Abstract
In the present review we summarize the relationship between the amino acid, tryptophan, the neurotransmitter, serotonin, and the indole, melatonin, with the rhythms of sleep/wake and the immune response along with the possible connections between the alterations in these rhythms due to aging and the so-called “serotonin and melatonin deficiency state.” The decrease associated with aging of the brain and circulating levels of serotonin and melatonin seemingly contributes to the alterations of both the sleep/wake cycle and the immune response that typically accompany old age. The supplemental administration of tryptophan, e.g. the inclusion of tryptophan-enriched food in the diet, might help to remediate these age-related alterations due to its capacity of raise the serotonin and melatonin levels in the brain and blood. Herein, we also summarize a set of studies related to the potential role that tryptophan, and its derived product melatonin, may play in the restoration of the aged circadian rhythms of sleep/wake and immune response, taking the ringdove (Streptopelia risoria) as a suitable model.
Keywords: immune function, melatonin, serotonin, sleep-wake cycle, ringdove, tryptophan
Introduction
Tryptophan is a polar, hydrophobic amino acid indispensable for protein synthesis. It is classified as an “essential” amino acid, i.e. it cannot be synthesized by the human organism and must therefore be ingested in the diet. Once tryptophan is consumed, it is readily absorbed into the capillaries in the intestinal wall. A small amount of the amino acid remains free while the majority of it (roughly 80%–90%) is transported bound to albumin through the blood and into the brain. This transport may be altered by the competition exerted by other free, neutral amino acids of high molecular weight, branched-chain amino acids, including valine, leucine and isoleucine, as well as phenylalanine and tyrosine, which bind to the same transporters.1,2
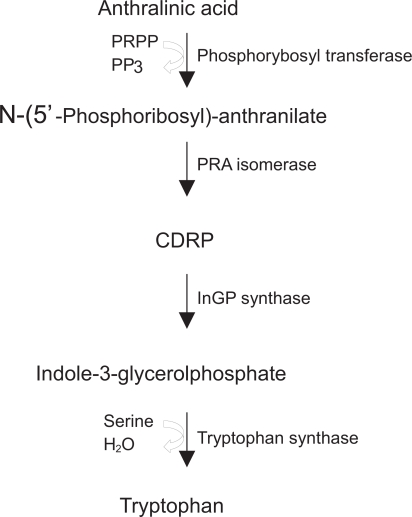
The metabolism of tryptophan is complex. It is involved in a variety of metabolic pathways and requires a suitable quantity of biopterin, magnesium or vitamin B6, which is involved in the conversion of the amino acid into serotonin and in the metabolism of other by-products, such as kynurenine. The main precursor of tryptophan is the anthralinic acid or anthranilate, a compound that after a series of chemical reactions is transformed into indole-3-glycerolphosphate. The enzyme tryptophan synthase converts this latter compound into glyceraldehyde 3-phosphate in a two-step reaction with the intermediary indole bound to the active site of the enzyme and with the intervention of serine (Fig. 1). The contribution of tryptophan to energetic metabolism is double since on one hand it is ketogenic, i.e. it forms acetyl coenzyme A, and on the other it is glucogenic, as it produces alanine.3
Figure 1.
Scheme of the conversion of anthranilic acid to tryptophan. PRPP, phosphoribosylpyrophosphate; PP3, pyrophosphate; PRA, N-(5’Phosphoribosyl)-anthranilate; CDRP, 1-(o-Carboxyphenylamino)-1-deoxyribulose-5-phosphate; InGP, indole-3-glycerolphosphate.
Tryptophan is transported to the liver where it is metabolized. Thereafter, in consecutive reactions it is transformed into nicotinic acid and other subproducts that either are stored or serve as a basis for important substances including quinolinate, picolinate or glutarate.4
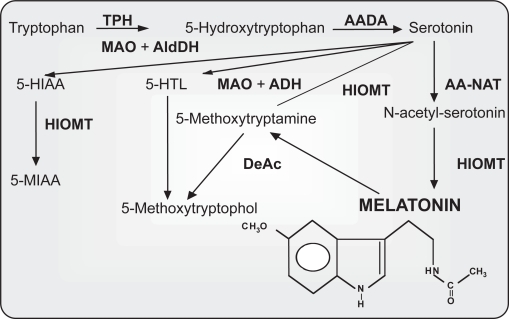
The amino acid tryptophan is the precursor of several important products including serotonin or melatonin (Fig. 2). These molecules are biogenic amines of low molecular weight that belong to the indole group. It has been observed that the synthesis of melatonin in the pineal gland diminishes with aging.5–7 This is believed to be due to degenerative changes in the neural structures (postganglionic neurons) innervating the pineal gland and central nervous system, rather than to the degeneration of the pineal tissue per se,8 as well as to a reduction in the quantity of the necessary precursor, serotonin.7,9,10 However, the number or sensitivity of melatonin receptors throughout the organism may decline with age as a result of the usual degenerative processes.11 The result is the development of the so-called “serotonin and melatonin deficiency state.”12–14 This age-related state seemingly contributes to the alterations of both the sleep-wake cycle and the immune responsivity that characterizes aging. Thus, the consumption of tryptophan as a pharmacological agent or as part of a diet rich in this amino acid may attenuate age-related changes in circadian organization, the immune system, sleep, and other disorders, due to its ability to elevate circulating levels of serotonin and melatonin.15–18
Figure 2.
Pathways of indole metabolism in photosensitive pineal cells. Enzymes: AADA, aromatic L-amino acid decarboxylase; AA-NAT, aralkylamine N-acetyltransferase; DeAc, deacetylase; HIOMT, hydroxyindole-O-methyltransferase; MAO, monoamine oxidase; TPH, tryptophan hydroxylase. Indoles: N-acetyl-serotonin; 5-HIAA, 5-Hydroxyindoleacetic acid; 5-HTL, 5-hydroxytryptophol; 5-MIAA, 5-methoxyindole-3-acetic. Chemical structure of melatonin is shown at the bottom of the figure (taken from Paredes, 2007,178 modified).
This review is constructed to provide a concise view of the effects of tryptophan, serotonin and melatonin on the sleep-wake cycle and the immune system responses, to identify possible links between the impairment of these rhythms and the reduction in serotonin and melatonin levels in the aging organism, and to illustrate the potential restorative role that tryptophan may play against the age-related afore-mentioned circadian alterations. Finally, we present the ring dove (Streptopelia risoria) as a suitable model for the study of the aged sleep-wake or activity-rest rhythms and immunosenescence and summarize the results obtained in this animal species in the circadian rhythm research field over the last decade.
Tryptophan, Serotonin, Melatonin, and the Sleep-wake Cycle
The initiation of investigations related to the hypnotic effects that tryptophan exerts on the human sleep dates back to the 70s and 80s,19,20 when it was observed that this amino acid augmented the propensity to sleep. During this interval tryptophan was used as a successful therapeutic agent to combat chronic insomnia.21 More recently, the consolidation properties of tryptophan for the sleep-wake rhythm of newborns have been tested. It has been shown that being fed with nutritionally dissociated milk formulas, i.e. a diurnal formula with low content of tryptophan and carbohydrates and a high amount of protein, supplemented with the nucleotides cytidine 5-monophosphate, guanosine 5-monophosphate and inosine 5-monophosphate, and a nocturnal formula that contains high levels of tryptophan and carbohydrates, a low level of protein, with the nucleotides adenosine 5-monophosphate and uridine 5-monophosphate, improved the total hours of sleep, the efficiency of sleep, the minutes of nocturnal immobility, and reduced both the number of nocturnal awakenings and the sleep latency of newborns.22,23 This is of special importance at a stage of life where an appropriate rest period is directly related to an optimal development of both the nervous and immune systems.
