Abstract
Genital human papillomavirus (HPV) infection is the most common sexually transmitted infection with an estimated worldwide prevalence of 9–13% and approximately 6 million people being infected each year. Mostly acquired during adolescence or young adulthood, HPV presents clinically as anogenital warts and may progress to precancerous lesions and cancers of the cervix, vagina, vulva, penis and anus, and oropharynx. HPV infection is considered to contribute to almost 100% cervical cancers and at least 80% of anal and 40–60% of vulvar, vaginal, and penile cancers. At present, two prophylactic HPV vaccines are commercially available and both are prepared from purified L1 structural proteins. These proteins self-assemble to form virus-like particles that induce a protective immunity. Gardasil® is a quadrivalent vaccine against HPV types 6, 11, 16, and 18 and is recommended for use in females 9–26 years of age, for the prevention of cervical, vulvar, and vaginal cancers and intraepithelial neoplasia and condyloma acuminata and recently for vaccination in boys and men 9–26 years of age for the prevention of genital warts. Cervarix™ is a bivalent vaccine approved for the prevention of cervical cancer and precancerous lesions caused by HPV 16 and 18, in females 10–25 years. HPV vaccines are safe and efficacious against type-specific HPV-induced anogenital warts, precancerous lesions, and cervical cancer. The vaccines are most effective when given before the onset of sexual activity and provide long-term protection. Effective vaccination coverage in young adolescent females will substantially reduce the incidence of these anogenital malignancy-related morbidity and mortality. There is need to generate India-specific data on HPV epidemiology and HPV vaccination efficacy as well as continue worldwide surveillance and development of newer vaccines.
Keywords: Cervical cancer, human papilloma virus vaccine, India, trials
INTRODUCTION
Genital human papillomavirus (HPV) infection is a common infection and is primarily transmitted by sexual contact. It is the most common sexually transmitted infection (STI) with around 630 million people already infected and approximately 6 million people being infected each year.[1] The prevalence of HPV increases with age from 14 to 24 years and then declines.[2] Up to 80% of women will acquire an HPV infection in their lifetime.[3] The cumulative risk of acquiring cervical HPV infection in women with only one sexual partner is 46% at 3 years after the first sexual encounter.[4] Majority of HPV infections are transient and subclinical and undergo subsequent clearance by the immune system. Persistence of infection results in development of anogenital warts as well as precancerous lesions and cancers of the anogenital tract and oropharynx. Anogenital warts are very common in sexually active adolescents and young adults with an annual incidence rate ranging from 182 to 229/100,000 population in developed countries such as USA, UK, and France.[5] Precancerous lesions associated with HPV infection may involve the cervix [cervical intraepithelial neoplasia or cervical intraepithelial neoplasia (CIN) and adenocarcinoma in situ or adenocarcinoma in situ (AIS)], vagina ([vaginal intraepithelial neoplasia or vaginal intraepithelial neoplasia (VaIN)], vulva [vulvar intraepithelial neoplasia or vulvar intraepithelial neoplasia (VIN)], or anus (anal intraepithelial neoplasia or AIN). Among the HPV-induced cancers, cervical cancer tops the list followed by cancer of the vagina, vulva, penis, and anus and a subset of head and neck cancers.[6]
In 2005, there were about 500,000 cases of cervical cancer and 260,000 related deaths worldwide.[6] As per an estimate, the global burden of cervical cancer by the year 2050 will be more than 1 million new cases every year.[7] Cervical cancer incidence rates vary from 1 to 50 per 100,000 females; rates are highest in Latin America and the Caribbean, sub-Saharan Africa, and south-central and South-East Asia.[6] In India, cervical cancer ranks number one among cancer in females with an annual incidence of more than 132,000 and around 740,00 deaths every year.[8]
The HPV virus and types
HPV, a member of the Papillomaviridae family of viruses, is a non-enveloped, double-stranded deoxyribonucleic acid virus. The HPV genome is enclosed in a capsid shell composed of major (L1) and minor (L2) structural proteins. More than 100 HPV genotypes are known, of which approximately 40 infect the anogenital region and around 13 are considered high risk, associated with anogenital and oropharyngeal cancers.[2] Low-risk HPV types 6 and 11 cause 90% of external anogenital warts and low-grade changes in cervical cells.[9] Other low-risk HPV types include HPV 40, 42, 43, 44, 54, 61, 70, 72, and 81.[10] The high-risk types 16 and 18 are known to cause about 70% of all cases of invasive cervical cancer.[11] HPV 16 has the greatest oncogenic potential and continues to be the dominant oncogenic type worldwide. Other oncogenic HPV types including 31, 33, 35, 45, 52, and 58 are phylogenetically related to HPV 16/18 and account for an additional 18% of all cases.[12] In India, HPV 16, 18, 31, 33, and 45 account for more than 90% of cervical cancer cases.[13]
Immunology of natural HPV infection and oncogenesis
HPV exhibits a specific tropism for the squamous epithelium of the skin and mucosae and evades local immune responses by many mechanisms—lack of viral-induced necrosis or inflammation, lack of viremia, exclusive intraepithelial localization of the infection, lack of activation of Langerhan cells by the uptake of HPV capsids, and inhibition of interferon synthesis and receptor signalling, among others.[14] The host's humoral immune response to natural HPV infection is usually slow, weak, and variable. Neutralizing antibodies to HPV specifically recognize or react with L1 capsid proteins and are important for inhibition of early infection before viral entry into cells.[15] Among women infected with oncogenic virus types, only 50% develop antibodies to HPV infection, and seroconversion may take as long as 18 months.[16] Further, these antibodies are not necessarily protective against reinfection by the same HPV type over time.[6]
