Abstract
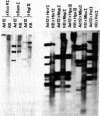
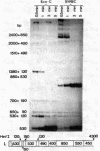
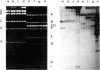
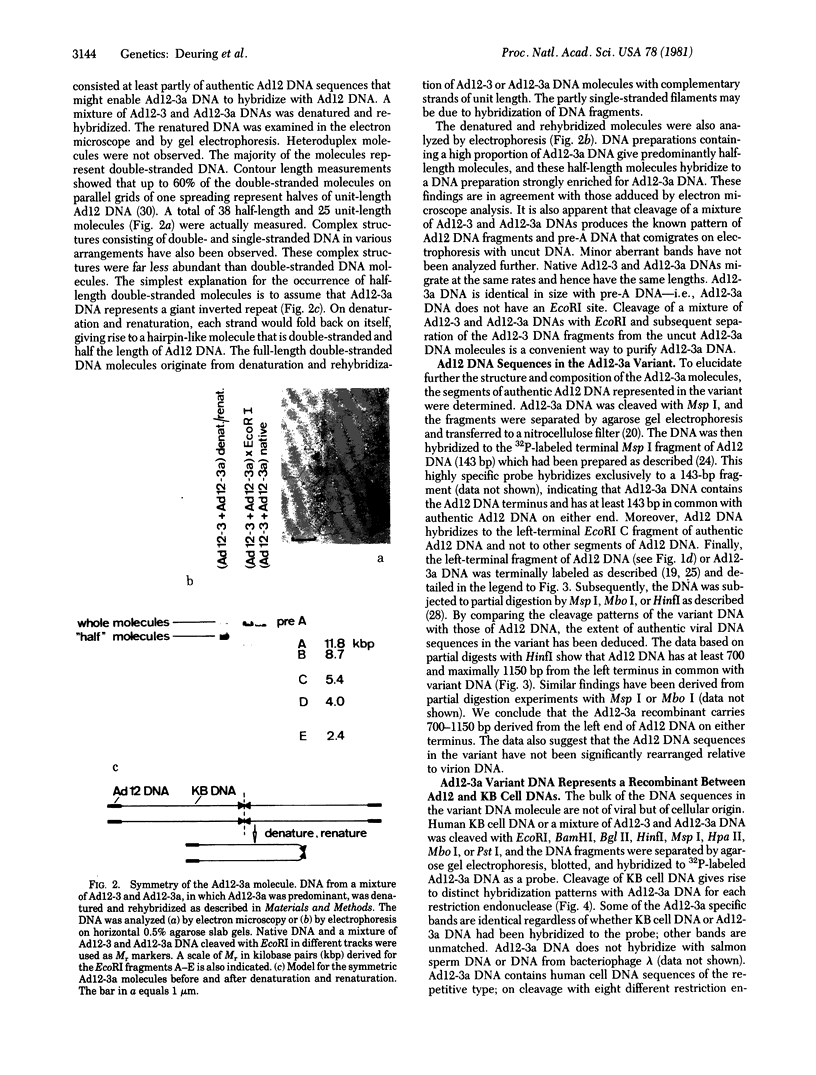
On purification of human adenovirus type 12 (Ad12) by equilibrium sedimentation in CsCl density gradients, two bands of particles, Ad12-3 and Ad12-3a, are observed. The particles from band Ad12-3a contain a recombinant of human host cell DNA and of Ad12 DNA. The human cell DNA sequences contain repetitive DNA recurring 200 to 500 times in cellular DNA. Ad12 DNA and the recombinant genomes exhibit the same or similar lengths. This finding suggests that a constant amount of DNA is packaged into complete Ad12 particles. On cleavage of KB cellular DNA with EcoRI, BamHI, HinfI, Msp I, Mbo I Pst I, or Bgl II, the 32P-labeled cellular DNA from Ad12-3a particles hybridizes on Southern blots to distinct bands of KB DNA. There is also less-specific background hybridization that is not observed in the control. The cellular DNA from Ad12-3a particles is not methylated, whereas the same cellular sequences in KB cell DNA appear to be extensively methylated. On denaturation and renaturation, the recombinant DNA molecules are converted to molecules half as long as Ad12 DNA, as determined by gel electrophoresis and electron microscopy. The recombinant DNA molecules were terminally labeled by exonuclease III treatment and subsequent refilling of the depleted segments with [32P]dNTPs by using DNA polymerase I (Klenow fragment). When these molecules were cleaved with EcoRI, BamHI, Msp I, or Pst I, only one terminal DNA fragment was found to be labeled. The results of partial digestion experiments using Msp I, HinfI, or Mbo I are consistent with a model in which 700-1150 base pairs from the left terminus of Ad12 DNA are linked to host cell DNA containing repetitious sequences, and this structure is symmetrically duplicated as a large inverted repeat of the type ABCDD′C′B′A′. The Ad12 DNA sequences are flanking the entire molecule, which consists mainly of human KB cell DNA. The recombinants appear to be stable on serial passage of the virus preparation for many years, although variations in the sequence of the recombinants occur. These symmetric recombinant (SYREC) molecules suggest a way to use adenovirus DNA as a eukaryotic vector. Their occurrence provides further evidence for the generation of virus-host DNA recombinants and may help elucidate the role this interaction may have in adenovirus replication and oncogenesis.
Keywords: symmetric DNA molecules, defective adenovirus DNA, recombination, hairpin molecules, end-labeling of adenorirus DNA
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Winocour E., Sachs L., Torten J. Hybridization between SV40 DNA and cellular DNA's. J Mol Biol. 1969 Sep 14;44(2):333–345. doi: 10.1016/0022-2836(69)90179-x. [DOI] [PubMed] [Google Scholar]
- BAXTER H., FRASER F. C. The production of congenital defects in the offspring of female mice treated with cortisone. A preliminary report. McGill Med J. 1950 Dec;19(4):245–249. [PubMed] [Google Scholar]
