ABSTRACT
Understanding the molecular pathogenesis of Coxiella burnetii, the causative agent of human Q fever, has historically been hindered by the technical difficulties of genetically manipulating obligate intracellular bacteria. The recent development of culture conditions suitable for axenic propagation of C. burnetii has paved the way for the application of a range of genetic techniques to address key questions within the field. Recent studies using mutational analysis have revealed that the C. burnetii Dot/Icm type 4 secretion system (T4SS) is an important virulence determinant that is essential for renovation of a lysosome into a mature Coxiella-containing vacuole (CCV) permissive of intracellular replication. Interestingly, a mutant of C. burnetii deficient in Dot/Icm function was found to be capable of replicating within the parasitophorous vacuole created by Leishmania amazonensis, which indicates that C. burnetii replication is not dependent on the cohort of Dot/Icm effector proteins per se but rather that the collective actions of effectors are required to create the specialized niche supportive of replication. Thus, a role for the Dot/Icm T4SS during the intracellular life cycle of C. burnetii has been more clearly defined by these studies, which demonstrate that advances in genetic analysis should allow future studies to focus on the intricacies of Dot/Icm effector functions that facilitate development of the unique CCV.
Commentary
Many intracellular bacterial pathogens have specialized secretion systems that deliver into host cells a specific catalogue of effector proteins that utilize a wide range of mechanisms to modulate cellular functions. Host cell manipulation allows the bacteria to model a specific intracellular niche compatible with bacterial replication. A sophisticated example of this is the type 4 secretion system (T4SS) termed Dot and Icm (1). The intracellular pathogens C. burnetii and Legionella pneumophila encode functionally analogous versions of the Dot/Icm system (2, 3). It is well established that the Dot/Icm system is essential for the ability of L. pneumophila to modulate transport of the vacuole in which the pathogen resides to evade lysosomal killing and create a specialized organelle derived from the endoplasmic reticulum (4). Comprehensive investigations of the importance of the Dot/Icm system in the intracellular survival and establishment of the unique Legionella-containing vacuole suggested that the Dot/Icm system is also a crucial virulence feature of C. burnetii. This hypothesis provided the impetus for several studies that aimed to identify and characterize putative effector proteins delivered into host cells by the C. burnetii Dot/Icm system, and over 60 different C. burnetii proteins have now been identified as translocated substrates of the Dot/Icm system (5–10). Yet, until recently, definitive roles for the C. burnetii Dot/Icm system during infection had not been demonstrated.
The development of a complex nutrient medium, acidified citrate cysteine medium (ACCM), and of culture parameters that mimic the low pH and reduced oxygen conditions within the CCV has resulted in the creation of conditions suitable for axenic growth of C. burnetii (11, 12). Axenic cultivation has facilitated the development of genetic tools to allow researchers in the field to finally address key questions regarding the pathogenesis and intracellular propagation of C. burnetii (13, 14). These advances enabled Beare et al. to use a genetic approach to address the importance of the Dot/Icm system during intracellular infection, and the results were presented in the July/August 2011 issue of mBio (5). The authors of that study isolated a C. burnetii mutant with a transposon insertion in icmD, a gene predicted to encode an essential inner membrane component of the Dot/Icm system. Analysis of the interaction between the icmD mutant and macrophage-like THP-1 cells has demonstrated that a functional Dot/Icm system is required for the translocation of Dot/Icm substrates, including the plasmid-encoded proteins CpeD and CpeE. Importantly, Dot/Icm function was found to be necessary for development of the large CCV, productive intracellular replication, and apoptosis protection of infected THP-1 cells. These data correlate nicely with those from another recent study demonstrating that a transposon insertion in the C. burnetii icmL gene abolished effector translocation and intracellular replication of C. burnetii in mammalian cells (6). Both studies demonstrated that the C. burnetii Dot/Icm system is not required for axenic growth or uptake by host cells.
