Abstract
Background:
The efficacy of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression has never been reported as yet in the Indian literature.
Aims:
To study the efficacy of rTMS in the treatment of depression and to evaluate its safety and tolerability.
Settings and Design:
A randomized, double-blind, sham-controlled trial was conducted at the Department of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore.
Materials and Methods:
23 patients with depression were randomized to receive either active (n=9) or sham (n=14) treatment with rTMS. Treatment consisted of six sessions of rTMS for 2 weeks (10 trains of pulses, intensity equal to motor threshold, 10 Hz frequency, train duration of 5 seconds, 1 minute inter-train duration). Response was assessed using Hamilton Depression Rating Scale (HDRS), Montgomery Åsberg Depression Rating Scale (MADRS) and Clinical Global Index (CGI). The safety and tolerability was assessed with side-effect checklist for electroconvulsive therapy. 50% reduction in HDRS scores from baseline was defined as treatment response. Outcome measures were analyzed by repeated measures analysis of variance. Chi-square test was used to analyze the categorical variables.
Results:
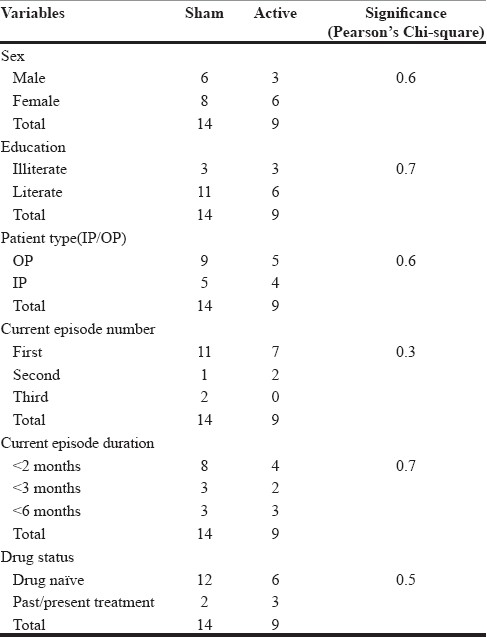
No statistical significance was seen on the baseline socio-demographic and illness characteristics (Pearson's Chi-square=0.5). Although HDRS (sham 22.0-12.4; active 22.8-12.7) and MADRS (sham 30.7-17.3; active 31.8-16.7) scores reduced by the end of 2 weeks treatment, it was not statistically significant. One patient developed manic symptoms early in the treatment.
Conclusions:
Treatment with rTMS did not show improvement at the end of 2 weeks. More studies with larger sample size and with higher rTMS dosages need to be done.
Keywords: Depression, electroconvulsive therapy, transcranial magnetic stimulation
INTRODUCTION
Over the last two decades, repetitive transcranial magnetic stimulation (rTMS) has documented therapeutic utility in various neuropsychiatric disorders such as depression,[1] schizophrenia,[2,3] mania[4,5] and obsessive-compulsive disorder.[6] Despite reports of both investigative and therapeutic utility of Transcranial magnetic stimulation (TMS) from various parts of the world, there was not a single publication on the same from the developing countries like India. Hence we undertook the first ever study from India with the aims and objectives of studying the efficacy of rTMS in the treatment of major depression, its adverse effect profile and whether rTMS could be a useful therapeutic option in the present scenario.
Background of rTMS
TMS is a non-invasive technique that delivers magnetic pulses to stimulate the human brain in vivo using a hand-held stimulating coil applied directly to the head.[7] This field passes unimpeded through the skull and induces electrical depolarization of the neurons.
rTMS is delivered either as a single pulse with a frequency <1 Hz or as paired pulses as in repetitive TMS (rTMS). Stimulation more than once per second (>1 Hz) is called as high-frequency rTMS and And one or less than once per second (≤1 Hz) is called as low frequency rTMS.[8] Low-frequency rTMS is thought to inhibit cortical excitation in certain regions, while high-frequency rTMS further activates neuronal excitation. In most rTMS protocols, a train of magnetic pulses is delivered for many milliseconds to several seconds at a frequency of 1-25 Hz. The pulses’ effects temporally summate to cause a greater change in neural activity than a single pulse, thereby effecting possible therapeutic changes.
Proposed mechanisms of rTMS-induced antidepressant response
Several lines of evidence suggest that major depressive disorder is most commonly associated with hypoexcitability over the left prefrontal cortex and hyperexcitability over the right prefrontal cortex. The strongest evidence in support of this contention and the reason why high-frequency (e.g. 10 Hz) rTMS was initially applied to the left dorsolateral prefrontal cortex (LDLPFC) and low-frequency (e.g. 1 Hz) rTMS was initially applied to the right DLPFC to obtain an antidepressant response is related to patients with left-sided strokes (the anatomic equivalent of hypoexcitability) who experience depression at much higher rates than in the general population and patients with right-sided strokes who experience manic symptoms at much higher rates than in the general population.