Persistent HPV infection may lead to CIN of moderate grade 2 or severe grade 3 or to AIS. Untreated CIN 2/3 and AIS have a high probability of progressing to invasive squamous cell cancer or adenocarcinoma of cervix, respectively. It is estimated that progression to CIN3 takes 7–15 years and progression to invasive cancer 20 years or more.[17] Risk factors for progression to high-grade dysplasia and cancer include persistence of HPV infection, infection with oncogenic HPV types, age more than 30 years, infection with multiple HPV types, and immunosuppression.[2]
HPV vaccines
In view of the morbidity and mortality associated with genital HPV-induced lesions and the poor immunity conferred by natural infection, the need for effective prophylactic vaccines has always been felt. At present, two prophylactic HPV vaccines are available internationally and both have been prepared from purified L1 structural proteins by recombinant technology. These proteins self-assemble to form virus-like particles (VLPs) that induce a protective host immune response. Compared with immunity-acquired following natural infection, the vaccine-induced immunity is much stronger, long lasting, and includes partial cross-protection to non-vaccine-related serotypes. The difference in the immune response generated by vaccination and natural infection is attributable to high immunogenicity of VLPs inducing much higher concentrations of neutralizing antibodies to L1, higher antigen dose in VLPs, and direct exposure of capsids to systemic immune responses.-[14] The mechanisms by which these vaccines induce protection have not been fully defined but apparently involve both cellular immunity and neutralizing immunoglobulin G antibodies.[15,18] HPV vaccines are designed for prophylactic use only; they do not clear existing HPV infection or treat HPV-related disease.[19]
The two commercially available vaccines, Gardasil®[20] and Cervarix™,[21] substantially differ in their composition [Table 1].
Table 1.
Salient differences between the two commercially marketed HPV vaccines

Indications and licensing
The list of currently FDA-approved indications for both the vaccines is given in Table 2. In India, both vaccines have been licensed for use in females (primary vaccination at 10–12 years, catch-up upto 26 years) since October 2008 (Gardasil®) and February 2009 (Cervarix™).
Table 2.
FDA-approved indications for Gardasil® and Cervarix™

Storage and administration
Both the vaccines are available as a sterile suspension in single-use glass vials or single-use prefilled syringes that should be maintained at 2–8°C (not to be frozen). Recommended route of administration is intramuscular with doses of 0.5 ml each time. The quadrivalent vaccine is given at baseline and repeated at 2 and 6 months. A minimum interval between successive doses of 4 weeks between the first and second dose, and 12 weeks between the second and third dose is recommended.[22] The bivalent vaccine is given at baseline and repeated at 1 and 6 months; the second dose may be administered between 1 and 2.5 months after the first dose if flexibility in the schedule is required.[23] If the vaccination schedule is interrupted, restarting the three-dose series is not necessary; remaining vaccine doses should be administered as close to the recommended schedule as possible.[6] Currently, a booster dose has not been recommended for any of the HPV vaccines following completion of the primary series.
Age of vaccination
The ideal time of vaccination for HPV vaccines would be before the onset of sexual activity, i.e., before the first exposure to HPV infection. It is currently recommended that HPV vaccine be administered to girls at 11–12 years, with catch-up vaccination for those who have not completed or initiated the series between 13 and 26 years.[24–26] The recommended age range for Gardasil® in males is 9–26 years.
Immunogenicity studies
After three doses of the vaccine, almost all adolescent and young females initially naive to the vaccine-related HPV types develop an antibody response.[27,28] Data available up to 5–6.4 years after vaccination have shown that antibody titres in vaccines peak after the third dose decline gradually and then level off by 24 months after the first dose, though they remain higher than in natural infection.-[6] Coadministration of HPV vaccines with most other vaccines has not shown any significant impairment of the immune response to any of the involved antigens.
Vaccine efficacy in young women
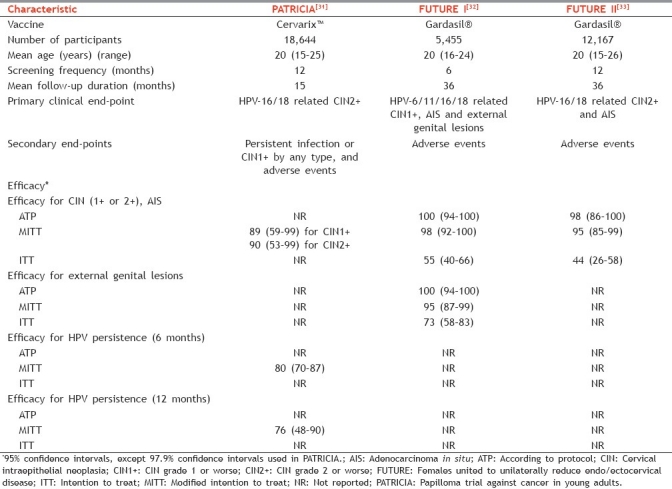
Results of multiple phase II and III studies are available for both vaccines. The USFDA recommended surrogate clinical end point for cervical cancer (development of CIN grade 2 or worse) has been used as the primary outcome measure in most HPV vaccine studies.[29] Published analyses have included variable study populations [Table 3].[29,30] Since obtaining cervical specimens from girls or young adolescents is considered unethical, clinical efficacy in younger girls (9–14 years) is extrapolated from immunobridging studies comparing vaccine immunogenicity in them with older females (15–26 years). Both vaccines have shown high efficacy rates for various clinical end points including condyloma, low- and high-grade CIN and AIS, as well as VaIN and VIN, associated with vaccine-related HPV types. The characteristics of three large phase III trials of Gardasil® (FUTURE I and II study) and Cervarix™ (PATRICIA study) conducted in young women and the prophylactic efficacies from these trials are summarized in Table 4. While the efficacy for prevention of HPV-16/18 related CIN-2/3 ranged from 90.4% to 98%, the overall HPV vaccine type-related CIN efficacy has ranged from 89.2% to 100%.[31–33] The quadrivalent vaccine also demonstrated 91% to 100% efficacy against HPV-6/11/16/18-related VIN-2/VIN-3 or VaIN-2/VaIN-3 and 96% to 100% efficacy against HPV6/11/16/18-associated condyloma.[32,33] High efficacy rates have been reported in the ATP analyses of most studies. However, efficacy has been lower in the MITT and ITT analyses [Table 4]. This may reflect, at least in part, lesser protection with single dose compared with three doses. More importantly, the lower efficacy in ITT (which includes women already exposed to vaccine-related HPV) clearly suggests that women naive to vaccine-related HPV types are likely to benefit the most with prophylactic vaccination.[29] Further analyses of the findings of FUTURE I/II and PATRICIA trials over longer follow-up periods have reinforced the efficacy of both HPV vaccines.[30,34]
Table 3.
