Abstract
The kynurenine pathway (KP) is the principle route of L-Tryptophan (TRP) metabolism, producing several neurotoxic and neuroprotective metabolic precursors before complete oxidation to the essential pyridine nucleotide nicotinamide adenine dinucleotide (NAD+). KP inhibition may prove therapeutic in central nervous system (CNS) inflammation by reducing the production of excitotoxins such as quinolinic acid (QUIN). However, KP metabolism may also be cytoprotective through the de novo synthesis of intracellular NAD+. We tested the hypothesis that the KP is directly involved in the maintenance of intracellular NAD+ levels and SIRT1 function in primary astrocytes and neurons through regulation of NAD+ synthesis. Competitive inhibition of indoleamine 2,3 dioxygenase (IDO), and quinolinic acid phosphoribosyltransferase (QPRT) activities with 1-methyl-L-Tryptophan (1-MT), and phthalic acid (PA) respectively, resulted in a dose-dependent decrease in intracellular NAD+ levels and sirtuin deacetylase-1 (SIRT1) activity, and correlated directly with reduced cell viability. These results support the hypothesis that the primary role of KP activation during neuroinflammation is to maintain NAD+ levels through de novo synthesis from TRP. Inhibition of KP metabolism under these conditions can compromise cell viability, NAD-dependent SIRT1 activity and CNS function, unless alternative precursors for NAD+ synthesis are made available.
Keywords: IDO, NAD+, sirtuins, astrocytes neurons 1-MT
Introduction
In recent years, the kynurenine pathway (KP) has generated considerable interest following the observation that KP metabolites may have significant and opposing actions on central neurons.1–3 One consistent finding in all neuroinflammatory diseases is a dramatic, immune-mediated increase in tryptophan (TRP) catabolism via the KP.4–10 In an effort to determine whether this biochemical phenomenon is related to the development of neuropathology, a number of recent studies have investigated the neurotoxic potential of the TRP metabolite quinolinic acid (QUIN) in inflammatory neurological disease such as Alzheimer’s disease (AD),11,12 Huntington’s disease (HD),13,14 Amyotropic Lateral Sclerosis (ALS),15 AIDS Dementia Complex (ADC)16,17 and multiple sclerosis (MS).18 As a result, the KP has been identified as a likely target for pharmaceutical intervention to perhaps slow down or prevent neuronal dysfunction associated with neuroinflammatory disease.19–23
A major aim is to attempt to rectify the balance of the KP with available inhibitors. However, the effect of KP inhibition on the biosynthesis of the ubiquitous pyridine nucleotide, nicotinamide adenine dinucleotide (NAD+) needs also to be evaluated. The KP has been established as the de novo pathway for NAD+ synthesis in the liver and kidney.22 We have recently shown that some KP metabolites, including 3-hydroxyanthranilic acid (3-HAA), 3-hydroxykynurenine (3-HK), and QUIN can promote NAD+ synthesis at nanomolar concentrations in human primary astrocytes and neurons.23,24 Moreover, NAD+ concentrations can be regenerated in rat-derived astrocytes using nicotinic acid (NA), nicotinamide (NM) or QUIN following H2O2 mediated NAD+ depletion.25 Given the importance for the KP for NAD+ synthesis, pharmacological modulation of the KP will significantly affect intracellular NAD+ levels.