Salient findings from review of trials of rTMS in depression
Since the first published studies showing that rTMS was effective at improving mood for patients with major depressive disorder,[9,10] more than 75 studies evaluating the efficacy of rTMS for treatment resistant depression (TRD) have been published. Unfortunately, all these studies are not comparable for the following reasons to draw uniform conclusions on the overall utility.
Most patients included in these studies were treatment resistant, who may represent a relatively heterogeneous subset of patients whose underlying disorder may be confounded by other comorbidities.
The stimulation parameters including frequency, intensity, and duration vary from study to study, precluding the proper determination of these parameters to optimize the therapeutic response.
The concomitant use of medications in these studies obfuscates the independent effects that rTMS may have on mood symptoms, making it unclear whether improvement was related to rTMS alone, medication, or the combination of both.
No consistent method for precisely localizing the prefrontal cortex was used, and as such different cortical areas may be stimulated between subjects and between studies, confounding treatment results.
Most rTMS studies described above typically involved 2-week treatment durations.
Although meta-analyses ideally provide better statistical and clinical findings than trials, the six[11–15] meta-analyses published between the years 1990 and 2003 have reported varying effect sizes and also were typically of modest clinical meaningfulness.
Safety of TMS
TMS can cause pain on the scalp at higher intensities which is related to the repetitive stimulation of peripheral facial and scalp muscles, resulting in muscle tension headaches in a proportion of subjects (approximately 5-20% depending on the study). These headaches respond to treatment with acetaminophen or aspirin. Magnetic stimulation also produces a high-frequency noise artifact that can cause short-term changes in hearing threshold. This is avoided when subjects and investigators wear earplugs.
The most critical immediate safety concern is that rTMS has resulted in seizures. The number of people who have received TMS or rTMS is unknown, but is likely to be several thousand worldwide. To date, seizures during rTMS are known to have occurred in seven individuals, including six normal volunteers. The TMS-induced seizures were self-limiting and did not seem to have permanent sequelae. The risk of seizure induction is related to the parameters of stimulation, and no seizures have been reported with single-pulse TMS or rTMS delivered at a slow frequency (<1 Hz). There is a growing understanding of the rTMS parameter combinations (magnetic intensity, pulse frequency, train duration, and inter-train interval) that result in spread of excitation, heralding impending seizure. Even if therapeutic benefits are convincingly shown, the seizure risk may limit the widespread and loosely supervised use of rTMS. In part for this reason, the therapeutic potential of slow frequency rTMS (<1Hz) deserves particular attention as used in our study.
Both TMS and rTMS can disrupt cognition during the period of stimulation. However, the safety concerns are about alterations in cognitive function beyond the period of stimulation. The limited investigation of short-term neuropsychological effects of TMS has not demonstrated significant changes. Little information is available about long-term effects. The technique has been in use for more than a decade without reports of long-term adverse consequences.
Impact of depression
According to World Health Organization (WHO), depression is the leading cause of disability as measured by years lived with a disability and the fourth leading contributor to the global burden of disease in 2000. Today, depression is already the second cause of disability-adjusted life years (DALYs) in the age category 15-44 years for both sexes combined. The Global Burden of Disease Study[16] reports that by 2020, depression is projected to reach the second place in the ranking of DALYs calculated for all ages and both sexes, and by 2030, depression will be the single biggest cause for burden, amounting to 15% out of all health conditions throughout the world, overtaking cardiovascular problems and cancer.
Though numerous effective treatments are available, as many as 30% of patients fail to respond to treatment[17] and about 60% experience a relapse. Specifically, antidepressant medication response varies from 20 to 25% with full remission of depression, 30-45% are partial responders, 25-35% do not respond and 10-25% percent show poor tolerance. Successful treatment, however, depends on a range of factors, including the choice of drug, its dosage, and duration of treatment, individual patient response and patient compliance. An estimated 50% of failed treatment is because patients stop taking their medication too soon.
Deficiencies of existing treatments
Antidepressant medication
The Sequenced Treatment Alternatives to Relieve Depression trial,[18–23] which is a semi-naturalistic treatment algorithm designed to model as closely as possible the sequence of treatment options most commonly used in clinical practice, provides useful conclusions on the limitations of existing antidepressant treatments.