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Biddle F. G., Fraser R. C. Cortisone-induced cleft palate in the mouse. A search for the genetic control of the embryonic response trait. Genetics. 1977 Feb;85(2):289–302. doi: 10.1093/genetics/85.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman W. W., Lee T. N., Nathans D. The evolution of new species of viral DNA during serial passage of simian virus 40 at high multiplicity. Virology. 1973 Aug;54(2):384–397. doi: 10.1016/0042-6822(73)90151-7. [DOI] [PubMed] [Google Scholar]
- Burger H., Doerfler W. Intracellular forms of adenovirus DNA. 3. Integration of the DNA of adenovirus type 2 into host DNA in productively infected cells. J Virol. 1974 May;13(5):975–992. doi: 10.1128/jvi.13.5.975-992.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlingham B. T., Brown D. T., Doerfler W. Incomplete particles of adenovirus. I. Characteristics of the DNA associated with incomplete adenovirions of types 2 and 12. Virology. 1974 Aug;60(2):419–430. doi: 10.1016/0042-6822(74)90336-5. [DOI] [PubMed] [Google Scholar]
- Butley M. S., Erickson R. P., Pratt W. B. Hepatic glucocorticoid receptors and the H-2 locus. Nature. 1978 Sep 14;275(5676):136–138. doi: 10.1038/275136a0. [DOI] [PubMed] [Google Scholar]
- Daniell E., Mullenbach T. Synthesis of defective viral DNA in HeLa cells infected with adenovirus type 3. J Virol. 1978 Apr;26(1):61–70. doi: 10.1128/jvi.26.1.61-70.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Hellmann W., Kleinschmidt A. K. The DNA of adenovirus type 12 and its denaturation pattern. Virology. 1972 Feb;47(2):507–512. doi: 10.1016/0042-6822(72)90290-5. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U., Hirsch-Kauffmann M. Intracellular forms of adenovirus deoxyribonucleic acid. I. Evidence for a deoxyribonucleic acid-protein complex in baby hamster kidney cells infected with adenovirus type 12. J Virol. 1972 Feb;9(2):297–308. doi: 10.1128/jvi.9.2.297-308.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Stabel S., Ibelgaufts H., Sutter D., Neumann R., Groneberg J., Scheidtmann K. H., Deuring R., Winterhoff U. Selectivity in integration sites of adenoviral DNA. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):551–564. doi: 10.1101/sqb.1980.044.01.057. [DOI] [PubMed] [Google Scholar]
- FRASER F. C., FAINSTAT T. D. Production of congenital defects in the off-spring of pregnant mice treated with cortisone; progress report. Pediatrics. 1951 Oct;8(4):527–533. [PubMed] [Google Scholar]
- FRASER F. C., WALKER B. E., TRASLER D. G. Experimental production of congenital cleft palate: genetic and environmental factors. Pediatrics. 1957 Apr;19(4 Pt 2):782–787. [PubMed] [Google Scholar]
- Gasser D. L. Genetic control of the immune response in mice. I. Segregation data and localization to the fifth linkage group of a gene affecting antibody production. J Immunol. 1969 Jul;103(1):66–70. [PubMed] [Google Scholar]