The first studies showing a relationship between serotonin and sleep appeared in the middle of the 50s. It was observed that reserpine, an antipsychotic, antihypertensive indole alkaloid dimin ished the concentration of serotonin in the brain and induced a sedative state analogous to sleep.24 It was also reported that the parenteral injection of L-5-hydroxytryptamine caused cortical synchronization and that inhibitors of the enzyme monoamine oxidase selectively suppressed paradoxical sleep or REM for long periods of time that could persist for days or even weeks.25 Serotonin subsequently appeared as a key factor in understanding some of the mechanisms involved in the sleep-wake cycle. In fact, subsequent investigations documented that the destruction of the raphe nuclei, an area with an abundance of serotonin-containing neurons, by means of coagulation, produced insomnia for lengthy periods of time (10–15 days). Thus, a relationship among insomnia intensity, the magnitude of the injury in the raphe nuclei and the amount of brain serotonin that remained in the telencephalon after the degeneration of the serotoninergic terminals was established. Particularly, a correlation between the destruction of the rostral raphe and the slow-wake sleep and the telencephalic serotonin, which decreased, and between paradoxical sleep and damage to the nucleus raphe magnus was identified.26,27 It was additionally pointed out that p-chlorophenylalanine inhibited the enzyme tryptophan hydroxylase, which in turn impaired the biosynthesis of serotonin and secondarily led to states of total insomnia.28
These experiments led to the elaboration of the so-called “monoaminergic theory of sleep.” This established that serotonin, or somnotonin as it was named by Koella,29 was the neurotransmitter or “neurohormone” of sleep, since it produced sleep by the inhibition of the reticular formation and locus ceruleus, the putative centers of wakefulness. Conversely, catecholamines were found to be responsible for awakening. The demonstration, however, that the electric activity of serotoninergic neurons as well as the release of serotonin increased during wakefulness and decreased with sleep was seemingly in clear contradiction with the afore-mentioned theory. In the late 80s, however, a relationship between the sleep/wake cycle and serotonin was again considered. More recent experiments suggest that during wakefulness, serotonin is responsible for initiating a cascade of post-synaptic genomic processes in hypnogenic neurons located in the pre-optic area.30 Through these processes, the release of the neurotransmitter during wakefulness leads to a homeostatic regulation of slow wave sleep,30 also acting as a positive modulator of melatonin synthesis.31
Among the diverse physiological functions in which melatonin has been involved, its role as regulator of the sleep/wake rhythms has attracted the attention of a number of sleep researchers in the last decade. The discovery that melatonin was mainly secreted at night and the tight relationship between the nocturnal increase of endogenous melatonin and the existing co-ordination of sleep as well as the pro-somnogenic effects that the pineal indole seemingly possessed, led many investigators to suggest that melatonin was likely implicated in the physiological regulation of sleep. With regard to this presumption, it was observed that the suppression of the production of melatonin using β-blockers correlated with insomnia,32–34 while the increase of the plasma levels of melatonin by reducing the activity of the enzymes that metabolize the indole in the liver resulted in an augmentation of the somnolence state.35
It was reported that during the wake period immediately prior to sleep, known as the wake-maintenance zone or “forbidden zone” for sleep,36 the propensity for sleep is reduced to a minimum and, at the same time, the activity of the neurons of the central nervous system is elevated.37,38 Thus, the transition from the wake stage to a period of high propensity for sleep coincides with the nocturnal elevation of the endogenous rhythm of melatonin.39 This increase seems to be temporally related to the opening of the so-called sleep gate.40,41
Taking into account the relationship between the endogenous secretion of melatonin and the opening of the entry into nocturnal sleep, it has been proposed that the role of the pineal indole does not involve an active induction of sleep, rather it consists of the inhibition of the mechanisms that generate the circadian period of wakefulness,42 presumably through the MT1 melatonin receptor43,44 and GABAergic activation45,46 at the central nervous system level.
Regarding the effects of the exogenous administration of melatonin on sleep and the circadian clock, there are a number of studies reporting that diurnal treatment with the indole produces drowsiness, 47–51 as well as raising the circulating levels of melatonin to values normally observed at night.52
For these reasons, melatonin, through its actions in the central nervous system, is seemingly a crucial substance for the co-ordination of the circadian mechanism of sleep. However, the recent discovery of melatonin receptors in other brain areas such as the hippocampus53 makes necessary further investigation to elucidate the exact role of melatonin on sleep in the different brain structures. Moreover, considering the rather high levels of melatonin in certain plant foodstuffs (Table 1),54 the consumption of melatonin through the diet may have significant benefits to human and animal health.
Table 1.
Levels of melatonin in representative common vegetables and fruits measured using different methods by Dubbels et al.175 (1), Hattori et al.176 (2), and Badria177 (3).
| Common name | Scientific name | Melatonin (1)a | Melatonin (2)b | Melatonin (3)c |
|---|---|---|---|---|
| Apple | Malus domestica | – | 47.6 ± 3.1 | 16.1 |
| Asparagus | Asparagus officinalis | – | 9.5 ± 3.2 | – |
| Banana | Musa ensete | – | – | 65.5 |
| Banana | Musa sapientum | 46.6 | – | – |
| Beetroot | Beta vulgaris | 0.2 | – | – |
| Cabbage | Brassica oleracea var. capitata | – | 107.4 ± 7.3 | 30.9 |
| Carrot | Daucus carota | – | 55.3 ± 11.9 | 49.4 |
| Corn | Zea mays | – | 1366.1 ± 465.1 | 187.8 |
| Cucumber | Cucumis sativus | 8.6 | 24.6 ± 3.5 | 59.2 |
| Garlic | Allium sativum | – | – | 58.7 |
| Ginger | Zingiber officinale | – | 583.7 ± 50.3 | 142.3 |
| Kiwi fruit | Actinidia chinensis | – | 24.4 ± 1.7 | – |
| Onion | Allium cepa | – | 31.5 ± 4.8 | 29.9 |
| Pineapple | Ananas comosus | – | 36.2 ± 8.4 | 27.8 |
| Pomegranate | Punica granatum | – | – | 16.8 |
| Radish | Raphanus sativus | – | – | 75.8 |
| Rice | Oryza sativa | – | 1006.0 ± 58.5 | 149.8 |
| Strawberry | Fragaria magna | – | 12.4 ± 3.1 | 13.6 |
| Tomato | Lycopersicon esculentum | 50.6 | 32.2 ± 2.4 | – |
| Tomato | Lycopersicon pimpinellifolium | 11.2 | – | 30.2 |
ng/100 g edible plant material (without peel). Levels measured by RIA and HPLC-MS.
pg/g tissue. Levels quantified by RIA.
ng/100 g. Levels measured by GC/MS analysis.
Tryptophan, Serotonin, Melatonin, and the Immune System
The concentration of the amino acid tryptophan is lower in psychologically depressed patients with respect to control individuals.55 This consequently produces a decrease in the levels of serotonin, a neurotransmitter that has frequently been implicated in depressive syndromes.56 Moreover, it has been observed that when depressive disorders appear, they are accompanied by an inflammatory response involving the immune system, which is inversely proportional to the concentration of tryptophan in plasma.57 This is also negatively correlated to the number of leukocytes and other components of the immune system including interleukin 6 (IL-6) and IL-8.58 It has been reported that individuals with sleep disturbances experience the same symptoms as patients suffering from depression, i.e. a diminution of the tryptophan levels in plasma and an augmentation of both IL-6 and IL-8, compared to healthy individuals.56,57 They also experience a decrease in the levels of IL-2.59 On the other hand, when interruption of sleep for 5 hours during the nocturnal period occurs, the levels of IL-1 and IL-2 are elevated. When somnolence is produced in excess, IL-6 and tumor necrosis factor α (TNF-α) are elevated,60 with a subsequent rise in the number of monocytes and neutorphils.61
Regarding the effect that the amino acid exerts on the phagocytic function, recent studies suggest an enhancement of phagocytosis after the oral administration of tryptophan. Particularly, it has been observed that administering the amino acid to rats causes incremental changes in circulating levels of melatonin as well as stimulating the antigenic capacity of ingestion of peritoneal macrophages obtained during the nocturnal period.15,62 An elevation of the phagocytic capacity at night has also been observed in otherwise untreated rats and mice.63–66 This suggests that the activation of the innate immune response after tryptophan consumption may be due to its conversion into the pineal indole. In fact, it has been shown that macrophages obtained from the peritoneal cavity of normal rats when incubated with tryptophan show an increase in arylalkylamine N-acetyltransferase activity which corresponds to a rise in melatonin production.67 Nevertheless, tryptophan is also the precursor of serotonin, a compound that may also play a role in the function of the innate immune system. Owing to the fact that receptors for serotonin exist in leukocytes and a transporter for this amine has been found in macrophages, mononuclear leukocytes, and B cells, this neurotransmitter may be a critical element for the connection between the nervous and immune systems.68,69 Some studies have shown that serotonin may also possess an antioxidative role.70–72 Serotonin has also been reported to inhibit leukocyte phagocytosis,73,74 especially when the concentrations of neurotransmitter used are in the pharmacological range.71 Since circadian variations of serotonin in plasma and different brain regions have been observed,75 this may somehow influence the circadian daily variations of the immune system.