In patients who may generally be expected to respond to antidepressant medication treatment, the likelihood of achieving remission of symptoms [defined by a Hamilton depression rating scale (HDRS) score of <8] after either one or two sequential treatment trials ranges over 50%. However, once prospective evidence of failure to achieve benefit has been demonstrated, the likelihood of good clinical outcome drops precipitously and hovers at exceedingly low levels after three prospective treatment failures.
The discontinuation rate due to treatment intolerance or adverse events ranged from 8.6 to 41.4%. In other words, as the expectations of efficacy diminished with increasing resistance to prior treatment, the non-adherence to, and likely intolerability of, treatment options increased quite dramatically. Overall, these data paint a picture of measurable but limited benefit with the most commonly used pharmaceutical treatments.
Relapse rates after one successful antidepressant trial ranged from 40.1% within 4.1 months follow-up to 71.1% within 3.3 months for those who failed to respond after four trials. Moreover, the persistence of acute response is more difficult to achieve with each successive failure. These observations highlight an often unrecognized fact: beyond the successful outcome with first-line treatment, tolerability and adherence to treatment loom large as major concerns.
Very few studies (17 antidepressant studies) in treatment-resistant patients with a controlled design are available till date. Current approaches to classification of treatment resistance are insufficient in that they do not comment fully on the relative clinical significance of the transition from lesser grades of treatment failure to more severe grades.[10,11]
Most placebo-controlled, registration quality, pharmaceutical, antidepressant studies are conducted in patient populations strongly biased in the direction of a more treatment-responsive sample.
In most pharmaceutical antidepressant studies, many clinical features that have a significant negative influence on the response to active and placebo treatments are used as exclusion conditions for entry.
Limitations of electroconvulsive therapy
Electroconvulsive therapy (ECT) is more effective than antidepressant medications, but because of its many drawbacks, is used mainly for severe cases of depression that do not respond to other treatment. For instance, one of the drawbacks of ECT is getting the electric current past the skull which is an excellent insulator. The skull “smears” the electricity so that it is impossible to control exactly where the current goes in the brain or the exact amount of it. This also makes it impossible to stop a seizure from spreading through the brain. ECT can cause deficits in delayed recall, a relatively specific cognitive effect. Depressed patients have preserved long-term memory, but suffer short-term memory impairment and frontal function alteration during maintenance ECT. Both unilateral and bilateral forms of ECT produce acceptable antidepressant response rates and also anterograde amnesia though the latter is only a transient side effect. There is also the need for anesthesia in modified ECT, and as well known, the anesthesia again entails its own complications apart from ECT. In addition, the stigma that is associated with ECT often limits its widespread acceptance as a treatment for depressive symptoms.
Psychological therapies
The two best psychological therapies for depression, cognitive-behavioral and interpersonal, have disadvantages, for example, these techniques may take 8-10 weeks to show a full effect (compared to about 6 weeks for medication) and they are not the first treatment choice for severely depressed individuals or those who are suicidal.
In this background, rTMS seems to be a promising alternative. Least number of side effects, excellent safety profile and lack of need for anesthesia have the potential of making this a popular treatment. Incorporating the limitations of previous studies and since there have been no reports of rTMS in depression from India, we planned to conduct the first randomized, double-blind, and placebo-controlled trial of rTMS to treat depression, in the National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore.
Rationale for this study
Treatment options that afford unambiguous advances in novelty of mechanism, tolerability, safety, or effectiveness clearly are needed. Clinical experience confirms that despite the common use of complex medication combinations and antidepressant augmentation strategies, a strong and consistent evidence base from controlled studies to support their routine use is weak. As mentioned above, virtually none of these interventions has been demonstrated to have efficacy in large, definitive, randomized, placebo-controlled clinical trials. Any future offering should ultimately be appraised in terms of the overall quality of its evidence base and its risk/benefit profile compared with current options.
MATERIALS AND METHODS
This was a prospective, hospital-based, randomized, double-blind, sham-controlled rTMS trial conducted over a period of 1 year from December 2003 to January 2004. Twenty-three patients were recruited from the outpatient department of NIMHANS, Bangalore.