Typical characteristics of different types of study populations analyzed in HPV vaccine trials (may differ in some aspects in different studies)
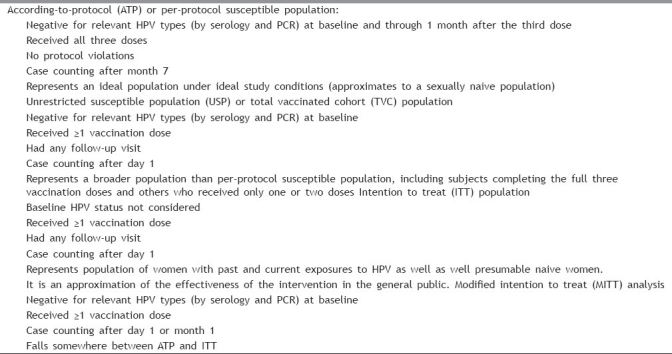
Table 4.
Outline and efficacy outcomes of three large phase III studies of HPV vaccines conducted in young women
The protective efficacy of both vaccines has been maintained throughout their respective observation periods (currently 5 years for Gardasil® and 8.4 years for Cervarix™).[35,36] A model estimated that antibody levels will remain detectable near lifelong in 99% of vaccinated females.[18,37] This suggests that a booster dose may not be required, although longer follow-up studies are warranted.
Gardasil® vs Cervarix™
Differences among the efficacy trials of the two vaccines in terms of choice of placebo recipients, immunological assays, and populations analyzed preclude direct comparison of results. A recent observer-blind head-to-head randomized controlled trial sponsored by GSK has compared the immonogenecity and safety of the two vaccines in 1106 women stratified by age (18–26, 27–35, and 36–45 years). At month 7 after first vaccination, analysis of women in the ATP cohort showed that the geometric mean titres of serum neutralizing antibodies ranged from 2.3- to 4.8-fold higher for HPV-16 and 6.8- to 9.1-fold higher for HPV-18 after vaccination with Cervarix™ compared with Gardasil®, across all age strata.[38] The incidence of adverse events was comparable between groups. The better immune response with Cervarix™ may reflect a longer duration of protection with HPV-16/18, although long-term studies are needed to confirm this. The obvious advantage of Gardasil® over Cervarix™ is the additional protection available for HPV-6/11-associated condyloma.
Cross protection to non-vaccine-type oncogenic HPV
The quadrivalent vaccine has shown statistically significant but limited protection against CIN2+ associated with non-vaccine-type oncogenic HPV (especially HPV-31, 45, and 33) that are phylogenetically related to HPV 16 and 18.[39–41] No statistically significant protection was detected against persistent infection with HPV-52 and 58. Cross-protection against incident infection, persistent infection and CIN2+ related to HPV-31 and HPV-45 has also been reported with the bivalent vaccine (with a 66 months of follow-up).[34,42] The added benefit of cross-protection may result in further reductions in incidence of cervical cancer and precancerous lesions following vaccination.