A substantial body of research has defined melatonin as a remarkable molecule with pleiotropic effects both of an endocrine and a non-endocrine nature on the immune system.76–78 The abolition of the daily rhythm of melatonin via either surgical or functional pinealectomy has been shown to directly correlate to weight loss of the thymus as well as to the abnormal involution of this immune organ; this is also accompanied by a depletion of lymphoblasts and an almost total absence of lymphocytes. 79 A reduction in the size of lymph nodes associated with follicular loss in the outer cortex80 together with an alteration of the activities of thymic polyamine biosynthetic amines have also been noted.81–83 Other immune organs such as the spleen or the bursa of Fabricius in birds are impaired following pinealectomy. In this respect, Brainard et al.79 showed a lack of evident germinal centers and an apparent inactivity of the red pulp in the Syrian hamster spleen, while Jankovic et al.84 found a delayed development not only in the bursa but also in the thymus and spleen of pinealectomized chicks. The absence of the pineal gland has also been reported to significantly reduce IL-2 production and NK activity85,86 and decrease the cellular and humoral immune response of both mammals and birds.87–90 When melatonin is administered to pinealectomized animals, the effects on immune system are typically reversed.
In vivo models have shown melatonin to be considered as a positive regulator of immune responses. The administration of melatonin results in the enhancement of antigen presentation by splenic macrophages in major histocompatibility complex II, IL-1 and TNF-α production,91 the increase in the generation of thymosin α1 through a rise in prothymosin α gene expression 92 as well as the production of IL-10.93 In mice, treatment with melatonin also upregulates macrophage-colony stimulating factor, TNF-α, transforming growth factor β and stem cell factor gene expression in peritoneal macrophages and the levels of IL-1β, interferon γ, macrophage-colony stimulating factor, TNF-α and stem cell factor in splenocytes.94
The pineal indole also possesses potential positive effects on several immune system pathologies including acute and chronic inflammation95,96 and syndromes provoked by certain viruses such as the encephalomyocarditis virus,97 lethal Semliki Forest virus and the attenuated non-invasive West Nile virus98 as well as the Venezuelan equine encephalomyelitis virus.99–101
The Serotonin and Melatonin Deficiency State Due to Ageing: Effects and Consequences on the Sleep/wake Cycle and the Immune System
Aging is associated with a reduction in the size of the brain. These changes are generally attributed to a loss of neurons in specific layers and regions of the brain, although there exists considerable interindividual variation.102 The loss of neurons has been shown to occur in the locus ceruleus, the main source of catecholaminergic neurons, and in the substantia nigra, where dopaminergic neurons are most abundant. This may contribute to age-related changes in homeostasis, sleep alterations, stability, movement, and cognitive function.103 Alterations tend to affect the myelinated axons (the white matter) at a much greater degree when compared to the neuron cell bodies in the grey matter.104 Aging has also been proposed to modify the permeability of the blood-brain barrier, which may have consequences in terms of porosity of this structure to different drugs or molecules and to cause a decline in the brain metabolism and blood flow.105
Neurotransmitter functions of serotonin are widely distributed in the central nervous system and are related to the regulation of a variety of behaviors. Serotonin is seemingly involved in the regulation of humor, anxiety, sleep, appetite, sexual function, brain blood flow and many other functions. The serotoninergic neurons are located in the raphe nuclei of the brain stem and their axons project to all brain areas, including the cerebral cortex, thalamus, the limbic system and the hypo-thalamus. In regard to this, any change in the number of serotonin receptors or in the endogenous levels of the neurotransmitter due to aging may have consequences on behaviour or cognitive function. Diverse studies have shown that alterations in serotinergic neurotransmission cause age-related alterations. Particularly, a reduction in the density of the serotonin type 2 receptor (5HT2A) has been described.106
The injection of altanserin, a high affinity ligand to 5HT2A receptor union sites, to young and old subjects showed that the specific union for this receptor was significantly reduced in old individuals compared to the young, as well as the number of receptors, whose loss was marked in certain brain regions.106 Since many antidepressant drugs typically relieve the symptoms of depression by blocking serotonin reuptake in order to facilitate an increase in serotonin activity, it is speculated that the high incidence of depression in the elderly may be attributed to the reduction in serotonin receptors. Moreover, it has been reported that serotonin receptors and transporters are less sensitive to hormone regulation, which responds to the deficiency associated to aging of the regulation of the hippocampal serotoningeric system exerted by corticosterone.107 This suggests that the age-related changes in the neurochemistry of serotonin may be a cause of the increased rates of depression and hypercortisolemia observed in the aged populations. In parallel, serotonin is known to increase the quality of slow wave sleep,30 as well as being a waking neurotransmitter.108 In addition, the close relationship between serotonergic activity and the adjustments of circadian phase has suggested that serotonin also plays a role in the endogenous regulation of the circadian clock.109 These findings point to the participation of serotonin neurotransmission in the behavioral alterations commonly observed in aged individuals and may have potential therapeutic implications.
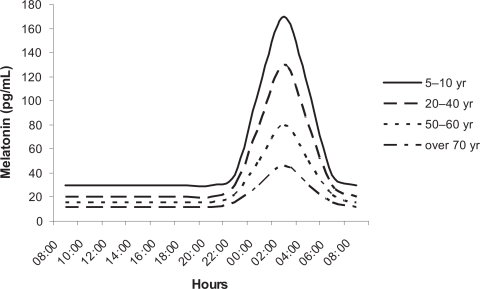
Blood melatonin levels show a clear circadian rhythm, with low levels during the day and high values at night, with these values being 10/15-fold greater that those measured in the diurnal period.110,111 In humans, the indole has been shown to gradually decrease during the increased life span, with the day/night rhythm being practically absent in individuals over 65-yr old (Fig. 3).112 This observation has also been reported when melatonin levels in young and old rats, gerbils, hamsters or ringdoves are compared.113,114 It is believed that the amplitude of the nocturnal melatonin rhythm is genetically determined as it shows important interindividual differences,115 even though in a given individual it exhibits a high degree of fidelity over time.116 Hence, some subjects produce significantly less melatonin in their life than others, which may be of importance for aging.13
Figure 3.
Diagrammatic representation of daily profiles of serum melatonin levels throughout lifespan (taken from Karasek and Reiter,13 modified).