Beare et al. developed an elegant genetic technique to demonstrate complementation of the icmD mutant that entailed using a Tn7-based transposon system to introduce the icmDJB operon into the chromosome in the glmS-CBU1788 intergenic region (5). Placing this operon under the control of an anhydrotetracycline-inducible promoter allowed investigators to examine the temporal requirements for expression of the Dot/Icm system. Strikingly, replication of the icmD mutant strain was achieved when icmDJB expression was induced 24 h after infection. This finding demonstrates that, in contrast to L. pneumophila, which requires translocation of effectors by the Dot/Icm system within minutes of uptake to promote vacuole remodeling prior to endocytic maturation (15), C. burnetii has adapted to withstand the antimicrobial activities of a lysosomal environment and can retain intracellular viability within a nonpermissive lysosome independently of Dot/Icm function. Thus, the Dot/Icm system can somehow change the nature of a phagolysosome to make it permissive for C. burnetii replication.
Characterization of C. burnetii Dot/Icm-deficient strains has allowed the comparison and contrast of both the temporal requirements and functional outcomes of the Dot/Icm systems in L. pneumophila and C. burnetii. There are key features shared by these two systems; for example, both L. pneumophila and C. burnetii Dot/Icm-deficient strains can replicate in vacuoles shared by Dot/Icm-competent counterparts (5, 16), which highlights the observation that the effectors translocated by the Dot/Icm system are needed to create a vacuolar environment that is generally permissive for replication of the bacteria. Genome analysis of L. pneumophila and C. burnetii revealed extensive plasticity in the effectors produced by different strains of these organisms (17, 18), suggesting that, as with L. pneumophila (19), a significant degree of functional redundancy is likely built into the C. burnetii Dot/Icm substrate repertoire.
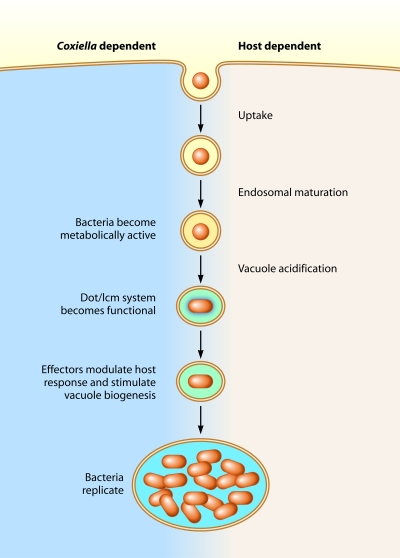
Comparative analysis revealed informative differences between the two Dot/Icm systems. The L. pneumophila Dot/Icm system is needed for the bacteria to immediately subvert host membrane transport pathways; because of this, the Dot/Icm system of L. pneumophila initiates effector translocation as soon as the extracellular bacteria make intimate contact with the plasma membrane of the host cell (20, 21). Because C. burnetii can utilize the host machinery for uptake and transport of the vacuole in which it resides through the endocytic pathway (22, 23), the bacteria should not need Dot/Icm-dependent functions to direct early membrane transport (Fig. 1). Consistent with this hypothesis, it appears that the C. burnetii Dot/Icm system is not functional until the bacteria have established residence in an acidified lysosome-derived vacuole several hours after uptake (6). L. pneumophila studies have shown that the ability of the bacteria to use the Dot/Icm system to communicate with the host cytosol to direct membrane transport leads to rapid detection by mammalian pattern recognition receptors in the cytosol that trigger a very robust innate immune response (24, 25). Thus, it is tempting to speculate that C. burnetii has evolved mechanisms to regulate the Dot/Icm system so that it does not deliver effectors during early endocytic transport stages; in this way, the bacteria may avoid innate immune signaling pathways that operate to detect pathogens with specialized secretion systems during the invasion process.
FIG 1 .
Host and pathogen functions important for C. burnetii infection.
Infection by C. burnetii is initiated though subversion of host cellular processes. Small-cell variants of C. burnetii (red circles) that are metabolically inactive represent the infectious forms internalized by host cells. Host cellular processes direct endocytosis of attached bacteria, and the resulting CCV matures along the endocytic pathway through sequential membrane fusion events. Acidification of the vacuole is a signal that stimulates C. burnetii to become metabolically active and develop into replicative forms called large-cell variants (red ovals). It is at this stage that translocation of bacterial effector proteins into the host cell mediated by the Dot/Icm T4SS can be detected. Analysis of C. burnetii mutants with a defective Dot/Icm function suggest that the combined activities of the effectors translocated into host cells by the Dot/Icm system are essential for remodeling of the CCV to form a spacious and fusogenic vacuole that permits C. burnetii replication and for blocking innate defenses of the host, such as apoptosis. Thus, there are several Coxiella-dependent features of the vacuole that require effector translocation by the Dot/Icm system.
