Abstract
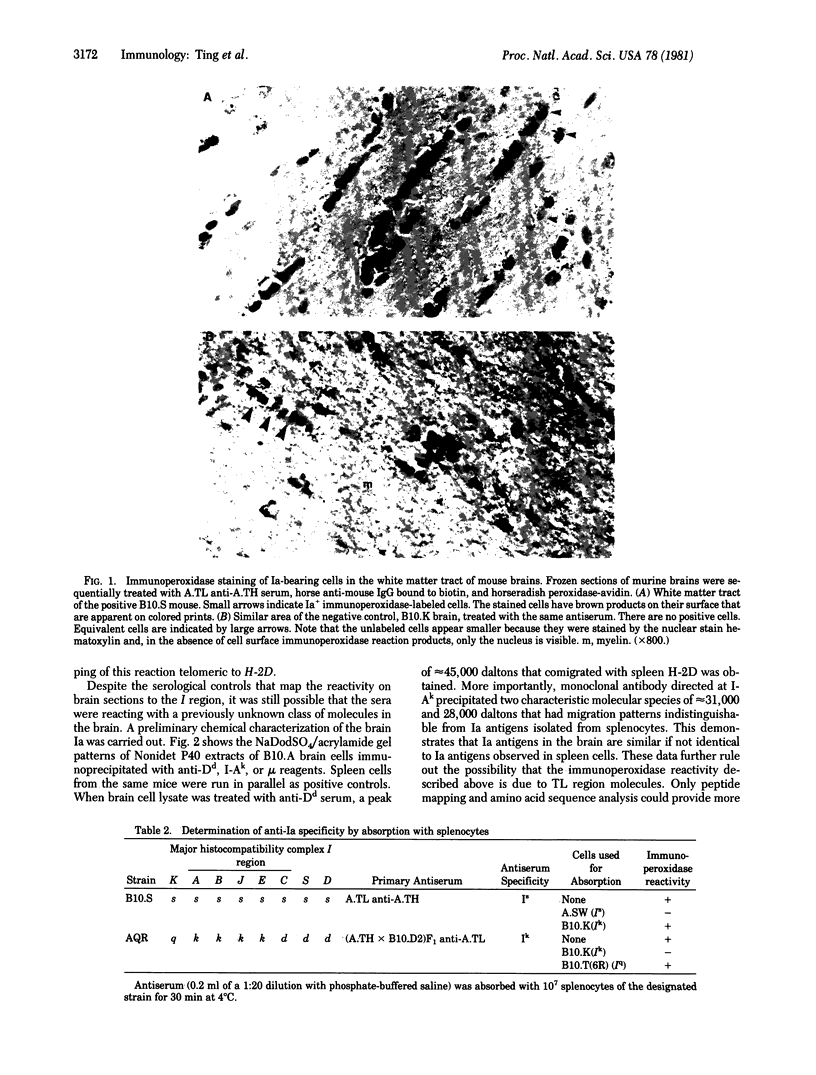
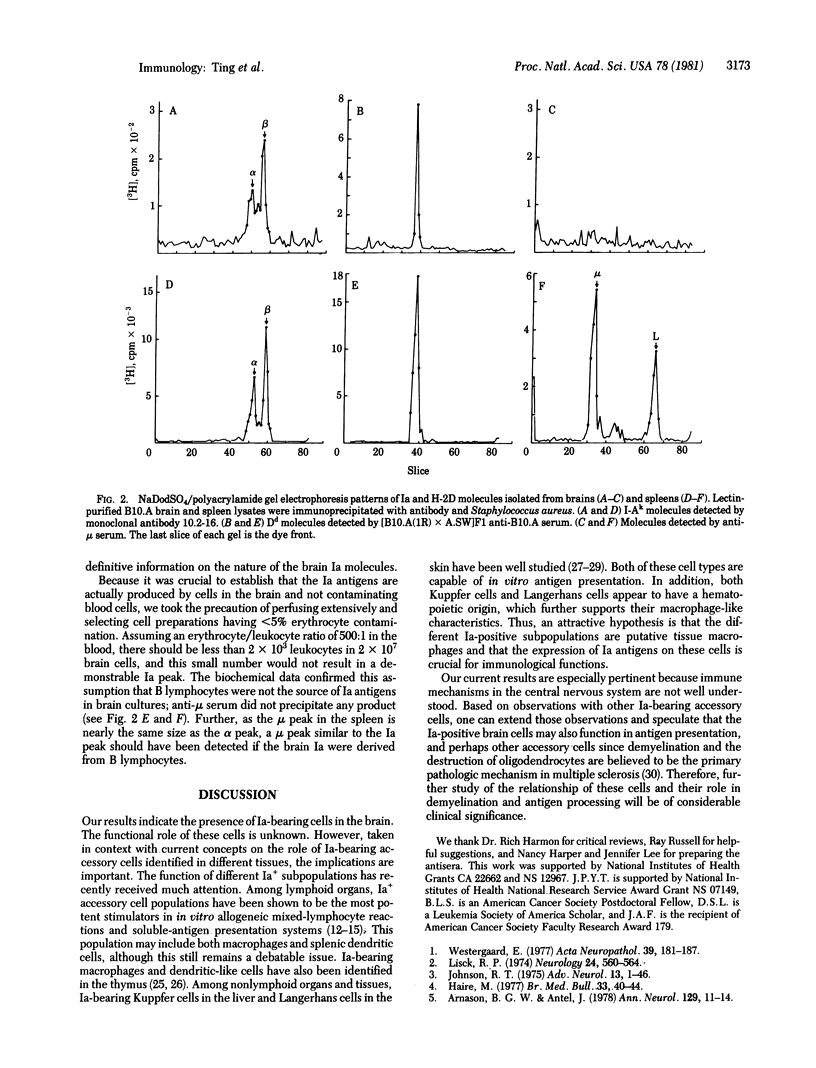
This paper provides biochemical and histochemical evidence that a fraction of murine brain cells express and synthesize Ia (Immune response-associated) antigens. Both I-A and I-E subregion products are detected on frozen sections of mouse brains by immunoperoxidase staining. Most of these Ia-bearing cells are located in white matter tracts and appear to be intrafascicular oligodendrocytes. In contrast, cells in the gray matter rarely display detectable Ia antigens on their cell surfaces. Specificity of the staining was confirmed by absorption studies. Biochemical evidence for the active synthesis of Ia antigens by brain cells was obtained by immunoprecipitation of [3H]leucine/tyrosine-labeled, NP-40-extracted cell lysates with monoclonal anti-Ia reagent. Both the alpha and beta subunits of Ia antigens were identified by NaDodSO4 electrophoresis. By contrast, anti-mu serum failed to precipitate any product, thus eliminating contaminant B lymphocytes as a source of Ia antigens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althaus H. H., Huttner W. B., Neuhoff V. Neurochemical and morphological studies of bulk-isolated rat brain cells. I. A new approach to the preparation of cerebral neurones. Hoppe Seylers Z Physiol Chem. 1977 Sep;358(9):1155–1159. [PubMed] [Google Scholar]
- Beller D. I., Unanue E. R. IA antigens and antigen-presenting function of thymic macrophages. J Immunol. 1980 Mar;124(3):1433–1440. [PubMed] [Google Scholar]
- Cowing C., Pincus S. H., Sachs D. H., Dickler H. B. A subpopulation of adherent accessory cells bearing both I-A and I-E or C subregion antigens is required for antigen-specific murine T lymphocyte proliferation. J Immunol. 1978 Nov;121(5):1680–1686. [PubMed] [Google Scholar]
- Cowing C., Schwartz B. D., Dickler H. B. Macrophage Ia antigens. I. macrophage populations differ in their expression of Ia antigens. J Immunol. 1978 Feb;120(2):378–384. [PubMed] [Google Scholar]