Vaccination of older females
Older women remain at risk of acquiring and developing persistent infection by high-risk HPV, leading to an increased risk of carcinoma when compared with younger women.[43] While sexually naive girls and young women will be the highest beneficiaries of prophylactic HPV vaccination, recent studies have shown vaccination benefits for older women as well, many of whom may have acquired transient infections in the past or had active infection at the time of vaccination. Muñoz et al. in their quadrivalent vaccine trial involving 3,819 older women (24–45 years old) observed 90% efficacy against combined incidence of vaccine HPV-related 6-month persistent infection, CIN 1-3 or external genital warts.[44] Further, Olsson et al. have demonstrated that even among women who had detectable serological evidence of vaccine-type-related HPV infection in the past but no DNA evidence of active infection at enrolment, prophylactic vaccination provided nearly 100% protection against CIN2+ associated with the vaccine HPV type with which the women had been previously infected.[45]
Vaccination of boys and men
Extension of routine HPV vaccination to males is a matter of debate. Vaccinated males will benefit from prevention of HPV-related disease (anogenital warts, AIN, and anal cancer). Giuliano et al. have reported an efficacy of 90.4% against external genital lesion and 85.6% against persistent infection by HPV-6/11/16/18 following administration of the quadrivalent vaccine to 4,065 healthy, predominantly heterosexual males 16-26 years of age.[46] Although less than 25% of HPV-related cancers occur in men, some subgroups, including men who have sex with men (MSM) and those with immunodeficiency, are at a markedly increased risk and are likely to benefit from vaccination.[47] In one study, involving MSM, Gardasil® provided 77.5% protection against development of AIN.[48] Men are also at a higher risk than women of developing oropharyngeal cancers, 50% of which may be HPV-related.[49] The argument that vaccinating boys could indirectly contribute to reduction of cervical cancer by “herd immunity” sounds logical. However, current analyses suggest that cost-effectiveness of vaccinating a girl far exceeds that of vaccinating a boy.[50]
Vaccinaton of immunocompromised individuals
Although HPV vaccine can be safely given to HIV-positive and other immunocompromised individuals, the efficacy has been found to be lesser compared with immunocompetent people.[6] Following administration of Gardasil® to 109 HIV-1-infected men in an open-label, multicenter clinical trial, seroconversion rates of upto 98%, 99%, 100% and 95% were observed for HPV types 6, 11, 16, and 18, respectively. No adverse effects (AEs) on CD4 counts and plasma HIV-1 RNA levels were observed.[51] In another trial, 126 HIV-infected children (7–12 years old) were blindly assigned to receive a dose of Gardasil® or placebo at 0, 8, and 24 weeks. Seroconversion to all four antigens occurred in more than 96% of vaccine recipients (irrespective of the baseline CD4 counts), compared with none in placebo recipients. Adverse events were infrequent, and there was no alteration of HIV viral load.[52] Thus, current evidence suggests that vaccination should be offered to all irrespective of their immunocompetence status.
Adverse effects
A recent systematic review and meta-analysis of seven clinical trials has shown that both vaccines are safe and well tolerated with no statistically significant difference in the risk for vaccine-related serious AEs between vaccine and control groups.-[12] Pain at injection site was the most frequently reported AE, ranging from 83% to 93.4% in vaccine groups versus 75.4–87.2% in control groups. Injection-site erythema and swelling were also common. Headache and fatigue were the most common vaccine-related systemic AEs observed in approximately 50–60% of vaccines. Observation of vaccines for 15 min after the injection is recommended, since an increased occurrence of syncope accompanied by tonic–clonic movements has been reported.[2] In June 2007, WHO's Global Advisory Committee on Vaccine Safety (GACVS) concluded that both vaccines had good safety profiles and this was confirmed, in a postmarketing surveillance of the quadrivalent vaccine in 2008.[6]
Contraindications and precautions
HPV vaccines are contraindicated in people with history of severe allergic reactions after a previous vaccine dose or to a vaccine component (yeast allergy for Gardasil®, latex allergy for Cervarix™ prefilled syringes). In individuals with severe acute illness, delaying HPV vaccination is recommended. A history of abnormal Pap smear or anogenital warts is not a contraindication and Pap smears or HPV testing are not required prior to vaccination.[2] Since vaccines do not contain live biological products or viral DNA, they are non-infectious.
Pregnancy and lactation
No adverse pregnancy outcomes or fetal risk in animals and data on women who became pregnant during the vaccine trials indicate no increased risk of adverse events (including congenital anomalies) compared with controls.[53,54] However, since adequate and well-controlled studies in pregnant women are lacking (pregnancy category B), vaccine should not be given to women known to be pregnant. Women who accidentally receive the vaccine while pregnant should delay further shots till pregnancy is over. A pregnancy test is not required prior to vaccine administration. Breastfeeding is not a contraindication although caution is recommended.[2]
Cervical cancer screening in vaccinated females
Although HPV-16/18 have been implicated in the causation of up to 70% cervical cancers, the remaining 30% cases are associated with other HPV types. A vaccinated female may subsequently become infected with a carcinogenic HPV type for which the current vaccines do not provide protection, and thus it has been recommended that cervical cancer screening in national programs for vaccinated females should remain the same as for non-vaccinated females.[55]
Impact of HPV vaccines on population health
Models predict that vaccination programmes for young adolescent females will substantially reduce the incidence of cervical cancers associated with vaccine-related HPV types if coverage is high (>70%) and vaccine-induced protection lasts for ≥10 years.[6] Considerable reductions in incidence may also be expected for cancers of the vagina, vulva, anus, and head and neck associated with HPV-16/18. Vaccination with the quadrivalent vaccine will substantially reduce the incidence of anogenital warts, low-grade cervical abnormalities caused by HPV-6/11 and, possibly, recurrent respiratory papillomatosis,[6] Since the vaccines protect females who are naive for the vaccine-related HPV types at the time of immunisation, a high coverage of young adolescent girls before first intercourse is expected to have a much larger impact than vaccinating older females.[56]
WHO recommendations