NAD+ acts as an essential cofactor for several enzyme catalysed reactions including alcohol, lactate and amino acid metabolism. NAD+ also serves as an electron transporter to power oxidative phosphorylation and ATP production.26 In genomic DNA, NAD+ is the sole substrate for the DNA nick sensor, poly(ADP-ribose) polymerase, (PARP).27 The PARP family of enzymes, particularly PARP-1, are DNA binding enzymes activated by free-radical mediated DNA strand breaks and play a crucial role in base excision repair.27,28 In addition to its role in PARP activity, NAD+ also serves as a substrate for a new class of enzymes known as sirtuins, or silent information regulator of gene transcription.29 SIRT1, the founding member of the sirtuin family of protein, has been shown to regulate gene silencing, and promote longevity.30
Given the importance of KP metabolism in de novo NAD+ synthesis in human brain cells, we tested the effect of KP inhibition on intracellular NAD+ levels and NAD-dependent SIRT1 activity in glial and neuronal cells. More specifically, we tested the hypothesis that indoleamine 2,3 dioxygenase (IDO), and quinolinic acid phosphoribosyltransferase (QPRT) activities, two important KP enzymes, can significantly regulate intracellular NAD+ synthesis and NAD-dependent SIRT1 activity in human astrocytes and neurons. Results from this study indicate a strong dependence on KP metabolism through IDO and QPRT regulation for the maintenance of NAD+ production and SIRT1 function. Therefore, caution should be advised when administering pharmacological inhibitors to TRP metabolism during neuroinflammatory conditions.
Materials and Methods
Reagents and chemicals
Dulbecco’s phosphate buffer solution (DBPS) and all other cell culture media and supplements were from Invitrogen (Melbourne, Australia) unless otherwise stated. Nicotinamide, bicine, β-nicotinamide adenine dinucleotide reduced form (β-NADH), 3-[-4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT), alcohol dehydrogenase (ADH), sodium pyruvate, TRIS, γ-globulins, L-tryptophan (TRP), 1-methyl-L-TRPtophan (1-MT), phthalic acid (PA), and catalase were obtained from Sigma-Aldrich (Castle-Hill, Australia). Phenazine methosulfate (PMS) was obtained from ICN Biochemicals (Ohio, USA). Bradford reagent was obtained from BioRad, Hercules (CA, USA).
Cell cultures
Human foetal brains were obtained from 16–19 week old foetuses collected following therapeutic termination with informed consent. Mixed brain cultures were prepared and maintained using a protocol previously described by Guillemin et al.31 Astrocytes and neurons were prepared from the mixed brain cell cultures, and maintained using a protocol previously described by Guillemin et al.32
KP inhibition in astrocytes and neurons
For IDO, QPRT and LDH activities, human primary astrocytes and neurons were incubated 10 μM, 100 μM, and 1000 μM 1-MT and PA, respectively (Table 1). For intracellular NAD+ concentrations, 1 μM, 10 μM, 50 μM, 100 μM, and 1000 μM of inhibitors was used. SIRT1 activity was measured using 100 μM of 1-MT and PA. Cultures were then incubated at 37 °C in 5% CO2 for 24 hours before analysis of IDO and QPRT activities, intracellular NAD+ levels, and extracellular LDH and SIRT1 activities. Experiments were performed with primary cultures derived from three different human foetal brains with each individual preparation tested in triplicate.
Table 1.
Inhibitors used in these experiments.
| Inhibitor | Chemical Structure | Action |
|---|---|---|
| 1-methyl-L-Tryptophan (1-MT) |  |
Competitive IDO inhibitor |
| Phthalic acid (PA) |  |
Competitive QPRT inhibitor |
NAD(H) microcycling assay
Intracellular NAD+ concentration following 24 hour incubation with the desired concentrations of KP inhibitors were measured spectrophotometrically using the thiazolyl blue microcycling assay established by Bernofsky and Swan33 adapted for 96 well plate format by Grant and Kapoor.25
Extracellular LDH activity
LDH activity following 24 hour incubation with the desired concentrations of KP inhibitors was assayed using a standard spectrophotometric technique described by Koh and Choi (1987).34
Indoleamine 2,3 dioxygenase activity
IDO activity was evaluated as previously described by.35 Briefly, cellular homogenates were homogenised at 4 °C with a Teflon pestle, resuspended in 50 mM K2HPO4/KH2PO4 buffer solution (pH 6.5), and centrifuged at 12,000 g for 30 min. The reaction mixture contained 20 mM ascorbic acid, 50 μM methylene blue, 200 μg catalase and 2 mM TRP. After adding 20 μg of cellular enzyme, the mixtures were incubated at 37 °C for 30 minutes. The product formed was read at 405 nm using the Model 680XR microplate reader (BioRad, Hercules, CA, USA).