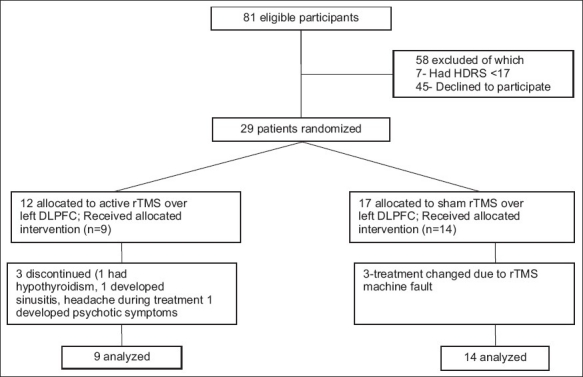
The study protocol was prepared according to the Consolidated Standards of Reporting Trials (CONSORT) Statement 2001 checklist and then approved by the institutional ethical committee. Informed consent prepared in their respective languages (English, Kannada, Tamil, Telugu, and Malayalam) was obtained from all participants before inclusion into the trial. Eighty-one patients were screened for inclusion into the trial, of whom only 23 completed the trial. Figure 1 shows the CONSORT diagram of flow of participants through the trial.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram showing the flow of participants through each stage of the trial (rTMS - Repetitive transcranial magnetic stimulation, DLPFC - Ddorsolateral pre-frontal cortex)
Randomization of subjects was done by a senior statistician using stratified random sampling method from a computer-generated random number table and this statistician was blind to the clinical status of the patients. Patients were randomized to receive either real rTMS or sham TMS based on the random number table by an independent clinician. The patients and the rater who applied the scales were kept blind to the randomization. The principal investigator and the patients were completely blind to the randomization status of the patient group (active or sham).
The sample consisted of 23 (excluding drop-outs) right-handed patients of both sexes, aged between 16 and 60 years, with a diagnosis of major depressive disorder (first episode or recurrent depressive disorder) and dysthymia according to the Structured Clinical Interview for DSM IV (SCID), administrated by the principal investigator and later re-confirmed by consensus following independent clinical interview by one of the two experienced senior psychiatrists; all of them were blind to the randomization status of the patients. The diagnosis was found to be stable at 1-month follow-up as re-assessed by one of the two above experienced psychiatrists. Drug-naïve patients or those already on antidepressant medications, those who had received adequate dosage (150 mg Imipramine equivalent or more or an equivalent dose of any SSRI) for adequate duration (4 weeks or longer) but still had not improved were also included and the medications were continued. They were continued on the same medicines during the treatment period and later.
Exclusion criteria consisted of other diagnoses such as bipolar affective disorder, presence of psychotic symptoms, mental retardation, organic brain syndrome, seizures, substance (alcohol, nicotine, etc.) use, electroconvulsive therapy received in the preceding 6 weeks, uncontrolled diabetes mellitus and systemic hypertension. Patients with any contraindications for the use of rTMS such as family history of seizures, current use of neuroleptics, raised intracranial tension, pregnancy, heart disease, cardiac pacemaker, medication pumps, intracardiac lines or metal in the cranium were also excluded.
Eligible patients (23) were assessed for their demographic and clinical information with the help of a structured proforma. Primary measures included the following scales.
The 17-item HDRS is one of the most widely used instruments for clinical assessment of depressive states in adults to document the depressive psychopathology. It was administered by the principal investigator and only those patients scoring >17 at baseline were included. HDRS total score <17 and highly suicidal patients with HDRS suicide item score >2 were excluded.
Montgomery Åsberg Depression Rating scale (MADRS) (Montgomery and Åsberg 1979), a clinician administered subscale of the comprehensive psychopathological rating scale, which is sensitive to changes in depressive symptoms over days during therapy, was also used. Both the primary measures have documented adequate reliability and validity from previous studies.
Secondary measures included the following tools. Clinical Global Impression-Severity of Illness (CGI-SI) (Guy 1976), a global ratings scale for estimating the severity of a psychiatric disorder independent of diagnosis, was used. The ratings were made on a 7-point scale.
Alcohol use disorders identification test (AUDIT), a 10-item questionnaire developed by WHO, was used to assess problem-drinking pattern in the last 1 year and also current disorders related to drinking alcohol. A score of 8 or more was used as the exclusion criterion to rule out problem drinking.
Family interview for genetic studies (FIGS), a semi-structured instrument for getting diagnostic information about relatives in the pedigrees, was used. There are three parts – general screening questions, the face sheet, symptom checklist. It was used to document family history of affective illness in the subjects.
Side-effect checklist for ECT (NIMHANS): Due to lack of standard rTMS side-effect checklist, we used the ECT side-effect checklist along with incorporation of an open-ended question about any adverse event that the patient might attribute after the start of rTMS. The tolerability to the treatment was also documented in the same questionnaire.
TMS equipment
The treatment was given using Magstim Standard Rapid Package. The figure-of-eight coil was used to deliver the magnetic pulses. The session software, designed to be used in conjunction with the machine, allowed automated delivery of stimuli according to a user-programmed session file.