The finding that the icmD mutant is able to replicate in a Leishmania amazonensis vacuole is both intriguing and enlightening. It was observed previously that C. burnetii and the trypanosomatid parasite L. amazonensis are able to productively inhabit the same vacuole (26). Here, the ability of Dot/Icm-deficient C. burnetii to replicate in the complete absence of the C. burnetii repertoire of Dot/Icm effector proteins implies that the environment within the lumen of the L. amazonensis parasitophorous vacuole must closely mimic that of the CCV and/or of ACCM and that the Dot/Icm-dependent activities needed to promote replication of C. burnetii are provided by L. amazonensis. It is clear that both L. amazonensis and C. burnetii do not alter the lysosomal pH or hydrolytic capacity of their respective replicative vacuoles and that both pathogens reside in fusogenic vacuoles reminiscent of a lysosome with a low luminal pH. It is plausible that L. amazonensis and Dot/Icm-competent C. burnetii both mediate lumenal alterations to either block an as-yet-undefined antimicrobial function of the lysosome or promote a new lysosomal activity that creates an environment conducive to replication. Another explanation for how the L. amazonensis vacuole allows Dot/Icm-independent C. burnetii replication is that it may provide specific nutritional requirements required for growth. Both organisms have several amino acid auxotrophies, which indicates that they likely modulate the intracellular environment to salvage essential metabolites (27, 28). For both organisms, it is presumed that the replicative vacuole is a nutritionally complex compartment containing a range of carbon sources, particularly amino acids, acquired by the continual fusion of the vacuole with a variety of organelles in the secretory and endolysosomal systems. These data may present an opportunity to extrapolate the finding that Dot/Icm effector proteins that act to facilitate the fusogenicity of the CCV also enable C. burnetii replication by providing essential nutrients. In addition, understanding the shared metabolic requirements of C. burnetii and L. amazonensis may help define the minimal requirements required for replication of each organism.
The advent of axenic culture and genetic tools for C. burnetii investigations has opened to an array of new possibilities and increased the understanding of the researchers in the field of how this intracellular pathogen interacts with its host. The confirmation that the Dot/Icm system plays an integral role in this host-pathogen interaction now paves the way for additional specialized studies of the functions of individual effector proteins in modulating the intracellular environment.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI064559 and AI041699 and Northeast Biodefense Center grant U54-AI057158.
Footnotes
Citation Newton HJ, Roy CR. 2011. The Coxiella burnetii Dot/Icm system creates a comfortable home through lysosomal renovation. mBio 2(5):e00226-11. doi:10.1128/mBio.00226-11.