Quinolinic acid phosphoribosyl transferase activity
QPRTase activity was determined by measuring the formation of nicotinic acid mononucleotide (NAMN) using a continuous UV spectrophotometric assay as previously described by Rahman et al (2009).36
SIRT1 deacetylase activity
SIRT1 deacetylase activity was evaluated on cellular homogenate using the Cyclex SIRT1/Sir2 Deacetylase Flourometric Assay Kit (CycLex, Nagano, Japan).
Bradford protein assay for the quantification of total protein
NAD+ concentration and extracellular LDH activity were adjusted for variations in cell number using the Bradford protein assay described by Bradford.37
Data analysis
Results obtained are presented as the means ± the standard error of the mean (SEM). Significant differences were verified using the two-tailed t-test with equal variance and 1-WAY ANOVA. Differences between treatment groups were considered significant if P was less than 0.05 (P < 0.05).
Results
Effect of 1-MT and PA on IDO and QPRT activities in human astrocytes and neurons
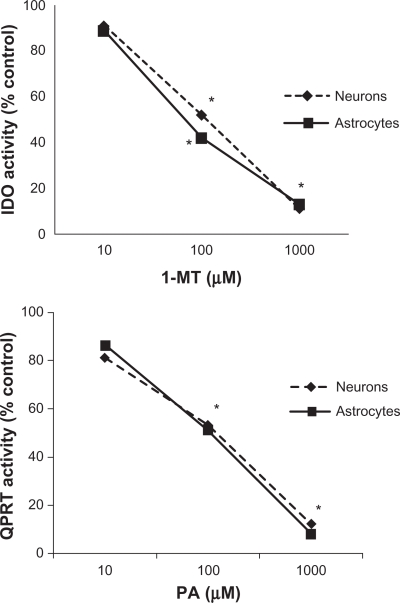
Consistent with previous studies, a dose-dependent inhibition of IDO activity was observed following treatment with 1-MT, a competitive inhibitor of IDO (Fig. 1A). A maximum reduction in IDO activity was observed in astrocytes and neurons treated with 1 mM 1-MT. Similarly, a dose-dependent inhibition of QPRT activity was reported following treatment with PA (Fig 1B). Likewise, a maximum inhibitory response was observed in human brain cells treated with 1 mM PA.
Figure 1.
A) Effect of 1-MT on IDO activity in human astrocytes and neurons. A dose-dependent inhibition of IDO activity was observed following treatment with 1-MT in human astrocytes and neurons. For astrocytes, no 1-MT (control) = 35.86 nmol kynurenine/hr/mg protein; 10 μM 1-MT = 31.15 ± 5.61 nmol kynurenine/hr/mg protein; 100 μM 1-MT = 14.70 ± 4.85 nmol kynurenine/hr/mg protein; 1000 μM 1-MT = 4.55 ± 1.93 nmol kynurenine/hr/mg protein; Significance *P < 0.05 compared to previous dose (n = 4 for each treatment group). For neurons, no 1-MT (control) = 27.22 ± 7.28 nmol kynurenine/hr/mg protein; 10 μM 1-MT = 24.77 ± 6.74 nmol kynurenine/hr/mg protein; 100 μM 1-MT = 14.15 ± 2.94 nmol kynurenine/hr/mg protein; 1000 μM 1-MT = 2.99 ± 1.42 nmol kynurenine/hr/mg protein; Significance *P < 0.05 compared to previous dose (n = 4 for each treatment group). B) PA on QPRT activity in human astrocytes and neurons. A dose-dependent inhibition of QPRT activity was observed following treatment with PA in human astrocytes and neurons. For astrocytes, no PA (control) = 41.33 ± 8.32 nmol kynurenine/hr/mg protein; 10 μM PA = 35.54 ± 3.22 nmol kynurenine/hr/mg protein; 100 μM PA = 21.08 ± 7.39 nmol kynurenine/hr/mg protein; 1000 μM PA = 3.31 ± 1.32 nmol kynurenine/hr/mg protein; Significance *P < 0.05 compared to previous dose (n = 4 for each treatment group). For neurons, no PA (control) = 21.55 ± 3.62 nmol kynurenine/hr/mg protein; 10 μM PA = 17.46 ± 3.49 nmol kynurenine/hr/mg protein; 100 μM PA = 11.42 ± 3.11 nmol kynurenine/hr/mg protein; 1000 μM PA = 2.59 ± 0.81 nmol kynurenine/hr/mg protein; Significance *P < 0.05 compared to previous dose (n = 4 for each treatment group).