Aging is a crucial factor in terms of sleep characteristics. The structure, depth, and continuity of sleep tend to change over the life span.117 Some reports have shown that more than a third of the elderly experience recurrent difficulty to maintain sleep118 due to impairment in both the quality and the quantity of sleep.119 Sleep onset latency usually increases together with the number and duration of awakenings, while sleep stability declines, and sleep consolidation is altered.117 It is therefore not surprising that the information provided by epidemiologic studies reveals that up to 40% of individuals over 65-yr old complain about sleep problems and 12%–25% suffer from persistent insomnia.120 The number of elderly people that have been prescribed sleep drugs or that use aids to facilitate nocturnal rest is estimated to be around 14%.121
Aging also causes alterations in the amplitude of the sleep/wake circadian rhythm.122 The temporal organization of sleep is impaired and the regulatory mechanisms of the sleep processes are attenuated.123 Several reasons suggested for these age-related changes are a reduction in the number of pinealocytes, changes in retina and in the suprachiasmatic nuclei or alterations in melatonin secretion.124
The effectiveness of the immune system decreases during aging. The lymphoid tissues of the spleen, bone marrow and thymus are progressively lost; this increases the incidence of infections, autoimmune diseases and cancer.125 With advancing age, the number and proliferative capacity in response to mitogen-stimulation of the diverse subpopulations of T-lymphocytes is reduced126,127 while apoptosis is elevated.128 Moreover, the synthesis and secretion of immunoglobulins is delayed, presumably due to a lack of appropriate levels of cytokines;129 this decreases the competence of antibodies in immunization against infectious agents.130 Antigen-presenting cells or accessory cells and phagocytes experience an age-related rise in the oxidative state,131 resulting in a reduced capability to adapt to environmental stress and in a reduction of the phagocytic parameters.114
It is known that the pineal gland influences the function of the neuroendocrine system and the efficacy of the immune system to recognize and react to any endogenous or exogenous factor.132 For this reason, it has been suggested that aging is a result of the deterioration of this key factor of the pineal gland due to a deficient melatonin secretion and a decline in the melatonin/serotonin ratio. This may impair several aspects of an individual’s neurophysiology. 48 It has been observed that early extirpation of the pineal gland produces substantial accumulations of lipid peroxidation products, oxidized DNA, reduced fluidity of cell membranes and elevated protein damage in many organs.133 These changes are a consequence of the loss of the endogenous melatonin rhythm. Impairment in melatonin synthesis is thought to likely play a role in the aging process since this indole participates in vital defence mechanisms including free radical scavenging and indirect antioxidative actions.134,135 Thus, melatonin is estimated to be responsible for the scavenging of ten or more reactive damaging agents.136 Furthermore, its initial, secondary, tertiary and quaternary derivatives are all potent scavengers that, together with melatonin, form a remarkable cascade of reactions referred to as melatonin’s antioxidative cascade.136,137 The detoxification of radical and radical products by melatonin and its derivatives are receptor-independent actions and only require that the scavenger be at the site where the radical product is generated.138 This is essential since highly reactive agents mediate damage in the immediate vicinity of where they are produced, i.e. the damage is site specific.138 Melatonin also has receptor-mediated actions which adds to the capability of this molecule in eradicating radicals and reducing oxidative stress.139,140 Thus, melatonin stimulates a number of antioxidative enzymes which metabolize reactive products to innocuous agents. The enzymes whose activities have been shown to be promoted by melatonin include both Cu/Zn and Mn superoxide dismutases, glutathione peroxidases and glutathione reductase.139,141,142 The effects of melatonin on the activities of the antioxidative enzymes are likely receptor-mediated and involve receptors on the plasma membrane and also presumably receptors/binding sites in the nucleus.140
The efficiency of sleep is also reduced as a result of low circulating levels of the pineal indole. These phenomena typically accompany advancing age. Melatonin may thus protect against the oxidation of essential molecules,143,144 which appear in significant numbers in aged organisms,145 and resist neurodegenerative disorders associated with the impairment produced in particular brain areas by free radicals.146 In fact, melatonin may possess benefits in Parkinson and Alzheimer diseases.147,148
Pinealectomy is believed to accelerate the aging process, causing high blood pressure, elevated alkaline phosphatase activity, modification in the synthesis of prostaglandins, and induction of REM. These alterations are seemingly counteracted by the administration of melatonin.149 Many studies support the idea that melatonin may be considered as an anti-aging and rejuvenating product. The evidence accumulated to date supports the hypothesis that the supplemental treatment with melatonin may be of benefit during aging.150,151
The Potential Restorative Role of Tryptophan of the Impaired in the Sleep/wake Cycle and Immune System that Accompany Aging: Streptopelia Risoria as a model
The ringdove (Sterptopelia risoria) is a species characterized by being diurnal and monophasic with sleep-wake cycles similar to those of human beings and, therefore, it represents a good model to investigate impairments in the circadian system due to age, including immune alterations.
The first study performed in the ringdove that showed a relationship between the pineal gland, melatonin and the immune system was that of Rodríguez and Lea.87 Pinealectomy produced a significant increase in the number of total white blood cells and total protein concentration in plasma in addition to altering different stages of the phagocytic process. Also, during an immunization study, a reduction in the percentage of leukocytes and lymphocytes and an increase in the percentage of heterophils accompanied by a rise in the concentration of serum corticosterone were observed 3 hr following treatment. For the immunological parameters, adherence capacity and latex bead ingestion were increased 3 hr after normal sleep serum injection and the nitroblue tetrazolium reduction test 3 and 24 hr after normal sleep serum treatment. In addition, the administration of normal sleep serum produced a significant increase in serum T3 and T4 concentrations 4 days following injection. These results indicate that the loss of melatonin due to pinealectomy has a marked effect on both the number and function of immune cells.
In reference to in vivo experiments with melatonin, a correlation between the circadian rhythm of the indole, phagocytosis, and superoxide anion levels has been reported.152 Thus, the elevated melatonin serum levels during the dark period coincide with an enhanced phagocytosis of inert particles and lower superoxide anion levels derived from the immune system. These effects where reinforced when the animals received melatonin orally, which also elevated circulating levels of the indole.153,154 Similar results were observed when the phagocytosed particle was a living organism (Candida albicans) with the effect being dose-dependent.153,155 In vitro experiments have reported similar results, with the chemoattractant ability for heterophils being significantly enhanced by the pineal indole156 as well as an augmentation of the phagocytic function and a decline in the free radical production.65 Melatonin also decreased the superoxide dismutase activity (an indicator of the metabolic burst) in heterophils after the ingestion of latex beads.157