- Cullen S. E., David C. S., Shreffler D. C., Nathenson S. G. Membrane molecules determined by the H-2 associated immune response region: isolation and some properties. Proc Natl Acad Sci U S A. 1974 Mar;71(3):648–652. doi: 10.1073/pnas.71.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C. S., Shreffler D. C., Frelinger J. A. New lymphocyte antigen system (Lna) controlled by the Ir region of the mouse H-2 complex. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2509–2514. doi: 10.1073/pnas.70.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelinger J. A., Neiderhuber J. E., David C. S., Shreffler D. C. Evidence for the expression of Ia (H-2-associated) antigens on thymus-derived lymphocytes. J Exp Med. 1974 Nov 1;140(5):1273–1284. doi: 10.1084/jem.140.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelinger J. G., Hood L., Hill S., Frelinger J. A. Mouse epidermal Ia molecules have a bone marrow origin. Nature. 1979 Nov 15;282(5736):321–323. doi: 10.1038/282321a0. [DOI] [PubMed] [Google Scholar]
- Haire M. Significance of virus antibodies in multiple sclerosis. Br Med Bull. 1977 Jan;33(1):40–44. doi: 10.1093/oxfordjournals.bmb.a071394. [DOI] [PubMed] [Google Scholar]
- Johnson R. T. The possible viral etiology of multiple sclerosis. Adv Neurol. 1975;13:1–46. [PubMed] [Google Scholar]
- Katz S. I., Tamaki K., Sachs D. H. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature. 1979 Nov 15;282(5736):324–326. doi: 10.1038/282324a0. [DOI] [PubMed] [Google Scholar]
- Lampert P. W. Autoimmune and virus-induced demyelinating diseases. A review. Am J Pathol. 1978 Apr;91(1):176–208. [PMC free article] [PubMed] [Google Scholar]
- Link H., Tibbling G. Principles of albumin and IgG analyses in neurological disorders. III. Evaluation of IgG synthesis within the central nervous system in multiple sclerosis. Scand J Clin Lab Invest. 1977 Sep;37(5):397–401. doi: 10.1080/00365517709091498. [DOI] [PubMed] [Google Scholar]