Effect of 1-MT and PA on intracellular NAD+ levels in human astrocytes and neurons
Importantly, the effect of decreasing IDO and QPRT activities on intracellular NAD+ levels in these cell types was highly correlated. NAD+ levels declined in a dose-dependent manner with increasing concentrations of 1-MT (Fig. 2A) and PA (Fig. 2B) respectively after 24 hours incubation.
Figure 2.
Effect of (A) 1-MT and (B) PA on intracellular NAD+ levels in human astrocytes and neurons. NAD+ levels significantly declined in a dose-dependent manner with increasing concentrations of (A) 1-MT and (B) PA respectively following 24 hours incubation with the selected inhibitor. Significance *P < 0.05 compared to previous dose (n = 4 for each treatment group).
Effect of 1-MT and PA on cellular viability in human astrocytes and neurons
The release of lactate dehydrogenase (LDH) into culture supernatant correlates with the amount of cell death and membrane damage, providing an accurate measure of cellular toxicity. Showing an inverse correlation with intracellular NAD+ levels, extracellular LDH activity was significantly increased with increased concentrations of either 1-MT (Fig. 3A), or PA (Fig. 3B) in both astrocytes and neurons after 24 hours incubation.
Figure 3.
Effect of (A) 1-MT and (B) PA on extracellular LDH activity in human astrocyte and neuron cultures. Extracellular LDH activities were significantly elevated in a dose-dependent manner with increasing concentrations of (A) 1-MT and (B) PA respectively, following 24 hours incubation with the selected inhibitor. Significance *P < 0.05 compared to previous dose (n = 4 for each treatment group).
Effect of 1-MT and PA on SIRT1 activity in human astrocytes and neurons
Reduced SIRT1 activity has been shown to decrease longevity in a range of organisms.
Addition of either 1-MT or PA at 100 μM significantly reduced SIRT1 activity in human astrocytes and neurons (Fig 4). 1-MT reduced SIRT1 activity by 51.4% and 56.8% in human astrocytes and neurons. In a similar fashion, PA reduced SIRT1 activity by 50.9% and 57.4% in human astrocytes and neurons. To help verify that the results obtained for enzyme activity were specific for SIRT1, we tested the effect of sirtinol, a synthetic SIRT1 inhibitor on SIRT1 activity.38 Our data shows that in the presence of sirtinol SIRT1 activity declined by 90% and 84% in human astrocytes and neurons.
Figure 4.
Effect of 1-MT and PA on SIRT1 activity in human astrocytes and neurons. Treatment with 1-MT or PA at 100 μM significantly reduced SIRT1 activity in human astrocytes and neurons. Significance *P < 0.05 compared to control (n = 4 for each treatment group).
Discussion
Numerous studies have shown that NAD+ turnover is increased during chronic oxidative stress and neuroinflammation.25,39–41 As neuroinflammation is associated with chronic increase in ROS activity, marked microglial infiltration, and subsequent PARP activation, NAD+ turnover is likely to be significantly increased.25,39–41 Therefore, a better understanding of the de novo NAD+ biosynthetic pathway in human brain cells will provide a basis for the regeneration of NAD+. To our knowledge, the role of the KP in NAD+ synthesis has not been studied in the CNS. If the KP is essential for NAD+ synthesis, then it is conceivable that inhibition will result in a decrease in NAD+ in human brain cells.