The concentration of malonaldehyde in cells is an index of induced oxidative damage to membrane lipids. The co-incubation of a heterophil suspension with or without inert particles (latex beads), as material to be phagocytosed, in combination with melatonin has been shown to clearly reduce the production of malonaldehyde. The enhancement of malonaldehyde levels produced by latex beads was also annulled in the samples incubated with melatonin.158
In stressful situations, an alteration in the endogenous circadian rhythm of melatonin in the ringdove has been described.159 This has also been reported in mammals, where a decreased MESOR and amplitude of the melatonin rhythm, and a significantly elevated MESOR of the corticosterone rhythm have been observed.66,160
Streptopelia risoria also experiences a “serotonin and melatonin deficiency state” during aging;7,10 this is associated with increased nocturnal activity and depressed immune function.7,154,161,162 Under these conditions, orally administered melatonin has been reported to improve nocturnal rest not only in old ringdoves, but also in young birds.161,162 This is likely to be a result of a decrease in the core temperature and an increase in the peripheral temperature observed after the oral administration of the indole in this species.162 Thus, melatonin may be used to palliate the reduction in the thermoregulatory responses and the capacity for thermal comfort reported in the elderly.163 This is of importance since sleep disorders are believed to be caused, at least partly, by changes in the circadian rhythms of temperature and melatonin.164
The oral administration of the indole restores some of the changes that aging produces in the innate immune response, with an enhancement in the phagocytic processes and a decrease in the production of free radicals, reflecting the scavenging properties of melatonin; this is most probably due to the restoration of the nocturnal rise of circulating melatonin due to its administration.153,154,161,165 This hypothesis is corroborated by previous findings showing that the incubation of ringdove heterophils obtained from old animals with the physiological concentrations of serum melatonin typical of young and mature birds induced a dose-dependent rise in both the phagocytic index and the candidicide capacity, together with a decline in superoxide anion levels.166 Furthermore, the incubation of old heterophils with the physiological concentrations of melatonin characteristic of young animals (50 and 300 pg/ml, diurnal and nocturnal, respectively) counteracted the enhancement of malonaldehyde levels caused by latex beads, with the effect being greater at the longer incubation time tested.167
Once the potential role of the pineal indole to reverse the age-related alteration in the activity/rest rhythms and immune impairment in the ringdove was documented, the next step was to test whether tryptophan, the precursor of melatonin and also of the neurotransmitter serotonin would have similar effects. Tryptophan administered in the diet is known to increase the availability of serotonin in the brain, improve the EEG delta potential, and elevate the amount of NREM.168 It has also been observed in mammals that orally ingested tryptophan increases brain levels of serotonin during the day and the circulating levels of melatonin during the immediately subsequent night.62 Likewise, tryptophan administration raised the circulating levels of both serotonin and melatonin in rats.15,18 In sexually immature ringdoves, the administration of the amino acid increased nocturnal rest, which seemingly correlated with the augmented circulating levels of melatonin caused by tryptophan treatment.169 Tryptophan significantly increases the hippocampal, striatal, and hypothalamic serotonin contents,10 and reduces the expression of c-fos in the suprachiasmatic nucleus.170 C-fos levels are high in several cerebral regions during spontaneous waking or sleep deprivation and fall after a few hours of sleep.171
In old ringdoves, the treatment with 300 mg of tryptophan per kg b.w. reduces nocturnal activity without affecting their diurnal activity, an effect accompanied by a general increase of serum serotonin levels16 This increase of serum serotonin indicates a higher availability of tryptophan which, after passing the blood–brain barrier, would be converted into serotonin.10,15,18,31,62 The elevated serotonin in the pineal gland serves as a substrate for melatonin synthesis, and increases in the levels of this molecule would reduce nocturnal activity of old ringdoves,16 improving their aging-impaired nocturnal rest.7 Tryptophan administered at the same dose and time also provoked an improvement of the circadian rhythm of temperature in this species.162
Regarding the innate immune response in old birds, treatment with tryptophan produced a significant diurnal and nocturnal augmentation in phagocytic parameters; the values reached during the night were significantly higher that those measured during the day.17 This is consistent with earlier findings demonstrating that giving the amino acid to mammals15,62 or birds,172 or melatonin itself to these species14,154,165,173,174 has a general immuno-enhancing effect. Moreover, a reduced production of superoxide anion radicals in old ringdoves was observed after tryptophan treatment.17 This change was presumably due to the rise of the circulating serum levels of melatonin produced by the exogenous administration of its precursor.16,17 This indicates that rising serum levels of melatonin are accompanied by a decline in the levels of superoxide anion radicals produced by heterophils, as reported previously. Also, tryptophan significantly limits the reduction in cell viability of heterophils exposed to hydrogen peroxide. 17 A similar effect was obtained when the cells are incubated with melatonin.154 Furthermore, both the oral administration of the amino acid and the indole lowered cytokine levels in aged birds.162
Concluding Remarks
A variety of studies on serotonin neurotransmission indicate that, as a consequence of aging, a reduction in the density of serotonin receptors and marked disturbance in the 5-hydroxindole acetic/serotonin turnover and in the responses of the receptors/transporters to the hormonal regulation occur.106,107 Many of these alterations result in a decreased serotonin binding. Furthermore, the production of melatonin suffers a dramatic decline with age.13 These neurochemical changes may have etiologic implications in the altered behavior observed in old individuals and an underlying cause of several geriatric conditions, including the impaired sleep/wake cycle and immunosenescence. The evidence obtained in the ringdove and other animal models suggests that the supplemental administration of tryptophan, e.g. the inclusion of tryptophan-enriched food in the diet, might help to remediate the reduction in serotonin and melatonin that normally occurs as animals age, and be consequently beneficial in the treatment of sleep problems and alterations in the innate immune response.
Acknowledgments
This investigation was supported by a research grant from Consejería de Infraestructuras y Desarrollo Tecnológico (Junta de Extremadura, 3PR05A053). S.D. Paredes was the beneficiary of a grant from Consejería de Economía, Comercio e Innovación—Fondo Social Europeo (Junta de Extremadura, POS07012).
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- 1.Steinberg LA, O’Connell NC, Hatch TF, et al. Tryptophan intake influences infants’ sleep latency. J Nutr. 1992;122:1781–91. doi: 10.1093/jn/122.9.1781. [DOI] [PubMed] [Google Scholar]
- 2.Jansman AJM, Kemp GWP, van Cauwenberghe S. Effect of the level of branch chain amino acids (BCAA) and tryptophan in the diet of performance of piglets. Book of Abstracts of the 51st EAAP congress; The Hague, The Nethelands. 2000. p. 396. [Google Scholar]
- 3.Gómez G, Llorca R. Aminoácidos. Biopsicología. 2000;3:548–76. [Google Scholar]