Motor threshold estimation
The 10-20 method was used to locate the motor area for the right adductor pollicis brevis (ABP). Each subject's motor evoked potential (MEP) threshold for the right ABP muscle was determined with the stimulating coil placed over the motor cortex. The optimal position on the scalp for producing MEPs is known to overlie the central sulcus. The MEP threshold was defined as the lowest stimulation intensity required to evoke at least 5 MEPs in at least 5 out of 10 consecutive trials. Each subject then received stimulation over LDLPFC at 100% of this intensity for the reminder of the study. The LDLPFC was defined as the region 5 cm rostral in the same sagittal plane as the optimal site for MEP production in the right ABP (George et al., 1997; Pascual-Leaone et al., 1996).[9,10]
Treatment parameters
Patients received six sessions of rTMS per week for 2 weeks every day except on Sundays. In each session, 10 trains of pulses (intensity equal to motor threshold, frequency of 10 Hz and train duration of 5 seconds), with an inter-train duration of 1 minute, were administered.
Identification of Active rTMS versus sham rTMS treatment
After confirming the area to be stimulated, an area 5 cm anterior to it was chosen to stimulate the left DLPFC. Active rTMS was given in this area with the coil placed parallel to the point identified to be stimulated. Sham rTMS was delivered using the same parameters, but holding the coil at 90° tangent to the skull at the same position.
Procedure
Out of 23 patients in two groups, 9 received active treatment and 14 received sham rTMS treatment. Once diagnosis was confirmed by independent psychiatrist, patients were offered the informed consent form by the principal investigator, and upon successful recruitment, a third psychiatrist allocated them into the treatment arm (active or sham) based on the randomization table. Treatment was offered on an outpatient or inpatient basis. At the baseline, assessments on HDRS, MADRS, CGI, and AUDIT and FIGS were administered by the principal investigator. A junior psychiatrist who had received in-depth training on the operation and administration of the rTMS equipment delivered the entire treatment session. Interim assessments on the HDRS, MADRS was done by the principal investigator on day 3, week 1, day 10, week 2 and at the end of 1 month after the last session. The side-effect checklist was applied at weekly intervals and at the end of 1 month. The CGI-I was also applied at the end of treatment. The patient consent was renewed again at the end of 2 weeks for all patients to accommodate for patient preferences in continuing the treatment further. At the end of 2 weeks treatment period, those who showed expected response (50% reduction in HDRS scores) continued to receive further rTMS sessions for another 2 weeks and in those failed to improve rTMS was stopped and antidepressant medications were started.
Statistical analysis
The data were analyzed using the Statistical Package for Social Sciences (SPSS version 10 for Windows computer program, SPSS Inc., Chicago, IL, USA). The alpha level was set at P<0.05 (two-tailed) for statistical hypothesis testing with exact probability levels for test statistics. The primary outcome measures of HDRS and MADRS were analyzed using repeated measures analysis of variance (RMANOVA). Since the repeated measures were not equidistant at two points of time of assessment, a multiple independent t-test was also used. The results of both the methods were almost the same and the difference noted was negligible. Fifty percent reduction in HDRS scores (George et al., 1997, 2000;[10] Garcia-Toro et al., 2001a[25])was taken as a cutoff to define response rates. Pearson's chi-square correlation was used to calculate between group differences in socio-demographic and clinical variables such as depressive episode number, episode duration, and previous medication status. The power of the study was calculated post-hoc using the study sample size, and the effect size was calculated using g (modified glass statistic with pooled sample standard deviation).
RESULTS
The proportions of males to females in both the groups were: active 9 (3 m/6 f) and sham 14 (6 m/8 f). The mean age groups of the patients in the two groups were active (mean 34 years; SD ±10.5) and sham (mean 37.2 years; SD ±11.8) and were not statistically significant (two-tailed significance = 0.50). The results of other parameters, namely, sex, education and inpatient or outpatient status, and the illness characteristics, namely, the number of depressive episodes, duration of these episodes and their antidepressant treatment status in both the groups, also did not differ statistically as shown in Table 1. Both the groups did not show any statistical difference in the distribution of major psychiatric diagnosis (Pearson's chi-square = 0.5).
Table 1.
Socio-demographic variables between two groups
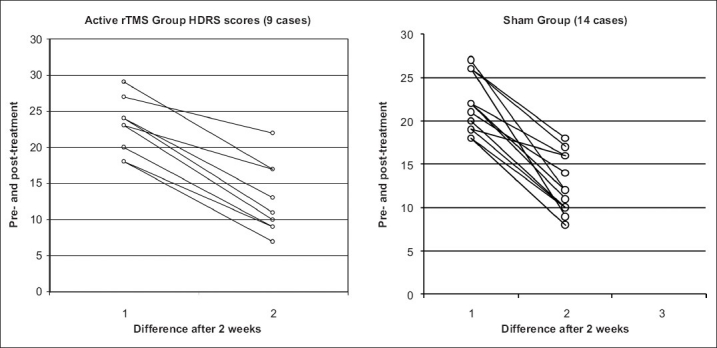
HDRS
The results show that the difference in the HDRS scores [Table 2] following treatment is nearly the same in both the active and sham groups and not statistically significant, meaning the nature of the treatment received in this sample does not favor active rTMS over sham rTMS in its antidepressant action.