In this study, we have shown for the first time, that competitive inhibition of IDO and QPRT, in both astrocytes and neurons results in a dose-dependent decrease in intracellular NAD+, highlighting the essential role of the KP in NAD+ production in human brain cells. KP inhibition leads to a decrease in NAD+ synthesis and a dose dependent increase in extracellular LDH activity indicating reduced cell viability and increased cell death. Importantly, while IDO and QPRT activites appear to be inhibited equally in both astrocytes and neurons, intracellular NAD+ levels and cell viability are significantly higher in astrocytes than neurons, suggesting that changes in KP metabolism have a greater effect on the neuronal population compared to glial cells.
As NAD+ serves as a substrate for SIRT1 activity, we have also shown herein that inhibition of KP metabolism can lead to a significant decline in SIRT1 activity in the astrocytes and neurons. This data supports an earlier study in which we showed that primary human astrocytes cultured in media deficient in TRP, NA and NM resulted in a 50% decrease in intracellular NAD+ levels after 24 hours.43 The decrease in NAD+ was partially ameliorated following supplementation of the culture media with TRP or NAD+ salvage pathway precursors, NA or NM.43
In mammalian cells, IDO represents the primary enzyme for oxidative TRP catabolism to kynurenine via the KP in both astrocytes, and neurons.44 Interferon-β1, an activator of IDO is currently used for the treatment of relapse-remitting MS due to the importance of the KP in promoting adaptive immunity through IDO-mediated down-regulation of T-cell proliferation. However, overactivation of the KP may lead to increased levels of QUIN and other neurotoxic metabolites generated by perivascular macrophages. Indeed, IDO over-expression and the accumulation of the neurotoxic TRP metabolite, QUIN has been implicated not only in the pathogenesis of MS, but also in the neurological deficits observed in later stages of the disease.18 Therefore, inhibition of IDO has been suggested as a potential therapeutic target to reduce QUIN toxicity in the MS brain.45 However, in a mouse model for MS, daily application of the IDO inhibitor, 1-MT aggravated disease progression indicating that IDO inhibition exacerbates disease.46 This is supported by our results showing that IDO inhibition reduces NAD+ synthesis and SIRT1 function, and therefore promoting cell death.
QPRT converts QUIN to NAMN and carbon dioxide in the presence if Mg2+ and 5-phosphoribosyl-1-pyrophosphate (PRPP).44 In the brain, QPRT is one of the rate-limiting enzymes of NAD+ synthesis from TRP, and therefore likely to influence QUIN levels in the CNS.47 We have previously shown that human cerebral neurons can take up exogenous QUIN but can only catabolise a small amount.48 This may be likely due to the rapid saturation of QPRT. Indeed we have shown that neuronal QPRT activity is saturated when QUIN concentration exceeds 500 nM.36 Thus, QPRT activity is essential for the maintenance of cellular energy metabolism and DNA repair. A reduction in QPRT activity can be envisioned to lead to an accumulation of QUIN, and likely to induce a cytotoxic cascade within astrocytes and neurons.31
As previously mentioned, SIRT1 belongs to a highly conserved gene family known as sirtuins, which encode NAD+-dependent histone and non-histone deacetylases promoting DNA stability and improved lifespan in yeasts and small mammals.29 SIRT1 also regulates the acetylation of a number of transcription factors, including the peroxisome proliferator-activated receptor-γ (PPAR γ), p53, and the FOXO family of transcription factors, all of which represent key metabolic regulators.49 Our data shows that SIRT1 activity goes hand in hand with those of NAD+ metabolism, suggesting a causal relationship between SIRT1 function and NAD+ synthesis. Other NAD+-dependent targets present in human brain cells include the cytosolic SIRT2, which is known to deacetylate tubulin, and the mitochondrial sirtuins (SIRT3, SIRT4, and SIRT5).50 Additional work is needed to determine the effect of KP inhibition on the activity of these proteins. However, as NAD+ is the substrate for sirtuins, it is likely that inhibition of IDO and QPRT may also negatively impact on the function of these proteins.
