Abstract
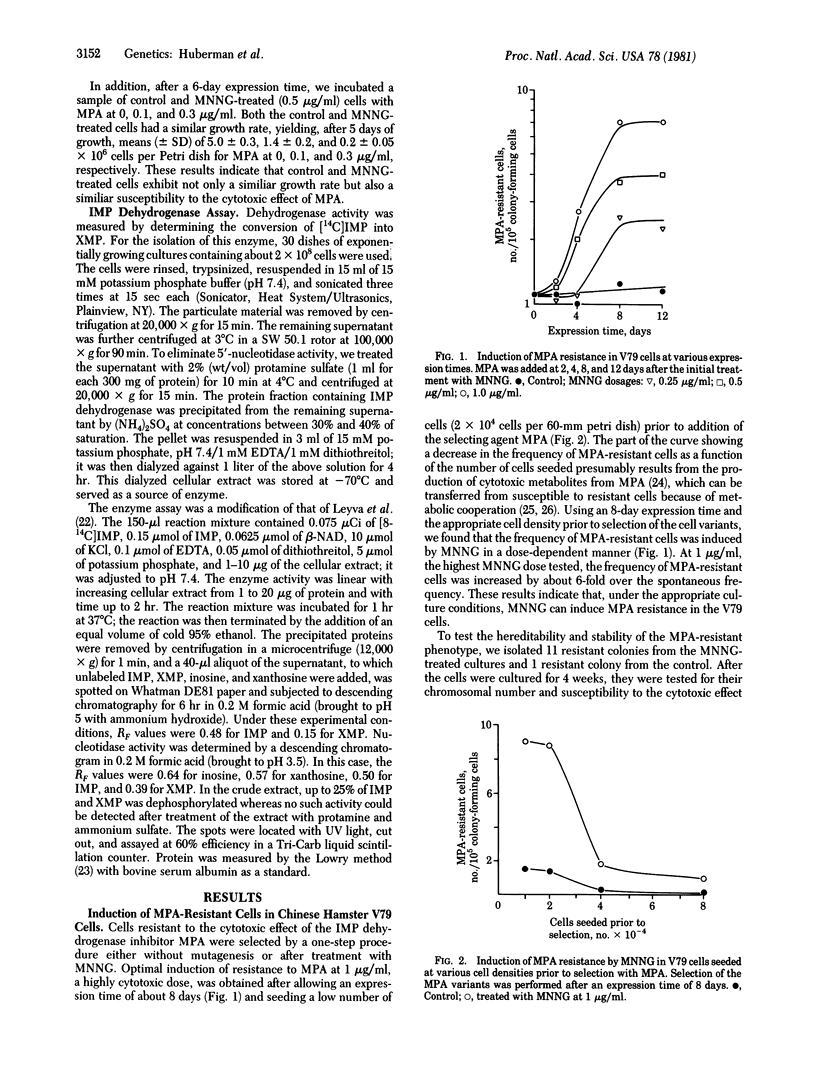
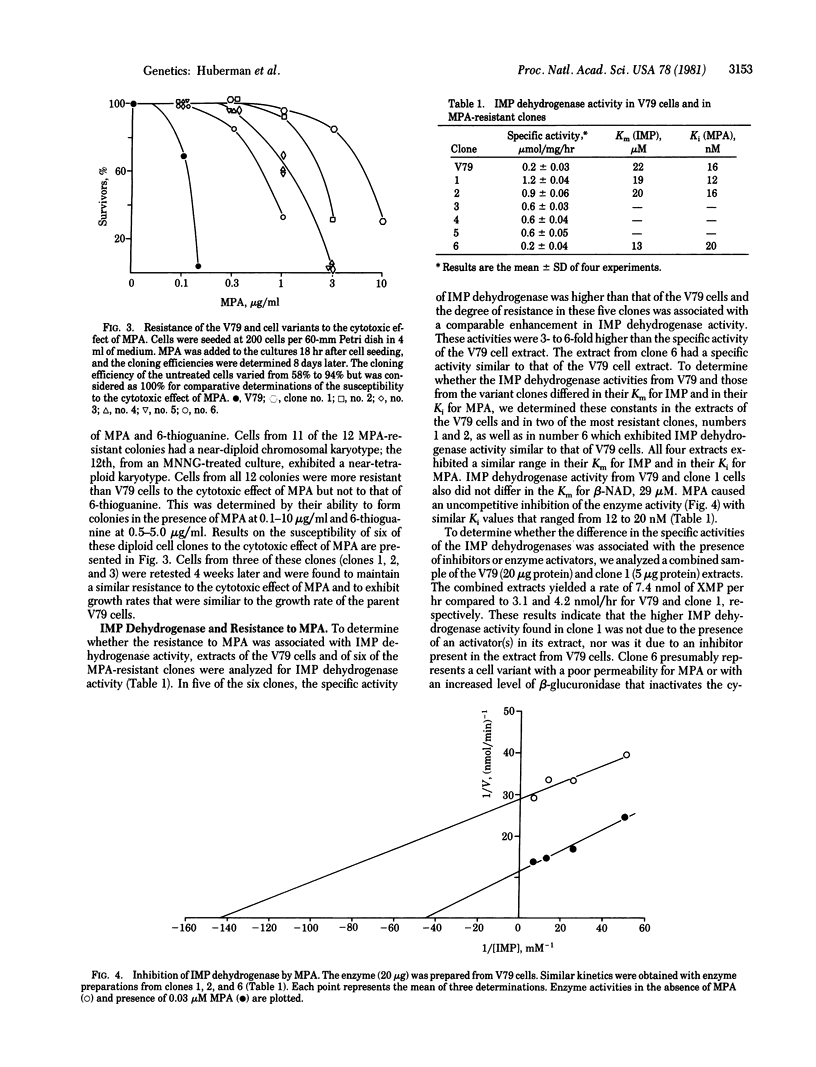
Cell variants resistant to the cytotoxic effect of mycophenolic acid, an inhibitor of IMP dehydrogenase (IMP:NAD+ oxidoreductase, EC 1.2.1.14), were selected by a one-step procedure from Chinese hamster V79 cells. The frequency of these variants was increased in a dose-dependent manner after treatment with the mutagen N-methyl-N'-nitro-N-nitrosoguanidine and after an expression time of 8 days. The degree of resistance in five of the six isolated cell variants was associated with a comparable increase in the specific activity of IMP dehydrogenase, which was 3- to 6-fold higher than that of the parent V79 cells. The IMP dehydrogenase activity from both the variants and the V79 cells had a similar affinity for the substrate IMP with a Km of about 20 microM and a similar response to mycophenolic acid with a Ki of 12-16 nM. It is suggested that cell variants with an altered regulation of IMP dehydrogenase activity may be helpful in studying the control of nucleic acid biosynthesis, cell growth, and carcinogenesis. Mycophenolic acid resistance also may be useful as a marker in short-term assays for the identification of potential chemical carcinogens.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott B. J., Horton D. R., Whitney J. G. Glucosylation of mycophenolic acid by Streptomyces aureofaciens. J Antibiot (Tokyo) 1980 May;33(5):506–509. doi: 10.7164/antibiotics.33.506. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- BIRKINSHAW J. H., RAISTRICK H., ROSS D. J. Studies in the biochemistry of micro-organisms. 86. The molecular constitution of mycophenolic acid, a metabolic product of Penicillium Brevi-compactum Dierckx. Part III. Further observations on the structural formula for mycophenolic acid. Biochem J. 1952 Mar;50(5):630–634. doi: 10.1042/bj0500630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. C., Ts'o P. O. Relationship between somatic mutation and neoplastic transformation. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3297–3301. doi: 10.1073/pnas.75.7.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck N., di Mayorca G. Somatic mutation as the basis for malignant transformation of BHK cells by chemical carcinogens. Nature. 1976 Dec 23;264(5588):722–727. doi: 10.1038/264722a0. [DOI] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975 May 15;255(5505):197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Kruh G. D. The HPRT locus. Cell. 1979 Jan;16(1):1–9. doi: 10.1016/0092-8674(79)90182-x. [DOI] [PubMed] [Google Scholar]
- Chan G. L., Little J. B. Induction of ouabain-resistant mutations in C3H 10T1/2 mouse cells by ultraviolet light. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3363–3366. doi: 10.1073/pnas.75.7.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E. H., Trosko J. E., Chang C. C. Mutational approaches to the study of carcinogenesis. J Toxicol Environ Health. 1977 Jul;2(6):1317–1334. doi: 10.1080/15287397709529533. [DOI] [PubMed] [Google Scholar]
- Franklin T. J., Cook J. M. The inhibition of nucleic acid synthesis by mycophenolic acid. Biochem J. 1969 Jul;113(3):515–524. doi: 10.1042/bj1130515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger C. Chemical oncogenesis in culture. Adv Cancer Res. 1973;18:317–366. doi: 10.1016/s0065-230x(08)60756-3. [DOI] [PubMed] [Google Scholar]
- Huberman E., Mager R., Sachs L. Mutagenesis and transformation of normal cells by chemical carcinogens. Nature. 1976 Nov 25;264(5584):360–361. doi: 10.1038/264360a0. [DOI] [PubMed] [Google Scholar]
- Huberman E., Sachs L. Mutability of different genetic loci in mammalian cells by metabolically activated carcinogenic polycyclic hydrocarbons. Proc Natl Acad Sci U S A. 1976 Jan;73(1):188–192. doi: 10.1073/pnas.73.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landolph J. R., Heidelberger C. Chemical carcinogens produce mutations to ouabain resistance in transformable C3H/10T1/2 Cl 8 mouse fibroblasts. Proc Natl Acad Sci U S A. 1979 Feb;76(2):930–934. doi: 10.1073/pnas.76.2.