Table 2.
Hamilton depression rating scale scores between two groups

The graphs in Figure 2 show the distribution of the HDRS across the two groups, clearly depicting the similar manner in which HDRS scores have changed following treatment.
Figure 2.
Comparison of changes in HDRS scores pre and post treatment between active and sham groups
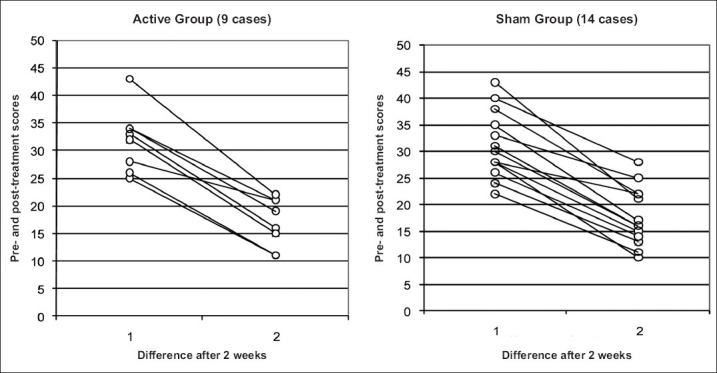
MADRS
Almost similar results [Table 3] are evident even in the MADRS scores also following treatment. The graphs in Figure 3 depict the comparison of MADRS scores between the two groups.
Table 3.
Montgomery Åsberg depression rating scale scores between two groups

Figure 3.
Comparison of changes in MADRS scores pre- and post-treatment between active and sham groups
CGI-SI
The objective evidence of change in depression as shown by Clinical Global Impression (CGI) scores between the two groups, active (mean 1.8; SD ±0.7) and sham (mean 1.4; SD ±0.5), did not differ statistically (two-tailed significance = 0.1).
Inter-rater reliability
The HDRS scores were assessed by another clinician at the baseline to ensure robustness in obtaining the best possible score which would reflect the severity of the depression. Analysis showed a value of 0.9, meaning very good reliability of the application of the scale.
Power estimation and effect size calculation
The study showed a power of 0.134 at the baseline and 0.059 at the end of 2 weeks and an effect size of 0.254 at baseline and 0.086 at the end of 2 weeks, indicating a very low power and a small to moderate effect size of the study.
DISCUSSION
There has been tremendous amount of progress that rTMS research has seen in the years after this study was conducted. But we have analyzed our results based on evidence prior to the study period, which gives more value to the conclusions that are drawn below.
Unlike most of the previous published randomized, double-blind, placebo-controlled trials of rTMS over LDLPFC in the treatment of depression, which have favored active rTMS over sham TMS in their antidepressant effect, our study showed no difference in the two groups at the end of 2 weeks and the changes in the depression scores were almost equal in both real and sham groups. It is difficult to pinpoint the exact reasons for this result. Two trials (Loo et al., Garcia-Toro et al.,) with the same result can provide useful insights that can be used to explain the negative result of our study. A careful examination of our study group yielded the following as the probable reasons.
Sample characteristics
A smaller sample size of 23 patients (even though we targeted at least 30).
Uneven distribution of this sample into the two groups following randomization (sham/active: 14/9). Loo and Garcia[24,25] had also identified the same reason of unequal sample size in the two arms for the poor response in their study. poor response in their study. This inequal distribution had contributed to the very low power of our study.
Only 4 subjects in our trial had received an antidepressant drug prior to receiving TMS and the remaining 14 were drug-naïve subjects. The problem of a relatively high placebo response rate (30-40%) seen with any novel antidepressant treatment tends to make the drug-naïve sham group patients also to respond and this is a major confounding factor which is difficult to overcome.
Treatment parameters
The issue of an ideal sham rTMS is still debatable and from our literature review,[26–28] we found that even the 90° sham used in our study is not a completely inert placebo tool as it can reduce the induced voltage only by 67-73% and it is believed that for these reasons an ideal sham which can simulate the real TMS closely can never be nonstimulating to the underlying cortex. This could explain the relatively equal antidepressant effect noticed in the sham group.
Clinical characteristics
Our sample was not homogenous in including both melancholic depressives and dysthymic subjects. It is well known that such heterogeneity varies the response to any existing standard antidepressant therapy and this could have also influenced our results.
We carried out more statistical analysis to look for any of the symptom clusters in the HDRS or MADRS scales across melancholic, anxiety and somatic symptom item scores which could explain our finding, but we did not find the same any in either of the scales.