WHO recommends that routine HPV vaccination should be included in national immunization programmes, provided that prevention of cervical cancer or other HPV-related diseases, or both, constitutes a public health priority; vaccine introduction is programmatically feasible; financially sustainable; and is cost effectiveness in the country.-[6] Programs should initially prioritize high coverage in the primary target population which should be selected based on data on the age of initiation of sexual activity and feasibility of reaching young adolescent girls through schools, or healthcare and community-based settings. Vaccination of secondary target populations of older adolescent females or young women is recommended only if this is feasible, affordable, cost-effective, does not divert resources from vaccinating the primary target population or effective cervical cancer screening programmes, and if a significant proportion of this target population is likely to be naive to vaccine-related HPV types.[6] The benefits of vaccination should be available to all irrespective of their HIV status. HPV vaccination of males is not recommended. The choice between the two vaccines should be based on the scale of the prevailing HPV problem, the target population, delivery strategies, safety concerns and the price, supply, and cold-chain requirements of the products.[6]
Cost-effectiveness and economic feasibility of HPV vaccination
In general, models show that a substantial reduction in costs associated with cervical cancer screening and follow-up of abnormal screening tests, diagnosis, and treatment of precancerous states and cancer is expected with nationwide programs that achieve high coverage in young adolescent girls, at least in countries where gross domestic product is high.[57] HPV vaccination may be cost-effective in low-income and middle-income countries (where quality screening is not widespread) if the cost per vaccinated girl (including three doses of vaccine and programmatic costs) is < US$ 10–25, which is substantially lower than current costs in high-income countries.[58] Quadrivalent HPV vaccination is expected to further reduce the costs associated with the diagnosis and treatment of genital warts in high-income settings.[6] The respective cost of a single 0.5-ml dose of Gardasil® and Cervarix™ is approximately $120 and $100 in the USA versus Rs 2,800 and Rs 3,300 in India. The cost in India for the entire three dose schedule turns out to be Rs 8,400 and Rs 9,900, respectively. The vaccine cost may drop substantially if the Government purchases vaccine in bulk by policy, or if Indian manufacturers are encouraged or enabled to manufacture vaccine.[59] The long-term cost-effectiveness of mass HPV vaccination needs to be specifically evaluated for India, comparing the expected economic burden incurred by cost of vaccines and infrastructure for the programme against the financial benefit of reduced health costs for diagnosis and treatment of CINs, cervical cancers and anogenital warts.
Indian scenario
Cervical cancer is the leading cause of cancer-related mortality in Indian females. Both HPV vaccines have been licensed for use in Indian females and have been recommended by the Indian Academy of Pediatrics (IAP) and the Federation of Obstetric and Gynaecological Societies of India (FOGSI). However, high-cost, low public awareness and a relatively conservative nature of the society are key barriers for successful implementation of the vaccination program in India.[60]
Social factors
The median age of initiation of sexual debut in Indian adolescents has been reported to range from 15 to 16 years to 17.37 ± 1.72 years with earliest debut seen as early as 13 years of age.[61] Thus, even in Indian females, the vaccine will be most effective if given at a younger age (prior to expected sexual debut). However, the concept of premarital sexual exposure is taboo in the Indian society and socio-cultural barriers exist to effective communication between physicians and parents regarding the sexual activities of their adolescent girls and boys. Explaining to the parents about importance of prophylactic vaccination of their children and their consent for the same is expected to be a difficult task and would require formulation of guidelines for effective counseling. It needs to be stressed upon that the risk of HPV infection and consequent cancer risk is not necessarily predicted by one's own sexual promiscuity alone as a woman is also at risk because of her partner's past or present sexual activities.[59]
Clinical trials in India
Only two HPV vaccination projects were initiated in India. One was a post-licensure observational study for operational feasibility of school-based and community-based vaccination in Khammam district (Andhra Pradesh, Gardasil®) and Vadodara (Gujarat, Cervarix™), conducted by the State Governments in collaboration with Indian Council of Medical Research and PATH (a US based non-profit non-governmental organization). The other was a multicentric clinical trial to investigate immunogenic efficacy of two doses (6 months apart) compared with conventional three doses (at 0-2-6 months) of Gardasil®, which if found successful would have resulted in 33% cost reduction.[59] Following media allegations of “vaccine-induced” deaths of four girls in Khammam, both studies have been suspended by the Union Government.[62–64] The deaths have since been investigated and confirmed as unrelated to the vaccine.[64] However, the studies have not been resumed (till the time of writing this article). The scepticism for the need and safety of HPV vaccines in the Indian context continues. To achieve effective prevention of HPV infection related morbidity and mortality by vaccination in India, the health authorities and Government should resort to more effective and sympathetic dialog with people to address their reasonable concerns and dispel their fears based on misinformation.[62]
Future trends
The quadrivalent and bivalent vaccines provide only limited cross-protection to development of persistent infection and CIN 2-3/AIS caused by non-vaccine HPV types. Thus, a multivalent vaccine against a multitude of HPVs will be a major breakthrough in providing near-complete prevention of HPV-related diseases, and indeed, efforts to develop a nine-type L1 VLP combination vaccine are ongoing.[14] Preclinical and human volunteer studies have also suggested that immunization against the minor capsid protein 2 with the candidate prophylactic/therapeutic vaccine HPV-16 L2E6E7 might work as a pan-HPV vaccine against different genotypes of HPVs.[65] Development of low-cost vaccines using plant species such as tobacco, potatoes, and tomatoes for the production of VLPs is also underway.[66–68] Therapeutic vaccines incorporating the E6E7 proteins such as the HPV-16 E6E7 ISCOMATRIX vaccine are being investigated for treatment of HPV-related anal intraepithelial neoplasia in HIV-infected men.