Given the importance of the KP for de novo synthesis of NAD+, the current study suggests that KP inhibition should be carried out with caution. While we have shown that IDO and QPRT inhibition can deplete intracellular NAD+ levels and reduce cell viability under normal physiological conditions, another study has shown a similar affect on NAD+ levels following IDO inhibition in primary murine astrocytes stimulated with IFN-γ, leading to a similar effect on cell viability.42 In the light of the growing importance of glial cell function and neuronal activity, inhibiting KP metabolism may be deletorious to de novo NAD+ synthesis and CNS function unless alternative precursors are made available.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Stone TW. Kynurenines in the CNS: from endogenous obscurity to therapeutic importance. Prog Neurobiol. 2001;64:185–218. doi: 10.1016/s0301-0082(00)00032-0. [DOI] [PubMed] [Google Scholar]
- 2.Stone TW. Purines and neuroprotection. Adv Exp Med Biol. 2002;513:249–80. doi: 10.1007/978-1-4615-0123-7_9. [DOI] [PubMed] [Google Scholar]
- 3.Heyes MP. The kynurenine pathway and neurologic disease. Therapeutic strategies. Adv Exp Med Biol. 1996;398:125–9. doi: 10.1007/978-1-4613-0381-7_20. [DOI] [PubMed] [Google Scholar]
- 4.Tuppo EE, Arias HR. The role of inflammation in Alzheimer’s disease. Int J Biochem Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 6.Stone TW. Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends Pharmacol Sci. 2000;21:149–54. doi: 10.1016/s0165-6147(00)01451-6. [DOI] [PubMed] [Google Scholar]
- 7.Stone TW. Endogenous neurotoxins from Tryptophan. Toxicon. 2001;39:61–73. doi: 10.1016/s0041-0101(00)00156-2. [DOI] [PubMed] [Google Scholar]
- 8.Stone TW, Addae JI. The pharmacological manipulation of glutamate receptors and neuroprotection. Eur J Pharmacol. 2002;447:285–96. doi: 10.1016/s0014-2999(02)01851-4. [DOI] [PubMed] [Google Scholar]
- 9.Stone TW, Forrest CM, Mackay GM, Stoy N, Darlington LG. Tryptophan, adenosine, neurodegeneration and neuroprotection. Metab Brain Dis. 2007 doi: 10.1007/s11011-007-9064-3. [DOI] [PubMed] [Google Scholar]
- 10.Stone TW, Mackay GM, Forrest CM, Clark CJ, Darlington LG. Tryptophan metabolites and brain disorders. Clin Chem Lab Med. 2003;41:852–9. doi: 10.1515/CCLM.2003.129. [DOI] [PubMed] [Google Scholar]
- 11.Guillemin GJ, Brew BJ. Implications of the kynurenine pathway and quinolinic acid in Alzheimer’s disease. Redox Rep. 2002;7:199–206. doi: 10.1179/135100002125000550. [DOI] [PubMed] [Google Scholar]
- 12.Guillemin GJ, Brew BJ, Noonan CE, Knight TG, Smythe GA, Cullen KM. Takai K, editor. Mass spectrometric detection of quinolinic acid in micro-dissected Alzheimer’s disease plaques. International Congress Series. 2007. pp. 404–8.