In conclusion, any reasonable evidence favoring the antidepressant efficacy of real rTMS can be ascertained only if some of the above-discussed limitations of this relatively safe treatment tool can be overcome.
Implications of the study
The study quality of our study was ensured from the start by following proper randomization of the subjects, taking informed consent for participation in the subject's own language, prepared in local languages, adequate blinding procedure being followed, and finally using rTMS treatment parameters based on existing literature evidence from previous rTMS trials conducted worldwide. All the patients tolerated the treatment well without any report of adverse effects and one patient opted for repeat rTMS when he had a recurrence of his depressive symptoms. Adequate safety measures were ensured to manage unexpected seizures while treatment was being delivered.
Limitations
One of the major limitation was the smaller sample size in our study. One of the major limitation in our study was the smaller sample size which was due to the shorter duration that was available for recruitment of subjects meeting the study criteria. Further it was practically difficult to obtain large number of purely melancholic group of depressed subjects within the one year period. It was also difficult to ensure a drug-naïve status in all the subjects, Unexpected delay was due to fault in the rTMS equipment and it took 2 months time before it could be used again. Other important limitations were lack of EMG machine to record MEPs and of standard treatment parameters on the therapeutic utilization of rTMS at the time of conducting this study.
Future implications
If the efficacy of rTMS in treating depression needs to be established on a par with other existing antidepressant modalities, the above-mentioned difficulties must be overcome, and if this can be done, this procedure is definitely a better option considering the following: its very good safety profile; it is well tolerated and convenient to use; it does not need anesthesia of ECT; its cost-effectiveness; and it is easy to administer for the clinician. Our study is the first of its kind in our country and there is definitely a positive trend toward its use in depression and it is worthwhile conducting more trials using larger sample sizes and more rigorous research design in our setting.
ACKNOWLEDGMENTS
I thank the staff of the Department of Psychiatry, NIMHANS, who had supported this project from the beginning. I thank all the patients and their families for wilfully participating in the trial. I thank my guide and co-guides for providing constant support and motivation.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Bortolomasi M, Minelli A, Fuggetta G, Perini M, Comencini S, Fiaschi A, et al. Long-lasting effects of high frequency repetitive transcranial magnetic stimulation in major depressed patients. Psychiatry Res. 2007;150:181–6. doi: 10.1016/j.psychres.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Prikryl R, Kasparek T, Skotakova S, Ustohal L, Kucerova H, Ceskova E. Treatment of negative symptoms of schizophrenia using repetitive transcranial magnetic stimulation in a double-blind, randomized controlled study. Schizophr Res. 2007;95:151–7. doi: 10.1016/j.schres.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Goyal N, Nizamie SH, Desarkar P. Efficacy of adjuvant high frequency repetitive transcranial magnetic stimulation on negative and positive symptoms of schizophrenia: preliminary results of a double-blind sham-controlled study. J Neuropsychiatry Clin Neurosci. 2007;19:464–7. doi: 10.1176/jnp.2007.19.4.464. [DOI] [PubMed] [Google Scholar]
- 4.Saba G, Rocamora JF, Kalalou K, Benadhira R, Plaze M, Lipski H, et al. Repetitive transcranial magnetic stimulation as an add-on therapy in the treatment of mania: A case series of eight patients. Psychiatry Res. 2004;128:199–202. doi: 10.1016/j.psychres.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Praharaj SK, Ram D, Arora A. Efficacy of high frequency (rapid) suprathreshold repetitive transcranial magnetic stimulation of right prefrontal cortex in bipolar mania: A randomized sham controlled study. J Affect Disord. 2009;117:146–50. doi: 10.1016/j.jad.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Alonso P, Pujol J, Cardoner N, Benlloch L, Deus J, Menchón JM, et al. Right prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: A double-blind, placebo-controlled study. Am J Psychiatry. 2001;158:1143–5. doi: 10.1176/appi.ajp.158.7.1143. [DOI] [PubMed] [Google Scholar]