- 4.Cooper JR, Bloom FE, Roth RH. The biochemical basis of neuropharmacology. 6th ed. New York: Oxford University Press; 1991. [Google Scholar]
- 5.Reiter RJ, Richardson BA, Johnson LY, et al. Pineal melatonin rhythm: reduction in aging Syrian hamsters. Science. 1980;210:1372–3. doi: 10.1126/science.7434032. [DOI] [PubMed] [Google Scholar]
- 6.Reiter RJ, Craft CM, Johnson JE, Jr, et al. Age-associated reduction in nocturnal melatonin levels in female rats. Endocrinology. 1981;109:1295–7. doi: 10.1210/endo-109-4-1295. [DOI] [PubMed] [Google Scholar]
- 7.Paredes SD, Terrón MP, Cubero J, et al. Comparative study of the activity/rest rhythms in young and old ringdove (Streptopelia risoria): correlation with serum levels of melatonin and serotonin. Chronobiol Int. 2006;23:779–93. doi: 10.1080/07420520600827145. [DOI] [PubMed] [Google Scholar]
- 8.Ruzsas C, Mess B. Melatonin and aging. A brief survey. Neuro Endocrinol Lett. 2000;21:17–23. [PubMed] [Google Scholar]
- 9.Pietraszek MH, Urano T, Serizawa S, et al. Circadian rhythm of serotonin: influence of age. Thromb Res. 1990;60:253–7. doi: 10.1016/0049-3848(90)90188-i. [DOI] [PubMed] [Google Scholar]
- 10.Garau C, Aparicio S, Rial RV, et al. Age-related changes in circadian rhythm of serotonin synthesis in ring doves: effects of increased tryptophan ingestion. Exp Gerontol. 2006a;41:40–8. doi: 10.1016/j.exger.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Zhdanova IV. Melatonin as a hypnotic: pro. Sleep Med Rev. 2005;9:51–65. doi: 10.1016/j.smrv.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Wurtman RJ. Age-related decreases in melatonin secretion—clinical consequences. J Clin Endocrinol Metab. 2000;85:2135–6. doi: 10.1210/jcem.85.6.6660. [DOI] [PubMed] [Google Scholar]
- 13.Karasek M, Reiter RJ. Melatonin and aging. Neuro Endocrinol Lett. 2002;23(Suppl):14–16. [PubMed] [Google Scholar]
- 14.Paredes SD, Barriga C, Rodríguez AB. Melatonin and tryptophan as therapeutic agents against the impairment of the sleep-wake cycle and immunosenescence due to aging in Streptopelia risoria. Neuro Endocrinol Lett. 2007a;28:757–60. [PubMed] [Google Scholar]
- 15.Sánchez S, Paredes SD, Martín MI, et al. Effect of tryptophan administration on circulating levels of melatonin and phagocytic activity. J Appl Biomed. 2004;2:169–177. [Google Scholar]
- 16.Paredes SD, Terrón MP, Cubero J, et al. Tryptophan increases nocturnal rest and affects melatonin and serotonin serum levels in old ringdove. Physiol Behav. 2007b;90:576–82. doi: 10.1016/j.physbeh.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Paredes SD, Terrón MP, Marchena AM, et al. Tryptophan modulates cell viability, phagocytosis and oxidative metabolism in old ringdoves. Basic Clin Pharmacol Toxicol. 2007c;101:56–62. doi: 10.1111/j.1742-7843.2007.00076.x. [DOI] [PubMed] [Google Scholar]
- 18.Mateos SS, Sánchez CL, Paredes SD, et al. Circadian levels of serotonina in plasma and brain after oral administration of tryptophan in rats. Basic Clin Pharmacol Toxicol. 2009;104:52–59. doi: 10.1111/j.1742-7843.2008.00333.x. [DOI] [PubMed] [Google Scholar]
- 19.Wyatt RJ, Engelman K, Kupfer DJ, et al. Effects of L-tryptophan (a natural sedative) on human sleep. Lancet. 1970;2:842–846. doi: 10.1016/s0140-6736(70)92015-5. [DOI] [PubMed] [Google Scholar]
- 20.Spinweber CL. L-tryptophan administered to chronic sleep-onset insomniacs: late-appearing reduction of sleep latency. Psychopharmacology (Berl) 1986;90:151–5. doi: 10.1007/BF00181230. [DOI] [PubMed] [Google Scholar]
- 21.Demisch K, Bauer J, Georgi K. Treatment of severe chronic insomnia with L-tryptophan and varying sleeping times. Pharmacopsychiatry. 1987;20:245–8. doi: 10.1055/s-2007-1017115. [DOI] [PubMed] [Google Scholar]
- 22.Cubero J, Narciso D, Aparicio S, et al. Improved circadian sleep-wake cycle in infants fed a day/night dissociated formula milk. Neuro Endocrinol Lett. 2006a;27:373–80. [PubMed] [Google Scholar]
- 23.Cubero J, Narciso D, Terrón P, et al. Chrononutrition applied to formula milks to consolidate infants’ sleep/wake cycle. Neuro Endocrinol Lett. 2007;28:360–6. [PubMed] [Google Scholar]
- 24.Brodie BB, Pletscher A, Shore PA. Evidence that serotonin has a role in brain function. Science. 1955;122:968. doi: 10.1126/science.122.3177.968. [DOI] [PubMed] [Google Scholar]
- 25.Jouvet M, Vimont P, Delorme F. [Elective suppression of paradoxal sleep in the cat by monoamine oxidase inhibitors] C R Seances Soc Biol Fil. 1965;159:1595–9. [PubMed] [Google Scholar]
- 26.Dahlstroem A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand Suppl. 1964;232:1–55. [PubMed] [Google Scholar]
- 27.Jouvet M. Biogenic amines and the states of sleep. Science. 1969;163:32–41. doi: 10.1126/science.163.3862.32. [DOI] [PubMed] [Google Scholar]
- 28.Koe BK, Weissman A. p-Chlorophenylalanine: a specific depletor of brain serotonin. J Pharmacol Exp Ther. 1966;154:499–516. [PubMed] [Google Scholar]
- 29.Koella WP. Serotonin and sleep. Exp Med Surg. 1969;27:157–68. [PubMed] [Google Scholar]
- 30.Jouvet M. Sleep and serotonin: an unfinished story. Neuropsychopharmacology. 1999;21:24S–7S. doi: 10.1016/S0893-133X(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 31.Huether G, Poeggeler B, Adler L, et al. Effects of indirectly acting 5-HT receptor agonists on circulating melatonin levels in rats. Eur J Pharmacol. 1993;283:249–54. doi: 10.1016/0014-2999(93)90854-b. [DOI] [PubMed] [Google Scholar]
- 32.Brismar K, Mogensen L, Wetterberg L. Depressed melatonin secretion in patients with nightmares due to beta-adrenoceptor blocking drugs. Acta Med Scand. 1987;221:155–8. doi: 10.1111/j.0954-6820.1987.tb01260.x. [DOI] [PubMed] [Google Scholar]
- 33.Brismar K, Hylander B, Eliasson K, et al. Melatonin secretion related to side-effects of beta-blockers from the central nervous system. Acta Med Scand. 1988;223:525–30. doi: 10.1111/j.0954-6820.1988.tb17690.x. [DOI] [PubMed] [Google Scholar]
- 34.Van Den Heuvel CJ, Reid KJ, Dawson D. Effect of atenolol on nocturnal sleep and temperature in young men: reversal by pharmacological doses of melatonin. Physiol Behav. 1997;61:795–802. doi: 10.1016/s0031-9384(96)00534-3. [DOI] [PubMed] [Google Scholar]
- 35.Hartter S, Wang X, Weigmann H, et al. Differential effects of fluvoxamine and other antidepressants on the biotransformation of melatonin. J Clin Psychopharmacol. 2001;21:167–74. doi: 10.1097/00004714-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Lavie P. Ultrashort sleep-waking schedule. III. ‘Gates’ and ‘forbidden zones’ for sleep. Electroencephalogr Clin Neurophysiol. 1986;63:414–25. doi: 10.1016/0013-4694(86)90123-9. [DOI] [PubMed] [Google Scholar]
- 37.Buysse DJ, Nofzinger EA, Germain A, et al. Regional brain glucose metabolism during morning and evening wakefulness in humans: preliminary findings. Sleep. 2004;27:1245–54. doi: 10.1093/sleep/27.7.1245. [DOI] [PubMed] [Google Scholar]
- 38.Long MA, Jutras MJ, Connors BW, et al. Electrical synapses coordinate activity in the suprachiasmatic nucleus. Nat Neurosci. 2005;8:61–6. doi: 10.1038/nn1361. [DOI] [PubMed] [Google Scholar]
- 39.Dijk DJ, Cajochen C. Melatonin and the circadian regulation of sleep initiation, consolidation, structure, and the sleep EEG. J Biol Rhythms. 1997;12:627–35. doi: 10.1177/074873049701200618. [DOI] [PubMed] [Google Scholar]
- 40.Tzischinsky O, Shlitner A, Lavie P. The association between the nocturnal sleep gate and nocturnal onset of urinary 6-sulfatoxymelatonin. J Biol Rhythms. 1993;8:199–209. doi: 10.1177/074873049300800303. [DOI] [PubMed] [Google Scholar]
- 41.Shochat T, Luboshitzky R, Lavie P. Nocturnal melatonin onset is phase locked to the primary sleep gate. Am J Physiol. 1997;273:R364–70. doi: 10.1152/ajpregu.1997.273.1.R364. [DOI] [PubMed] [Google Scholar]
- 42.Lavie P. Melatonin: role in gating nocturnal rise in sleep propensity. J Biol Rhythms. 1997;12:657–65. doi: 10.1177/074873049701200622. [DOI] [PubMed] [Google Scholar]
- 43.Liu C, Weaver DR, Jin X, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 44.Hunt AE, Al-Ghoul WM, Gillette MU, et al. Activation of MT(2) melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am J Physiol Cell Physiol. 2001;280:C110–8. doi: 10.1152/ajpcell.2001.280.1.C110. [DOI] [PubMed] [Google Scholar]