CONCLUSIONS
HPV vaccines are safe and efficacious against type-specific HPV-induced anogenital warts, precancerous lesions, and cervical cancer. The vaccines are most effective when given before the onset of sexual activity and provide long-term protection. While new clinical trials and follow-up of older trials will yield more information on issues such as efficacy, safety, duration of protection, need for booster dose, current evidence supports the introduction of HPV vaccination as part of a coordinated strategy to prevent cervical cancer, and other HPV-related diseases. India-specific guidelines need to be based on cost-effectiveness and feasibility of implementing HPV vaccination as a part of national immunisation schedule. Vaccination alone will not be successful unless it is coupled with education about healthy sexual behavior and information about the diagnosis and treatment of precancerous lesions and cancer.
Multiple Choice Questions
-
Q.1
What percentage of invasive cervical cancers are attributable to infection with HPV-16 and 18?
-
a.25%
-
b.55%
-
c.70%
-
d.100%
-
a.
-
Q.2
Which of the following statements regarding the development of immune response to natural HPV infection with oncogenic types in women is incorrect?
-
a.More than 90% develop significant antibody titres
-
b.Antibodies to HPV specifically recognize L1 capsid proteins
-
c.Seroconversion may take upto 18 months
-
d.Antibodies may not be protective against subsequent infection by the same HPV type
-
a.
-
Q.3
The currently recommended route of administration and dosing schedule for the quadrivalent HPV vaccine is
-
a.Subcutaneous; three doses at 0, 1, and 6 months
-
b.Subcutaneous; three doses at 0, 2, and 6 months
-
c.Intramuscular; three doses at 0, 2, and 6 months
-
d.Intramuscular; two doses at 0 and 6 months
-
a.
-
Q.4
The bivalent HPV vaccine is not indicated for the prophylaxis of
-
a.Condyloma acuminata
-
b.CIN 1
-
c.CIN 2/3
-
d.Cervical cancer
-
a.
-
Q.5
An important recommended precaution for physicians administering HPV vaccines is that
-
a.Patient with past history of anogenital warts should not be vaccinated
-
b.Vaccinated individuals should be observed for 15 minutes after the injection
-
c.Vaccination should be deferred in individuals with mild fever
-
d.HIV positive individuals should not be vaccinated
-
a.
Answers
-
1.
c. 70%
-
2.
a. More than 90% develop significant antibody titres
-
3.
c. Intramuscular; three doses at 0, 2, and 6 months
-
4.
a. Condyloma acuminata
-
5.
b. Vaccinated individuals should be observed for 15 minutes after the injection
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.WHO. Vaccines against human papillomavirus. [Last accessed on 2011 June 4]. Available from: http://www.who.int/vaccines/en/hpvrd.shtml/shtml/shtml .
- 2.Forcier M, Musacchio N. An overview of human papillomavirus infection for the dermatologist: Disease, diagnosis, management, and prevention. Dermatol Ther. 2010;23:458–76. doi: 10.1111/j.1529-8019.2010.01350.x. [DOI] [PubMed] [Google Scholar]
- 3.Brown DR, Shew ML, Qadadri B, Neptune N, Vargas M, Tu W, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191:182–92. doi: 10.1086/426867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins S, Mazloomzadeh S, Winter H, Blomfield P, Bailey A, Young LS, et al. High incidence of cervical human papillomavirus infection in women during their first sexual relationship. BJOG. 2002;109:96–8. doi: 10.1111/j.1471-0528.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 5.Hsueh PR. Human papillomavirus, genital warts, and vaccines. J Microbiol Immunol Infect. 2009;42:101–6. [PubMed] [Google Scholar]
- 6.Human papillomavirus vaccines. WHO position paper. Wkly Epidemiol Rec. 2009;84:118–31. [PubMed] [Google Scholar]
- 7.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(Suppl 8):S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 8.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 9.Stanley M. Prophylactic HPV vaccines: Prospects for eliminating ano-genital cancer. Br J Cancer. 2007;96:1320–3. doi: 10.1038/sj.bjc.6603695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiological classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 11.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int J Cancer. 2007;121:621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 12.Lu B, Kumar A, Castellsagué X, Giuliano AR. Efficacy and Safety of Prophylactic Vaccines against Cervical HPV Infection and Diseases among Women: A Systematic Review and Meta- Analysis. BMC Infect Dis. 2011;11:13. doi: 10.1186/1471-2334-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre). Human Papillomavirus and Related Cancers in India. Summary Report. 2010. [Last accessed on 2011 Jun 4]. Available from: http://apps.who.int/hpvcentre/statistics/dynamic/ico/country_pdf/IND.pdf .
- 14.Mariani L, Venuti A. HPV vaccine: An overview of immune response, clinical protection, and new approaches for the future. J Transl Med. 2010;8:105. doi: 10.1186/1479-5876-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanley M, Lowy DR, Frazer I. Prophylactic HPV vaccines: Underlying mechanisms. Vaccine. 2006;24(Suppl 3):S106–13. doi: 10.1016/j.vaccine.2006.05.110. [DOI] [PubMed] [Google Scholar]
- 16.Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181:1911–9. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 17.Bosch FX, de Sanjose S. Chapter 1: Human papilloma virus and cervical cancer burden and assessment of causality. J Natl Cancer Inst Monogr. 2003;31:3–13. doi: 10.1093/oxfordjournals.jncimonographs.a003479. [DOI] [PubMed] [Google Scholar]
- 18.Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine. Vaccine. 2007;25:4931–9. doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 19.Ault KA Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: A combined analysis of four randomized clinical trials. Lancet. 2007;369:1861–8. doi: 10.1016/S0140-6736(07)60852-6. [DOI] [PubMed] [Google Scholar]
- 20.Food and Drug Administration. Product approval-prescribing information [package insert] Gardasil® [human papillomavirus quadrivalent (types 6, 11, 16, and 18) vaccine, recombinant], Merck and Co, Inc: Food and Drug Administration. 2009. [Last accessed on 2011 Jun 4]. Available from: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM094042 .