- 7.Eitan R, Lerer B. Nonpharmacological, somatic treatments of depression: electroconvulsive therapy and novel brain stimulation modalities. Dialogues Clin Neurosci. 2006;8:241–58. doi: 10.31887/DCNS.2006.8.2/reitan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wassermann EM, Wedegaertner FR, Ziemann U, George MS, Chen R. Crossed reduction of human motor cortex excitability by 1-Hz transcranial magnetic stimulation. Neurosci Lett. 1998;250:141–4. doi: 10.1016/s0304-3940(98)00437-6. [DOI] [PubMed] [Google Scholar]
- 9.Pascual-Leone A, Rubio B, Pallardo F, Catalá MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–7. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- 10.George MS, Wassermann EM, Williams WA, Steppel J, Pascual-Leone A, Basser P, et al. Changes in mood and hormone levels after rapid-rate transcranial magnetic stimulation (rTMS) of the prefrontal cortex. J Neuropsychiatry Clin Neurosci. 1996;8:172–80. doi: 10.1176/jnp.8.2.172. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman RE, Boutros NN, Hu S, Berman RM, Krystal JH, Charney DS. Transcranial magnetic stimulation and auditory hallucinations in schizophrenia. Lancet. 2000;355:1073–5. doi: 10.1016/S0140-6736(00)02043-2. [DOI] [PubMed] [Google Scholar]
- 12.Wassermann EM, Wedegaertner FR, Ziemann U, George MS, Chen R. Crossed reduction of human motor cortex excitability by 1-Hz transcranial magnetic stimulation. Neurosci Lett. 1998;250(3):141–4. doi: 10.1016/s0304-3940(98)00437-6. [DOI] [PubMed] [Google Scholar]
- 13.McNamara B, Ray JL, Arthurs OJ, Boniface S. Transcranial magnetic stimulation for depression and other psychiatric disorders. Psychol Med. 2001;31:1141–6. doi: 10.1017/s0033291701004378. [DOI] [PubMed] [Google Scholar]
- 14.Holtzheimer PE, 3rd, Russo J, Avery DH. A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacol Bull. 2001;35:149–69. [PubMed] [Google Scholar]
- 15.Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. Int J Neuropsychopharmacol. 2002;5:73–103. doi: 10.1017/S1461145702002791. [DOI] [PubMed] [Google Scholar]
- 16.Murray CJ, Lopez AD, editors. Cambridge, MA: Harvard University Press; 1996. The Global Burden of Disease: A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and Projected to 2020. [Google Scholar]
- 17.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 18.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement based care in STAR*D: Implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 19.Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Eng J Med. 2006;354:1231–42. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- 20.Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, et al. Medication augmentationafter the failure of SSRIs for depression. N Eng J Med. 2006;354:1243–52. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 21.Fava M, Rush AJ, Wisniewski SR, Nierenberg AA, Alpert JE, McGrath PJ, et al. A comparison of mirtazapineand nortriptyline following two consecutive failed medication treatments for depressed outpatients: A STAR*D report. Am J Psychiatry. 2006;163:1161–72. doi: 10.1176/ajp.2006.163.7.1161. [DOI] [PubMed] [Google Scholar]
- 22.Nierenberg AA, Fava M, Trivedi MH, Wisniewski SR, Thase ME, McGrath PJ, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: A STAR*D report. Am J Psychiatry. 2006;163:1519–30. doi: 10.1176/ajp.2006.163.9.1519. [DOI] [PubMed] [Google Scholar]
- 23.McGrath PJ, Stewart JW, Fava M, Trivedi MH, Wisniewski SR, Nierenberg AA, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: A STAR*D report. Am J Psychiatry. 2006;163:1531–41. doi: 10.1176/ajp.2006.163.9.1531. [DOI] [PubMed] [Google Scholar]
- 24.Loo C, Mitchell P, Sachdev P, McDarmont B, Parker G, Gandevia S. Double-blind controlled investigation oftranscranial magnetic stimulation for the treatment of resistant major depression. Am J Psychiatry. 1999;156:946–8. doi: 10.1176/ajp.156.6.946. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Toro M, Mayol A, Arnillas H, Capllonch I, Ibarra O, Crespí M, et al. Modest adjunctive benefit with transcranial magnetic stimulation in medication-resistant depression. J Affect Disord. 2001;64:271–5. doi: 10.1016/s0165-0327(00)00223-8. [DOI] [PubMed] [Google Scholar]
- 26.Loo CK, Mitchell PB, Croker VM, Malhi GS, Wen W, Gandevia SC, et al. Double-blind controlled investigation of bilateral prefrontal transcranial magnetic stimulation for the treatment of resistant major depression. Psychol Med. 2003;33:33–40. doi: 10.1017/s0033291702006839. [DOI] [PubMed] [Google Scholar]
- 27.Lisanby SH, Arango V, Underwood MD, Perara T, Dwork AJ, Sackeim HA. Hippocampal plasticity following chronic repetitive transcranial magnetic stimulation [abstract] J ECT. 2000;16:74–5. [Google Scholar]
- 28.Lisanby SH, Gutman D, Luber B, Schroeder C, Sackeim HA. Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biol Psychiatry. 2001;49:460–3. doi: 10.1016/s0006-3223(00)01110-0. [DOI] [PubMed] [Google Scholar]