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva A., Holmes E. W., Jr, kelley W. N. Effect of 6-mercaptopurine on inosinic acid dehydrogenase in cultured human fibroblasts. Biochem Pharmacol. 1976 Mar 1;25(5):527–532. doi: 10.1016/0006-2952(76)90382-8. [DOI] [PubMed] [Google Scholar]
- Lowe J. K., Brox L., Henderson J. F. Consequences of inhibition of guanine nucleotide synthesis by mycophenolic acid and virazole. Cancer Res. 1977 Mar;37(3):736–743. [PubMed] [Google Scholar]
- Revel M., Groner Y. Post-transcriptional and translational controls of gene expression in eukaryotes. Annu Rev Biochem. 1978;47:1079–1126. doi: 10.1146/annurev.bi.47.070178.005243. [DOI] [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- Sehgal P. B., Lyles D. S., Tamm I. Superinduction of human fibroblast interferon production: further evidence for increased stability of interferon mRNA. Virology. 1978 Aug;89(1):186–198. doi: 10.1016/0042-6822(78)90051-x. [DOI] [PubMed] [Google Scholar]
- Subak-Sharpe H., Bürk R. R., Pitts J. D. Metabolic co-operation between biochemically marked mammalian cells in tissue culture. J Cell Sci. 1969 Mar;4(2):353–367. doi: 10.1242/jcs.4.2.353. [DOI] [PubMed] [Google Scholar]
- Swaneck G. E., Nordstrom J. L., Kreuzaler F., Tsai M. J., O'Malley B. W. Effect of estrogen on gene expression in chicken oviduct: evidence for transcriptional control of ovalbumin gene. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1049–1053. doi: 10.1073/pnas.76.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M. J., Hoffman D. H., Esterman M. A. Metabolism and biochemistry of mycophenolic acid. Cancer Res. 1972 Sep;32(9):1803–1809. [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Weber G., Olah E., Lui M. S., Kizaki H., Tzeng D. Y., Takeda E. Biochemical commitment to replication in cancer cells. Adv Enzyme Regul. 1980;18:3–26. doi: 10.1016/0065-2571(80)90005-9. [DOI] [PubMed] [Google Scholar]