- 21.Food and Drug Administration. Product approval-prescribing information [package insert] Cervarix™ [human papillomavirus bivalent (types 16 and 18) vaccine, recombinant], GlaxoSmithKline Biologicals: Food and Drug Administration. 2009. [Last accessed on 2011 Jun 4]. Available from: http://www.fda.gov/biologicsbloodvaccines/vaccines/approvedproducts/ucm186957.htm .
- 22.Merck USA. Highlights of prescribing information: GARDASIL® [human papillomavirus quadrivalent (Types 6, 11, 16, and 18) vaccine, Recombinant] 2008. [Last accessed on 2011 Jun 4]. Available from: http://www.merck.com/product/usa/pi_circulars/g/Gardasil®_pi.pdf .
- 23.GlaxoSmithKline Australia. Cervarix™® product information: Human papillomavirus vaccine type 16 and 18 (Recombinant AS04 adjuvanted) 2007. [Last accessed on 2011 Jun 4]. Available from: http://www.gsk.com.au/resources.ashx/vaccineproductschilddataproinfo/94/FileName/7A14FBAEA16635A2DD7A68ED78E8FDDC/P1_Cervarix™.pdf .
- 24.The American Academy of Pediatrics, Committee on Infectious Diseases. Policy statement: Recommended childhood and adolescent immunization schedules – United States, 2010. Pediatrics. 2010;125:195–6. doi: 10.1542/peds.2009-3194. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix™) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2010;59:626–9. [PubMed] [Google Scholar]
- 26.Human papillomavirus (HPV) vaccine: A position statement of the Society for Adolescent Medicine. J Adolesc Health. 2006;39:620. doi: 10.1016/j.jadohealth.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Villa LL. Overview of the clinical development and results of a quadrivalent HPV (types 6, 11, 16, 18) vaccine. Inte J Infect Dis. 2007;11(Suppl 2):S17–25. doi: 10.1016/S1201-9712(07)60017-4. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen C, Petaja T, Strauss G, Rumke HC, Poder A, Richardus JH, et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health. 2007;40:564–71. doi: 10.1016/j.jadohealth.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Schiller JT, Castellsaguéb X, Villac LL, Hildesheimd A. An Update of Prophylactic Human Papillomavirus L1 Virus-Like Particle Vaccine Clinical Trial Results. Vaccine. 2008;26(Suppl 10):K53–61. doi: 10.1016/j.vaccine.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dillner J, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, et al. FUTURE I/II Study Group. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: Randomised controlled trial. BMJ. 2010;341:c3493. doi: 10.1136/bmj.c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. HPV PATRICIA study group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: An interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 32.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 33.The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 34.Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus -16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): Final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 35.Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95:1459–66. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKeage K, Romanowski B. AS04-adjuvanted human papillomavirus (HPV) types 16 and 18 vaccine (Cervarix™®): A review of its use in the prevention of premalignant cervical lesions and cervical cancer causally related to certain oncogenic HPV types. Drugs. 2011;71:465–88. doi: 10.2165/11206820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Fraser C. Modelling the long-term antibody response of a human papillomavirus virus-like particle type 16 prophylactic vaccine. Vaccine. 2007;25:4324–33. doi: 10.1016/j.vaccine.2007.02.069. [DOI] [PubMed] [Google Scholar]
- 38.Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, et al. Comparison of the immunogenicity and safety of Cervarix™ and Gardasil® human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin. 2009;10:705–19. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 39.Smith JF, Brownlow M, Brown M, Kowalski R, Esser MT, Ruiz W, et al. Antibodies from women immunized with Gardasil® cross-neutralize HPV 45 pseudovirions. Hum Vaccin. 2007;3:109–15. doi: 10.4161/hv.3.4.4058. [DOI] [PubMed] [Google Scholar]
- 40.Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J Infect Dis. 2009;199:926–35. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 41.Wheeler CM, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16-26 years. J Infect Dis. 2009;199:936–44. doi: 10.1086/597309. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins D. A review of cross-protection against oncogenic HPV by an HPV-16/18 AS04-adjuvanted cervical cancer vaccine: Importance of virological and clinical endpoints and implications for mass vaccination in cervical cancer prevention. Gynecol Oncol. 2008;110:S18–25. doi: 10.1016/j.ygyno.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 43.Poppe WA, Simon PH, De Ridder MR. Why consider human papillomavirus vaccination in older women? Gynecol Obstet Invest. 2010;70:237–43. doi: 10.1159/000314012. [DOI] [PubMed] [Google Scholar]
- 44.Muñoz N, Manalastas R, Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24-45 years: A randomised, double-blind trial. Lancet. 2009;373:1949–57. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 45.Olsson SE, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009;5:696–704. doi: 10.4161/hv.5.10.9515. [DOI] [PubMed] [Google Scholar]