- Gasser D. L., Silvers W. K. Genetic determinants of immunological responsiveness. Adv Immunol. 1974;18:1–66. doi: 10.1016/s0065-2776(08)60307-7. [DOI] [PubMed] [Google Scholar]
- Goldman A. S., Katsumata M., Yaffe S. J., Gassner D. L. Palatal cytosol cortisol-binding protein associated with cleft palate susceptibility and H-2 genotype. Nature. 1977 Feb 17;265(5595):643–645. doi: 10.1038/265643a0. [DOI] [PubMed] [Google Scholar]
- Gunthert U., Schweiger M., Stupp M., Doerfler W. DNA methylation in adenovirus, adenovirus-transformed cells, and host cells. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3923–3927. doi: 10.1073/pnas.73.11.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarskjöld M. L., Winberg G. Encapsidation of adenovirus 16 DNA is directed by a small DNA sequence at the left end of the genome. Cell. 1980 Jul;20(3):787–795. doi: 10.1016/0092-8674(80)90325-6. [DOI] [PubMed] [Google Scholar]
- Haseman J. K., Hogan M. D. Selection of the experimental unit in teratology studies. Teratology. 1975 Oct;12(2):165–171. doi: 10.1002/tera.1420120209. [DOI] [PubMed] [Google Scholar]
- Iványi P. Some aspects of the H-2 system, the major histocompatibility system in the mouse. Proc R Soc Lond B Biol Sci. 1978 Jun 5;202(1146):117–158. doi: 10.1098/rspb.1978.0060. [DOI] [PubMed] [Google Scholar]
- Kalter H. The Inheritance of Susceptibility to the Teratogenic Action of Cortisone in Mice. Genetics. 1954 Mar;39(2):185–196. doi: 10.1093/genetics/39.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. List of congenic lines of mice. I. Lines with differences at alloantigen loci. Transplantation. 1973 Jan;15(1):137–153. doi: 10.1097/00007890-197301000-00021. [DOI] [PubMed] [Google Scholar]
- Klenow H., Overgaard-Hansen K., Patkar S. A. Proteolytic cleavage fo native DNA polymerase into two different catalytic fragments. Influence of assay condtions on the change of exonuclease activity and polymerase activity accompanying cleavage. Eur J Biochem. 1971 Oct 14;22(3):371–381. doi: 10.1111/j.1432-1033.1971.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Lafuse W., Edidin M. Influence of the mouse major histocompatibility complex, H-2, on liver adenylate cyclase activity and on glucagon binding to liver cell membranes. Biochemistry. 1980 Jan 8;19(1):49–54. doi: 10.1021/bi00542a008. [DOI] [PubMed] [Google Scholar]
- Lavi S., Rozenblatt S., Singer M. F., Winocour E. Acquisition of sequences homologous to host DNA by closed circular simian virus 40 DNA. II. Further studies on the serial passage of virus clones. J Virol. 1973 Sep;12(3):492–500. doi: 10.1128/jvi.12.3.492-500.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi S., Winocour E. Acquisition of sequences homologous to host deoxyribonucleic acid by closed circular simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):309–316. doi: 10.1128/jvi.9.2.309-316.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman R., Paul W. E., Humphrey W., Jr, Stimpfling J. H. H-2-linked immune response (Ir) genes. Independent loci for Ir-IgG and Ir-IgA genes. J Exp Med. 1972 Nov 1;136(5):1231–1240. doi: 10.1084/jem.136.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozner E. C., Sachs D. H., Shearer G. M., Terry W. D. B-cell alloantigens determined by the H-2 linked Ir region are associated with mixed lymphocyte culture stimulation. Science. 1974 Feb 22;183(4126):757–759. doi: 10.1126/science.183.4126.757. [DOI] [PubMed] [Google Scholar]
