympathetic ophthalmia. Eye (Lond) 2003;17:666–7. doi: 10.1038/sj.eye.6700423. [DOI] [PubMed] [Google Scholar]
- 9.Zamir E. Central serous retinopathy associated with adrenocorticotrophic hormone therapy.A case report and hypothesis. Arch Clin Exp Ophthalmol. 1997;235:339–44. doi: 10.1007/BF00937280. [DOI] [PubMed] [Google Scholar]
- 10.Facial palsy (Online) [Last accessed on 2010 May 9]. Available from: http://www.emedicine.medscape.com/article/791311-overview .
- 11.Wynn PA. Idiopathic central serous chorioretinopathy–A physical complication of stress? Occup Med (Chic III) 2001;51:139–40. doi: 10.1093/occmed/51.2.139. [DOI] [PubMed] [Google Scholar]