- 13.Guidetti P, Hemachandra Reddy P, Tagle DA, Schwarcz R. Early kynurenergic impairment in Huntington’s Disease and in a transgenic animal model. Neurosci Lett. 2000;283:233–5. doi: 10.1016/s0304-3940(00)00956-3. [DOI] [PubMed] [Google Scholar]
- 14.Guidetti P, Schwarcz R. 3-Hydroxykynurenine and quinolinate: pathogenic synergism in early grade Huntington’s disease. Adv Exp Med Biol. 2003;527:137–45. doi: 10.1007/978-1-4615-0135-0_16. [DOI] [PubMed] [Google Scholar]
- 15.Guillemin GJ, Meininger V, Brew BJ. Implications for the kynurenine pathway and quinolinic acid in amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:166–76. doi: 10.1159/000089622. [DOI] [PubMed] [Google Scholar]
- 16.Brew BJ, Pemberton L, Evans L, Heyes M. Quinolinic acid production by macrophages infected with demented and non-demented isolates of HIV. Clin Neuropathol. 1993;12:S1. [Google Scholar]
- 17.Guillemin GJ, Wang L, Brew BJ. Quinolinic acid selectively induces apoptosis of human astocytes: Potential role in AIDS dementia complex. Journal of Neuroinflammation. 2005;2:16. doi: 10.1186/1742-2094-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillemin GJ, Kerr SJ, Pemberton LA, et al. IFN-beta1 induces kynurenine pathway metabolism in human macrophages: Potential implications for multiple sclerosis treatment. Journal of Interferon and Cytokine Research. 2001;21:1097–101. doi: 10.1089/107999001317205231. [DOI] [PubMed] [Google Scholar]
- 19.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–20. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 20.Dale WE, Dang Y, Brown O. Tryptophan metabolism through kynurenine pathway in rat brain and liver slices. Free Radic Biol Med. 2000;29:191–8. doi: 10.1016/s0891-5849(00)00341-5. [DOI] [PubMed] [Google Scholar]
- 21.Braidy N, Grant R, Brew BJ, Adams S, Jayasena T, Guillemin GJ. Effects of Kynurenine pathway metabolites on intracellular NAD+ synthesis and cell death in human primary astrocytes and neurons. IJTR. 2009;2:61–9. doi: 10.4137/ijtr.s2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan PH, Bharath AK. Manipulation of indoleamine 2,3 dioxygenase; a novel therapeutic target for treatment of diseases. Exp Opin Ther Targ. 2009;13:987–1012. doi: 10.1517/14728220903018940. [DOI] [PubMed] [Google Scholar]
- 23.Wonodi I, Schwarcz R. Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in schizophrenia. Schiz Bull. 2010;36:211–8. doi: 10.1093/schbul/sbq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braidy N, Grant R, Adams S, Brew BJ, Guillemin G. Mechanism for quinolinic acid cytotoxicity in human astrocytes and neurons. Neurotox Res. 2009;16:77–86. doi: 10.1007/s12640-009-9051-z. [DOI] [PubMed] [Google Scholar]
- 25.Grant RS, Kapoor V. Murine glial cells regenerate NAD, after peroxide-induced depletion, using either nicotinic acid, nicotinamide, or quinolinic acid as substrates. J Neurochem. 1998;70:1759–63. doi: 10.1046/j.1471-4159.1998.70041759.x. [DOI] [PubMed] [Google Scholar]
- 26.Berger SJ, Sudar DC, Berger NA. Metabolic consequences of DNA damage: DNA damage induces alterations in glucose metabolism by activation of Poly(ADP-ribose) polymerase. Biochem Biophys Res Commun. 1986;134:227–32. doi: 10.1016/0006-291x(86)90551-6. [DOI] [PubMed] [Google Scholar]
- 27.Erdelyi K, Bakondi E, Gergely P, Szabo C, Virag L. Pathophysiologic role of oxidative stress-induced poly(ADP-ribose) polymerase-1 activation: focus on cell death and transcriptional regulation. Cell Mol Life Sci. 2005;62:751–9. doi: 10.1007/s00018-004-4506-0. [DOI] [PubMed] [Google Scholar]
- 28.Bouchard V, Rouleau M, Poirier GG. PARP-1, a determinant of cell survival in response to DNA damage. Experimental Hematology. 2003;31:446–54. doi: 10.1016/s0301-472x(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 29.Milne J, Denu JM. The Sirtuin family: Therapeutic targets to treat diseases of aging. Curr Pharm Des. 2008;12:11–7. doi: 10.1016/j.cbpa.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 30.Anastasiou D, Krek W. SIRT1: linking adaptive cellular responses to aging-associated changes in organismal physiology. Physiology (Bethesda) 2006;21:404–10. doi: 10.1152/physiol.00031.2006. [DOI] [PubMed] [Google Scholar]