- Lisak R. P., Behan P. O., Zweiman B., Shetty T. Cell-mediated immunity to myelin basic protein in acute disseminated encephalomyelitis. Neurology. 1974 Jun;24(6):560–564. doi: 10.1212/wnl.24.6.560. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Herzenberg L. A., Okumura K., Herzenberg L. A., McDevitt H. O. A new I subregion (I-J) marked by a locus (Ia-4) controlling surface determinants on suppressor T lymphocytes. J Exp Med. 1976 Sep 1;144(3):699–712. doi: 10.1084/jem.144.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Richman L. K., Klingenstein R. J., Richman J. A., Strober W., Berzofsky J. A. The murine Kupffer cell. I. Characterization of the cell serving accessory function in antigen-specific T cell proliferation. J Immunol. 1979 Dec;123(6):2602–2609. [PubMed] [Google Scholar]
- Rouse R. V., van Ewijk W., Jones P. P., Weissman I. L. Expression of MHC antigens by mouse thymic dendritic cells. J Immunol. 1979 Jun;122(6):2508–2515. [PubMed] [Google Scholar]
- Shreffler D. C., David C. S. The H-2 major histocompatibility complex and the I immune response region: genetic variation, function, and organization. Adv Immunol. 1975;20:125–195. doi: 10.1016/s0065-2776(08)60208-4. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Witmer M. D. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5132–5136. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl G., Katz S. I., Shevach E. M., Wolff-Schreiner E., Green I. Detection of Ia antigens on Langerhans cells in guinea pig skin. J Immunol. 1978 Feb;120(2):570–578. [PubMed] [Google Scholar]
- Sunshine G. H., Katz D. R., Feldmann M. Dendritic cells induce T cell proliferation to synthetic antigens under Ir gene control. J Exp Med. 1980 Dec 1;152(6):1817–1822. doi: 10.1084/jem.152.6.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J. E., Peters A. A third neuroglial cell type. An electron microscopic study. J Comp Neurol. 1968 Jun;133(2):269–288. doi: 10.1002/cne.901330207. [DOI] [PubMed] [Google Scholar]
- Westergaard E. The blood-brain barrier to horseradish peroxidase under normal and experimental conditions. Acta Neuropathol. 1977 Aug 31;39(3):181–187. doi: 10.1007/BF00691695. [DOI] [PubMed] [Google Scholar]