- 45.Golombek DA, Pevet P, Cardinali DP. Melatonin effects on behavior: possible mediation by the central GABAergic system. Neurosci Biobehav Rev. 1996;20:403–12. doi: 10.1016/0149-7634(95)00052-6. [DOI] [PubMed] [Google Scholar]
- 46.Tenn CC, Niles LP. The antidopaminergic action of S-20098 is mediated by benzodiazepine/GABA(A) receptors in the striatum. Brain Res. 1997;756:293–6. doi: 10.1016/s0006-8993(97)00244-8. [DOI] [PubMed] [Google Scholar]
- 47.Waldhauser F, Saletu B, Trinchard-Lugan I. Sleep laboratory investigations on hypnotic properties of melatonin. Psychopharmacology (Berl) 1990;100:222–6. doi: 10.1007/BF02244410. [DOI] [PubMed] [Google Scholar]
- 48.Grad BR, Rozencwaig R. The role of melatonin and serotonin in aging: update. Psychoneuroendocrinology. 1993;14:283–95. doi: 10.1016/0306-4530(93)90025-g. [DOI] [PubMed] [Google Scholar]
- 49.Nave R, Peled R, Lavie P. Melatonin improves evening napping. Eur J Pharmacol. 1995;275:213–6. doi: 10.1016/0014-2999(94)00769-4. [DOI] [PubMed] [Google Scholar]
- 50.Hughes RJ, Badia P. Sleep-promoting and hypothermic effects of daytime melatonin administration in humans. Sleep. 1997;20:124–31. [PubMed] [Google Scholar]
- 51.Satomura T, Sakamoto T, Shirakawa S, et al. Hypnotic action of melatonin during daytime administration and its comparison with triazolam. Psychiatry Clin Neurosci. 2001;55:303–4. doi: 10.1046/j.1440-1819.2001.00868.x. [DOI] [PubMed] [Google Scholar]
- 52.Dollins AB, Zhdanova IV, Wurtman RJ, et al. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc Natl Acad Sci U S A. 1994;91:1824–8. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Savaskan E, Ayoub MA, Ravid R, et al. Reduced hippocampal MT2 melatonin receptor expression in Alzheimer’s disease. J Pineal Res. 2005;38:10–16. doi: 10.1111/j.1600-079X.2004.00169.x. [DOI] [PubMed] [Google Scholar]
- 54.Paredes SD, Korkmaz A, Manchester LC, et al. Phytomelatonin: A review. J Exp Bot. 2009a doi: 10.1093/jxb/ern284. [DOI] [PubMed] [Google Scholar]
- 55.Yatham LN, Liddle PF, Shiah IS, et al. Effects of rapid tryptophan depletion on brain 5-HT(2) receptors: a PET study. Br J Psychiatry. 2001;178:448–53. doi: 10.1192/bjp.178.5.448. [DOI] [PubMed] [Google Scholar]
- 56.Maes M, Bosmans E, De Jongh R, et al. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–8. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 57.Song C, Lin A, Bonaccorso S, et al. The inflammatory response system and the availability of plasma tryptophan in patients with primary sleep disorders and major depression. J Affect Disord. 1998;49:211–9. doi: 10.1016/s0165-0327(98)00025-1. [DOI] [PubMed] [Google Scholar]
- 58.Maes M, Lin A, Bosmans E, et al. Serotonin-immune interactions in detoxified chronic alcoholic patients without apparent liver disease: activation of the inflammatory response system and lower plasma total tryptophan. Psychiatry Res. 1998;78:151–61. doi: 10.1016/s0165-1781(98)00010-9. [DOI] [PubMed] [Google Scholar]
- 59.Uthgenannt D, Schoolmann D, Pietrowsky R, et al. Effects of sleep on the production of cytokines in humans. Psychosom Med. 1995;57:97–104. doi: 10.1097/00006842-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 60.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 61.Dinges DF, Douglas SD, Zaugg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93:1930–9. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Esteban S, Nicolaus C, Garmundi A, et al. Effect of orally administered L-tryptophan on serotonin, melatonin, and the innate immune response in the rat. Mol Cell Biochem. 2004;267:39–46. doi: 10.1023/b:mcbi.0000049363.97713.74. [DOI] [PubMed] [Google Scholar]
- 63.Barriga C, Martín MI, Tabla R, et al. Circadian rhythm of melatonin, corticosterone and phagocytosis: effect of stress. J Pineal Res. 2001;30:180–7. doi: 10.1034/j.1600-079x.2001.300307.x. [DOI] [PubMed] [Google Scholar]
- 64.Barriga C, Martín MI, Ortega E, et al. Physiological concentrations of melatonin and corticosterone in stress and their relationship with phagocytic activity. J Neuroendocrinol. 2002a;14:691–5. doi: 10.1046/j.1365-2826.2002.00823.x. [DOI] [PubMed] [Google Scholar]
- 65.Rodríguez AB, Terrón MP, Duran J, et al. Physiological concentrations of melatonin and corticosterone affect phagocytosis and oxidative metabolism of ring dove heterophils. J Pineal Res. 2001;31:31–38. doi: 10.1034/j.1600-079x.2001.310105.x. [DOI] [PubMed] [Google Scholar]
- 66.Paredes SD, Sánchez S, Parvez H, et al. Altered circadian rhythms of corticosterone, melatonin, and phagocytic activity in response to stress in rats. Neuro Endocrinol Lett. 2007d;28:489–95. [PubMed] [Google Scholar]
- 67.Martins E, Jr, Ferreira AC, Skorupa AL, et al. Tryptophan consumption and indoleamines production by peritoneal cavity macrophages. J Leukocyte Biol. 2004;75:1116–1121. doi: 10.1189/jlb.1203614. [DOI] [PubMed] [Google Scholar]
- 68.Mossner R, Lesch KP. Role of serotonin in the immune system and in neuroimmune interactions. Brain Behav Immun. 1998;12:249–71. doi: 10.1006/brbi.1998.0532. [DOI] [PubMed] [Google Scholar]
- 69.Hofstetter HH, Mossner R, Lesch KP, et al. Absence of reuptake of serotonin influences susceptibility to clinical autoimmune disease and neuroantigen-specific interferon-gamma production in mouse EAE. Clin Exp Immunol. 2005;142:39–44. doi: 10.1111/j.1365-2249.2005.02901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schuff-Werner P, Splettstosser W, Schmidt F, et al. Serotonin acts as a radical scavenger and is oxidized to a dimer during the respiratory burst of human mononuclear and polymorphonuclear phagocytes. Eur J Clin Invest. 1995;25:477–84. doi: 10.1111/j.1365-2362.1995.tb01733.x. [DOI] [PubMed] [Google Scholar]
- 71.Schuff-Werner P, Splettstoesser W. Antioxidative properties of serotonin and the bactericidal function of polymorphonuclear phagocytes. Adv Exp Med Biol. 1999;467:321–5. doi: 10.1007/978-1-4615-4709-9_41. [DOI] [PubMed] [Google Scholar]
- 72.Betten A, Dahlgren C, Hermodsson S, et al. Serotonin protects NK cells against oxidatively induced functional inhibition and apoptosis. J Leukoc Biol. 2001;70:65–72. [PubMed] [Google Scholar]
- 73.Nannmark U, Sennerby L, Bjursten LM, et al. Inhibition of leukocyte phagocytosis by serotonin and its possible role in tumor cell destruction. Cancer Lett. 1992;62:83–6. doi: 10.1016/0304-3835(92)90202-7. [DOI] [PubMed] [Google Scholar]
- 74.Salman-Tabcheh S, Guerin MC, Torreilles J. Potential role of the peroxidase-dependent metabolism of serotonin in lowering the polymorphonuclear leukocyte bactericidal function. Free Radic Res. 1996;24:61–8. doi: 10.3109/10715769609088000. [DOI] [PubMed] [Google Scholar]
- 75.Sánchez S, Sánchez C, Paredes SD, et al. Circadian variations of serotonin in plasma and different brain regions of rats. Mol Cell Biochem. 2008;317:105–111. doi: 10.1007/s11010-008-9836-z. [DOI] [PubMed] [Google Scholar]
- 76.Carrillo-Vico A, Guerrero JM, Lardone PJ, et al. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27:189–200. doi: 10.1385/ENDO:27:2:189. [DOI] [PubMed] [Google Scholar]
- 77.Carrillo-Vico A, Reiter RJ, Lardone PJ, et al. The modulatory role of melatonin on immune responsiveness. Curr Opin Investig Drugs. 2006;7:423–31. [PubMed] [Google Scholar]
- 78.Berger J. A two-clock model of circadian timing in the immune system of mammals. Pathol Biol (Paris) 2008;56:286–91. doi: 10.1016/j.patbio.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 79.Brainard GC, Watson-Whitmeyer M, Knobler RL, et al. Neuroendocrine regulation of immune parameters. Photoperiod control of the spleen in Syrian hamsters. Ann N Y Acad Sci. 1988;540:704–6. doi: 10.1111/j.1749-6632.1988.tb27219.x. [DOI] [PubMed] [Google Scholar]