- Mak I., Ezoe H., Mak S. Structure and function of adenovirus type 12 defective virions. J Virol. 1979 Oct;32(1):240–250. doi: 10.1128/jvi.32.1.240-250.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak S. Defective virions in human adenovirus type 12. J Virol. 1971 Apr;7(4):426–433. doi: 10.1128/jvi.7.4.426-433.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marković R. D., Eisen H. J., Parchman L. G., Barnett C. A., Litwack G. Evidence for a physiological role of corticosteroid binder IB. Biochemistry. 1980 Sep 30;19(20):4556–4564. doi: 10.1021/bi00561a003. [DOI] [PubMed] [Google Scholar]
- McDevitt H. O., Bodmer W. F. HL-A, immune-response genes, and disease. Lancet. 1974 Jun 22;1(7869):1269–1275. doi: 10.1016/s0140-6736(74)90021-x. [DOI] [PubMed] [Google Scholar]
- McDevitt H. O., Bodmer W. F. Protein clinical manifestations of primary tumors of the heart. Am J Med. 1972 Jan;52(1):1–8. doi: 10.1016/0002-9343(72)90002-2. [DOI] [PubMed] [Google Scholar]
- Melchers I., Rajewsky K., Shreffler D. C. Ir-LDHB: map position and functional analysis. Eur J Immunol. 1973 Dec;3(12):754–761. doi: 10.1002/eji.1830031204. [DOI] [PubMed] [Google Scholar]
- Meruelo D., Edidin M. Association of mouse liver adenosine 3':5'-cyclic monophosphate (cyclic AMP) levels with histocompatibility-2 genotype. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2644–2648. doi: 10.1073/pnas.72.7.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo D., Edidin M. The biological function of the major histocompatibility complex: hypotheses. Contemp Top Immunobiol. 1980;9:231–253. doi: 10.1007/978-1-4615-9131-3_9. [DOI] [PubMed] [Google Scholar]
- Miller K. K. Commercial dietary influences on the frequency of cortisone-induced cleft palate in C57B1/6J mice. Teratology. 1977 Jun;15(3):249–251. doi: 10.1002/tera.1420150306. [DOI] [PubMed] [Google Scholar]
- Neumann R., Doerfler W. Integration of adenovirus type 2 DNA at a limited number of cellular sites in productively infected cells. J Virol. 1981 Mar;37(3):887–892. doi: 10.1128/jvi.37.3.887-892.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo D. A., Vladutiu A. O. Estrogen receptor in uteri of mice of different H-2 genotypes. Experientia. 1979 Aug 15;35(8):1103–1104. doi: 10.1007/BF01949969. [DOI] [PubMed] [Google Scholar]
- Prage L., Höglund S., Philipson L. Structural proteins of adenoviruses. 8. Characterization of incomplete particles of adenovirus type 3. Virology. 1972 Sep;49(3):745–757. doi: 10.1016/0042-6822(72)90531-4. [DOI] [PubMed] [Google Scholar]