- 46.Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Jr, Penny ME, Aranda C, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011;364:401–11. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palefsky JM. Human papillomavirus-related disease in men: Not just a women's issue. J Adolesc Health. 2010;46(Suppl):S12–9. doi: 10.1016/j.jadohealth.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palefsky JM. Monte Carlo, Monaco: European Research Organisation on Genital Infection and Neoplasia Conference; 2010. Efficacy of Gardasil in men aged 16-26 years naive to vaccine HPV types at baseline: The latest data. Paper presented at: [Google Scholar]
- 49.Sturgis EM, Dahlstrom KR. HPV vaccination. Inaccurate assumptions about oropharyngeal cancer. BMJ. 2009;339:b4525. doi: 10.1136/bmj.b4525. [DOI] [PubMed] [Google Scholar]
- 50.Nelson B. A new front in the debate over HPV vaccines for boys: Studies hint at broader benefits from Gardasil®. Cancer Cytopathol. 2010;118:413–4. doi: 10.1002/cncy.20128. [DOI] [PubMed] [Google Scholar]
- 51.Wilkin T, Lee JY, Lensing SY, Stier EA, Goldstone SE, Berry JM, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis. 2010;202:1246–53. doi: 10.1086/656320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levin MJ, Moscicki AB, Song LY, Fenton T, Meyer WA, 3rd, Read JS, et al. IMPAACT P1047 Protocol Team.Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune Defic Syndr. 2010;55:197–204. doi: 10.1097/QAI.0b013e3181de8d26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Segal L, Wilby OK, Willoughby CR, Veenstra S, Deschamps M. Evaluation of the intramuscular administration of Cervarix™ vaccine on fertility, pre- and post-natal development in rats. Reprod Toxicol. 2011;31:111–20. doi: 10.1016/j.reprotox.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Forinash AB, Yancey AM, Pitlick JM, Myles TD. Safety of the HPV Bivalent and Quadrivalent Vaccines During Pregnancy. Ann Pharmacother. 2011;45:258–62. doi: 10.1345/aph.1P396. [DOI] [PubMed] [Google Scholar]
- 55.Rambout L, Hopkins L, Hutton B, Fergusson D. Prophylactic vaccination against human papillomavirus infection and disease in women: A systematic review of randomized controlled trials. CMAJ. 2007;177:469–79. doi: 10.1503/cmaj.070948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JJ, Andres-Beck B, Goldie SJ. The value of including boys in an HPV vaccination programme: A cost-effectiveness analysis in a low-resource setting. Br J Cancer. 2007;97:1322–8. doi: 10.1038/sj.bjc.6604023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Techakehakij W, Feldman RD. Cost-effectiveness of HPV vaccination compared with Pap smear screening on a national scale: A literature review. Vaccine. 2008;26:6258–65. doi: 10.1016/j.vaccine.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 58.Goldie SJ, O’Shea M, Diaz M, Kim SY. Benefits, cost requirements and cost-effectiveness of the HPV 16, 18 vaccine for cervical cancer prevention in developing countries: policy implications. Reprod Health Matters. 2008;16:86–96. doi: 10.1016/S0968-8080(08)32409-4. [DOI] [PubMed] [Google Scholar]
- 59.Choudhury P, John TJ. Human papilloma virus vaccines and current controversy. Indian Pediatr. 2010;47:724–5. [PubMed] [Google Scholar]
- 60.Bharadwaj M, Hussain S, Nasare V, Das BC. HPV and HPV vaccination: Issues in developing countries. Indian J Med Res. 2009;130:327–33. [PubMed] [Google Scholar]
- 61.Giri PA, Shirol SB, Kasbe AM. A comparative study to assess the knowledge and practices regarding sexual health among the migrants and non-migrants in Mumbai city. Int J Collab Res Intern Med Public Health. 2011;3:341–52. [Google Scholar]
- 62.Larson HJ, Brocard P, Garnett G. The India HPV-vaccine suspension. Lancet. 2010;376:572–3. doi: 10.1016/S0140-6736(10)60881-1. [DOI] [PubMed] [Google Scholar]
- 63.Anon Update: PATHS's HPV vaccine project in India. 2010. Apr 27, [Last accessed on 2011 Jun 4]. Available from: http://www.path.org/news/an100422-hpvindia.php .
- 64.Sinha K. Four deaths not due to flawed cervical cancer vaccine trial. Times of India. 2010. Apr 09, [Last accessed on 2011 Jun 4]. Available from: http://timesofindia.indiatimes.com/india/Four-deaths-not-due-to-flawed-cervicalcancer-vaccine-trial/articleshow/5776065.cms .
- 65.Gambhira R, Gravitt PE, Bossis I, Stern PL, Viscidi RP, Roden RB. Vaccination of healthy volunteers with human papillomavirus type 16 L2E7E6 fusion protein induces serum antibody that neutralizes across papillomavirus species. Cancer Res. 2006;66:11120–4. doi: 10.1158/0008-5472.CAN-06-2560. [DOI] [PubMed] [Google Scholar]
- 66.Biemelt S, Sonnewald U, Galmbacher P, Willmitzer L, Müller M. Production of human papillomavirus type 16 virus-like particles in transgenic plants. J Virol. 2003;77:9211–20. doi: 10.1128/JVI.77.17.9211-9220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Varsani A, Williamson AL, Rose RC, Jaffer M, Rybicki EP. Expression of Human papillomavirus type 16 major capsid protein in transgenic Nicotiana tabacum cv.Xanthi. Arch Virol. 2003;148:1771–86. doi: 10.1007/s00705-003-0119-4. [DOI] [PubMed] [Google Scholar]
- 68.Warzecha H, Mason HS, Lane C, Tryggvesson A, Rybicki E, Williamson AL, et al. Oral immunogenicity of human papillomavirus-like particles expressed in potato. J Virol. 2003;77:8702–11. doi: 10.1128/JVI.77.16.8702-8711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