- 31.Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM. Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol Appl Neurobiol. 2005;31:395–404. doi: 10.1111/j.1365-2990.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 32.Guillemin GJ, Kerr SJ, Smythe GA, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem. 2001;78:1–13. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 33.Bernofsky C, Swan M. An improved cycling assay for nicotinamide adenine dinucleotide. Anal Biochem. 1973;53:452–8. doi: 10.1016/0003-2697(73)90094-8. [DOI] [PubMed] [Google Scholar]
- 34.Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987;20:83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- 35.Takikawa O, Yoshida R, Kido R, Hayaishi O. Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J Biol Chem. 1986;261:3648–53. [PubMed] [Google Scholar]
- 36.Rahman A, Ting K, Cullen KM, Brew BJ, Braidy N, Guillemin GJ. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLOSONE. 2009;4:e6344. doi: 10.1371/journal.pone.0006344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem. 1976;53:452–8. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 38.Pallas M, Verdaguer E, Tajes M, Gutierrez-Cuesta J, Camins A. Modulation of sirtuins: new targets for antiageing. Recent Patents CNS Drug Discov. 2008;3:61–9. doi: 10.2174/157488908783421492. [DOI] [PubMed] [Google Scholar]
- 39.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–205. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 40.Furukawa A, Tada-Oikawa S, Kawanishi S. H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell Physiol Biochem. 2007;20:45–54. doi: 10.1159/000104152. [DOI] [PubMed] [Google Scholar]
- 41.Braidy N, Guillemin G, Grant R. Promotion of cellular NAD+ anabolism: Therapeutic potential for oxidative stress in ageing and Alzheimer’s disease. Neurotox Res. 2007;13:173–84. doi: 10.1007/BF03033501. [DOI] [PubMed] [Google Scholar]
- 42.Grant RS, Naif H, Espinosa M, Kapoor V. IDO induction in IFN-gamma activated astroglia: a role in improving cell viability during oxidative stress. Redox Rep. 2007;5:101–4. doi: 10.1179/135100000101535357. [DOI] [PubMed] [Google Scholar]
- 43.Grant R. Kynurenine pathway metabolism is involved in the maintenance of the intracellular NAD+ concentration in human primary astrocytes. IJTR. 2010;3:151–6. doi: 10.4137/ijtr.s4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magni G, Amici A, Emanuelli M, Raffaelli N, Ruggieri S. Enzymology of NAD+ synthesis. Adv Enzymol Relat Areas Mol Biol. 2010;73:135–82. xi. doi: 10.1002/9780470123195.ch5. [DOI] [PubMed] [Google Scholar]
- 45.Kwidzinski E, Bechmann I. IDO expression in the brain: a double-edged sword. J Mol Med. 2007 doi: 10.1007/s00109-007-0229-7. [DOI] [PubMed] [Google Scholar]
- 46.Sakurai K, Zou JP, Tschetter JR, Ward JM, GM S. Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;129:186–96. doi: 10.1016/s0165-5728(02)00176-5. [DOI] [PubMed] [Google Scholar]
- 47.Foster AC, Schwarcz R. Characterization of quinolinic acid phosphoribosyltransferase in human blood and observations in Huntington’s disease. J Neurochem. 1985;45:199–205. doi: 10.1111/j.1471-4159.1985.tb05493.x. [DOI] [PubMed] [Google Scholar]
- 48.Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49:15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- 49.Fiege J, Auwerx J. Transcriptional targets of sirtuins in the coordination of mammalian physiology. Curr Opin Cell Biol. 2005:20. doi: 10.1016/j.ceb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michishita E, Park J, Burneskis J, Barrett J, Horikawa I. Evolutionary conserved and nonconserved cellular localisations and functions of human SIRT proteins. Mol Biol Chem. 2005;16:4623–35. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]