- RICHARDSON C. C., LEHMAN I. R., KORNBERG A. A DEOXYRIBONUCLEIC ACID PHOSPHATASE-EXONUCLEASE FROM ESCHERICHIA COLI. II. CHARACTERIZATION OF THE EXONUCLEASE ACTIVITY. J Biol Chem. 1964 Jan;239:251–258. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Salomon D. S., Pratt R. M. Involvement of glucocorticoids in the development of the secondary palate. Differentiation. 1979;13(3):141–154. doi: 10.1111/j.1432-0436.1979.tb01577.x. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stabel S., Doerfler W., Friis R. R. Integration sites of adenovirus type 12 DNA in transformed hamster cells and hamster tumor cells. J Virol. 1980 Oct;36(1):22–40. doi: 10.1128/jvi.36.1.22-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuitje A. R., Veltkamp E., Maat J., Heyneker H. L. The nucleotide sequence surrounding the replication origin of the cop3 mutant of the bacteriocinogenic plasmid Clo DF13. Nucleic Acids Res. 1980 Apr 11;8(7):1459–1473. doi: 10.1093/nar/8.7.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci U S A. 1980 Jan;77(1):253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter D., Westphal M., Doerfler W. Patterns of integration of viral DNA sequences in the genomes of adenovirus type 12-transformed hamster cells. Cell. 1978 Jul;14(3):569–585. doi: 10.1016/0092-8674(78)90243-x. [DOI] [PubMed] [Google Scholar]
- Tibbetts C. Viral DNA sequences from incomplete particles of human adenovirus type 7. Cell. 1977 Sep;12(1):243–249. doi: 10.1016/0092-8674(77)90202-1. [DOI] [PubMed] [Google Scholar]
- Tolun A., Aleström P., Pettersson U. Sequence of inverted terminal repetitions from different adenoviruses: demonstration of conserved sequences and homology between SA7 termini and SV40 DNA. Cell. 1979 Jul;17(3):705–713. doi: 10.1016/0092-8674(79)90277-0. [DOI] [PubMed] [Google Scholar]
- Tyan M. L., Miller K. K. Genetic and environmental factors in cortisone induced cleft palate. Proc Soc Exp Biol Med. 1978 Sep;158(4):618–621. doi: 10.3181/00379727-158-40259. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Doerfler W. Persistent infection of Muntiacus muntjak cells with adenovirus type 2 and abortive infection with adenovirus type 12. Virology. 1980 Feb;101(1):72–80. doi: 10.1016/0042-6822(80)90484-5. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Neumann R., Kuhlmann I., Sutter D., Doerfler W. DNA methylation and viral gene expression in adenovirus-transformed and -infected cells. Nucleic Acids Res. 1980 Jun 11;8(11):2461–2473. doi: 10.1093/nar/8.11.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadell G., Hammarskjöld M. L., Varsanyi T. Incomplete virus particles of adenovirus type 16. J Gen Virol. 1973 Sep;20(3):287–302. doi: 10.1099/0022-1317-20-3-287. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner G., zur Hausen H. Deletions and insertions in adenovirus type 12 DNA after viral replication in Vero cells. Virology. 1978 May 1;86(1):66–77. doi: 10.1016/0042-6822(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Yoshiike K. Studies on DNA from low-density particles of SV40. I. Heterogeneous defective virions produced by successive undiluted passages. Virology. 1968 Mar;34(3):391–401. doi: 10.1016/0042-6822(68)90059-7. [DOI] [PubMed] [Google Scholar]