REFERENCES
- 1. Nagai H, Kubori T. 2011. Type IVB secretion systems of Legionella and other Gram-negative bacteria. Front. Microbiol. 2:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zamboni DS, McGrath S, Rabinovitch M, Roy CR. 2003. Coxiella burnetii express type IV secretion system proteins that function similarly to components of the Legionella pneumophila Dot/Icm system. Mol. Microbiol. 49:965–976 [DOI] [PubMed] [Google Scholar]
- 3. Zusman T, Yerushalmi G, Segal G. 2003. Functional similarities between the icm/dot pathogenesis systems of Coxiella burnetii and Legionella pneumophila. Infect. Immun. 71:3714–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hubber A, Roy CR. 2010. Modulation of host cell function by Legionella pneumophila type IV effectors. Annu. Rev. Cell Dev. Biol. 26:261–283 [DOI] [PubMed] [Google Scholar]
- 5. Beare PA, et al. 2011. Dot/Icm type IVB secretion system requirements for Coxiella burnetii growth in human macrophages. mBio 2:e00175-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carey KL, Newton HJ, Luhrmann A, Roy CR. 2011. The Coxiella burnetii Dot/Icm system delivers a unique repertoire of type IV effectors into host cells and is required for intracellular replication. PLoS Pathog. 7:e1002056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen C, et al. 2010. Large-scale identification and translocation of type IV secretion substrates by Coxiella burnetii. Proc. Natl. Acad. Sci. U. S. A. 107:21755–21760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan X, Lührmann A, Satoh A, Laskowski-Arce MA, Roy CR. 2008. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science 320:1651–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Voth DE, et al. 2011. The Coxiella burnetii cryptic plasmid is enriched in genes encoding type IV secretion system substrates. J. Bacteriol. 193:1493–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Voth DE, et al. 2009. The Coxiella burnetii ankyrin repeat domain-containing protein family is heterogeneous, with C-terminal truncations that influence Dot/Icm-mediated secretion. J. Bacteriol. 191:4232–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Omsland A, Cockrell DC, Fischer ER, Heinzen RA. 2008. Sustained axenic metabolic activity by the obligate intracellular bacterium Coxiella burnetii. J. Bacteriol. 190:3203–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Omsland A, et al. 2009. Host cell-free growth of the Q fever bacterium Coxiella burnetii. Proc. Natl. Acad. Sci. U. S. A. 106:4430–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beare PA, et al. 2009. Characterization of a Coxiella burnetii ftsZ mutant generated by Himar1 transposon mutagenesis. J. Bacteriol. 191:1369–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beare PA, Sandoz KM, Omsland A, Rockey DD, Heinzen RA. 2011. Advances in genetic manipulation of obligate intracellular bacterial pathogens. Front. Microbiol. 2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roy CR, Berger KH, Isberg RR. 1998. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 28:663–674 [DOI] [PubMed] [Google Scholar]
- 16. Coers J, Monahan C, Roy CR. 1999. Modulation of phagosome biogenesis by Legionella pneumophila creates an organelle permissive for intracellular growth. Nat. Cell Biol. 1:451–453 [DOI] [PubMed] [Google Scholar]
- 17. Beare PA, et al. 2009. Comparative genomics reveal extensive transposon-mediated genomic plasticity and diversity among potential effector proteins within the genus Coxiella. Infect. Immun. 77:642–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cazalet C, et al. 2004. Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat. Genet. 36:1165–1173 [DOI] [PubMed] [Google Scholar]
- 19. O’Connor TJ, Adepoju Y, Boyd D, Isberg RR. 2011. Minimization of the Legionella pneumophila genome reveals chromosomal regions involved in host range expansion. Proc. Natl. Acad. Sci. U. S. A. 108:14733–14740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Charpentier X, et al. 2009. Chemical genetics reveals bacterial and host cell functions critical for type IV effector translocation by Legionella pneumophila. PLoS Pathog. 5:e1000501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagai H, et al. 2005. A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc. Natl. Acad. Sci. U. S. A. 102:826–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aguilera M, et al. 2009. Actin dynamics and Rho GTPases regulate the size and formation of parasitophorous vacuoles containing Coxiella burnetii. Infect. Immun. 77:4609–4620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Romano PS, Gutierrez MG, Berón W, Rabinovitch M, Colombo MI. 2007. The autophagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell. Microbiol. 9:891–909 [DOI] [PubMed] [Google Scholar]
- 24. Shin S, et al. 2008. Type IV secretion-dependent activation of host MAP kinases induces an increased proinflammatory cytokine response to Legionella pneumophila. PLoS Pathog. 4:e1000220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shin S, Roy CR. 2008. Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell. Microbiol. 10:1209–1220 [DOI] [PubMed] [Google Scholar]
- 26. Veras PS, et al. 1995. Entry and survival of Leishmania amazonensis amastigotes within phagolysosome-like vacuoles that shelter Coxiella burnetii in Chinese hamster ovary cells. Infect. Immun. 63:3502–3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McConville MJ, Naderer T. Metabolic pathways required for the intracellular survival of Leishmania. Annu. Rev. Microbiol., in press [DOI] [PubMed] [Google Scholar]
- 28. Omsland A, et al. 2011. Isolation from animal tissue and genetic transformation of Coxiella burnetii are facilitated by an improved axenic growth medium. Appl. Environ. Microbiol. 77:3720–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]