- 80.Maestroni GJ, Hertens E, Galli P, et al. Melatonin-induced T-helper cell hematopoietic cytokines resembling both interleukin-4 and dynorphin. J Pineal Res. 1996;21:131–9. doi: 10.1111/j.1600-079x.1996.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 81.Scalabrino G, Ferioli ME, Nebuloni R, et al. Effects of pinealectomy on the circadian rhythms of the activities of polyamine biosynthetic decarboxylases and tyrosine adminotransferase in different organs of the rat. Endocrinology. 1979a;104:377–84. doi: 10.1210/endo-104-2-377. [DOI] [PubMed] [Google Scholar]
- 82.Scalabrino G, Ferioli ME, Basagri M, et al. Endocrine regulation of thymic biosynthetic polyamine decarboxylases in adult rat. Am J Physiol. 1979b;237:E6–10. doi: 10.1152/ajpendo.1979.237.1.E6. [DOI] [PubMed] [Google Scholar]
- 83.Fraschini F, Ferioli ME, Nebuloni R, et al. Pineal gland and polyamines. J Neural Transm. 1980;48:209–21. doi: 10.1007/BF01243505. [DOI] [PubMed] [Google Scholar]
- 84.Jankovic BD, Knezevic Z, Kojic L, et al. Pineal gland and immune system. Immune functions in the chick embryo pinealectomized at 96 hours of incubation. Ann N Y Acad Sci. 1994;719:398–409. doi: 10.1111/j.1749-6632.1994.tb56845.x. [DOI] [PubMed] [Google Scholar]
- 85.del Gobbo V, Libri V, Villani N, et al. Pinealectomy inhibits interleukin-2 production and natural killer activity in mice. Int J Immunopharmacol. 1989;11:567–73. doi: 10.1016/0192-0561(89)90187-2. [DOI] [PubMed] [Google Scholar]
- 86.Libri V, Del Gobbo V, Villani N, et al. Influence of pineal gland lesion on interleukin-2 production and natural killer activity in C57BL/6 mice. Pharmacol Res. 1990;22(Suppl 3):52. doi: 10.1016/s1043-6618(09)80024-8. [DOI] [PubMed] [Google Scholar]
- 87.Rodríguez AB, Lea RW. Effect of pinealectomy upon the nonspecific immune response of the ring-dove (Streptopelia risoria) J Pineal Res. 1994;16:159–66. doi: 10.1111/j.1600-079x.1994.tb00096.x. [DOI] [PubMed] [Google Scholar]
- 88.Yellon SM. Daily melatonin treatments regulate the circadian melatonin rhythm in the adult Djungarian hamster. J Biol Rhythms. 1996;11:4–13. doi: 10.1177/074873049601100101. [DOI] [PubMed] [Google Scholar]
- 89.Haldar C, Singh R, Guchhait P. Relationship between the annual rhythms in melatonin and immune system status in the tropical palm squirrel Funambulus pennanti. Chronobiol Int. 2001;18:61–69. doi: 10.1081/cbi-100001174. [DOI] [PubMed] [Google Scholar]
- 90.Moore CB, Siopes TD, Steele CT, et al. Pineal melatonin secretion, but not ocular melatonin secretion, is sufficient to maintain normal immune responses in Japanese quail (Coturnix coturnix japonica) Gen Comp Endocrinol. 2002;126:352–8. doi: 10.1016/s0016-6480(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 91.Pioli C, Caroleo MC, Nistico G, et al. Melatonin increases antigen presentation and amplifies specific and non specific signals for T-cell proliferation. Int J Immunopharmacol. 1993;15:463–8. doi: 10.1016/0192-0561(93)90060-c. [DOI] [PubMed] [Google Scholar]
- 92.Molinero P, Soutto M, Benot S, et al. Melatonin is responsible for the nocturnal increase observed in serum and thymus of thymosin alpha1 and thymulin concentrations: observations in rats and humans. J Neuroimmunol. 2000;103:180–8. doi: 10.1016/s0165-5728(99)00237-4. [DOI] [PubMed] [Google Scholar]
- 93.Raghavendra V, Singh V, Kulkarni SK, et al. Melatonin enhances Th2 cell mediated immune responses: lack of sensitivity to reversal by naltrexone or benzodiazepine receptor antagonists. Mol Cell Biochem. 2001;221:57–62. doi: 10.1023/a:1010968611716. [DOI] [PubMed] [Google Scholar]
- 94.Liu F, Ng TB, Fung MC. Pineal indoles stimulate the gene expression of immunomodulating cytokines. J Neural Transm. 2001;108:397–405. doi: 10.1007/s007020170061. [DOI] [PubMed] [Google Scholar]
- 95.Reiter RJ, Tan DX, Osuna C, et al. Actions of melatonin in the reduction of oxidative stress: A review. J Biomed Sci. 2000;7:444–58. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 96.d’Emmanuele di Villa Bianca R, Marzocco S, Di Paola R, et al. Melatonin prevents lipopolysaccharide-induced hyporeactivity in rat. J Pineal Res. 2004;36:146–54. doi: 10.1046/j.1600-079x.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 97.Maestroni GJ, Conti A, Pierpaoli W. Role of the pineal gland in immunity. III. Melatonin antagonizes the immunosuppressive effect of acute stress via an opiatergic mechanism. Immunology. 1988;63:465–9. [PMC free article] [PubMed] [Google Scholar]
- 98.Ben-Nathan D, Maestroni GJ, Lustig S, et al. Protective effects of melatonin in mice infected with encephalitis viruses. Arch Virol. 1995;140:223–30. doi: 10.1007/BF01309858. [DOI] [PubMed] [Google Scholar]
- 99.Bonilla E, Valero-Fuenmayor N, Pons H, et al. Melatonin protects mice infected with Venezuelan equine encephalomyelitis virus. Cell Mol Life Sci. 1997;53:430–34. doi: 10.1007/s000180050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bonilla E, Rodon C, Valero N, et al. Melatonin prolongs survival of immunodepressed mice infected with the Venezuelan equine encephalomyelitis virus. Trans R Soc Trop Med Hyg. 2001;95:207–10. doi: 10.1016/s0035-9203(01)90170-1. [DOI] [PubMed] [Google Scholar]
- 101.Bonilla E, Valero N, Chacín-Bonilla L, et al. Melatonin increases interleukin-1beta and decreases tumor necrosis factor alpha in the brain of mice infected with the Venezuelan equine encephalomyelitis virus. Neurochem Res. 2003;28:681–6. doi: 10.1023/a:1022897314108. [DOI] [PubMed] [Google Scholar]
- 102.Timiras P. Physiological basis of aging and geriatrics. 2nd ed. Boca Raton: CRC Press; 1994. [Google Scholar]
- 103.Reeves S, Bench C, Howard R. Ageing and the nigrostriatal dopaminergic system. Int J Geriatr Psychiatry. 2002;17:359–70. doi: 10.1002/gps.606. [DOI] [PubMed] [Google Scholar]
- 104.Mrak RE, Griffin ST, Graham DI. Aging-associated changes in human brain. J Neuropathol Exp Neurol. 1997;56:1269–75. doi: 10.1097/00005072-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 105.Mattson MP. Cellular and neurochemical aspects of aging human brain. In: Hazzard WR, Blass JP, Ettinger WH, Halter JB, Ouslander JG, editors. Principles of geriatric medicine and gerontology. 5th ed. New York: McGraw-Hill; 1999. pp. 1193–1208. [Google Scholar]
- 106.Meltzer CC, Smith G, Price JC, et al. Reduced binding of [18F]altanserin to serotonin type 2A receptors in aging: persistence of effect after partial volume correction. Brain Res. 1998;813:167–71. doi: 10.1016/s0006-8993(98)00909-3. [DOI] [PubMed] [Google Scholar]
- 107.Maines LW, Keck BJ, Smith JE, et al. Corticosterone regulation of serotonin transporter and 5-HT1A receptor expression in the aging brain. Synapse. 1999;32:58–66. doi: 10.1002/(SICI)1098-2396(199904)32:1<58::AID-SYN8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 108.Ursin R. Serotonin and sleep. Sleep Med Rev. 2002;6:55–69. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- 109.Glass JD, DiNardo LA, Ehlen JC. Dorsal raphe nuclear stimulation of SCN serotonin and circadian phase-resetting. Brain Res. 2000;859:224–32. doi: 10.1016/s0006-8993(00)01963-6. [DOI] [PubMed] [Google Scholar]
- 110.Arendt J. Melatonin and the mammalian pineal gland. London: Chapman and Hall; 1995. [Google Scholar]
- 111.Karasek M. Melatonin in humans—where we are 40 years after its discovery. Neuro Endocrinol Lett. 1999;20:179–88. [PubMed] [Google Scholar]
- 112.Karasek M, Reiter RJ, Cardinali DP, et al. Future of melatonin as a therapeutic agent. Neuro Endocrinol Lett. 2002;23(Suppl 1):118–121. [PubMed] [Google Scholar]
- 113.Myers BL, Badia P. Changes in circadian rhythms and sleep quality with aging: mechanisms and interventions. Neurosci Biobehav Rev. 1995;19:553–71. doi: 10.1016/0149-7634(95)00018-6. [DOI] [PubMed] [Google Scholar]
- 114.Rodríguez AB, Barriga C, Paredes SD, et al. Age, melatonin and the immune system. In: Pandalai SG, editor. Recent Research Developments in Molecular and Cellular Biochemistry. Vol. 2, Part II. Trivandrum: Research Sign Post; 2005. pp. 255–87. [Google Scholar]
- 115.Bergiannaki JD, Soldatos CR, Paparrigopoulos TJ, et al. Low and high melatonin excretors among healthy individuals. J Pineal Res. 1995;18:159–64. doi: 10.1111/j.1600-079x.1995.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 116.Arendt J. Melatonin. Clin Endocrinol (Oxf) 1988;29:205–29. doi: 10.1111/j.1365-2265.1988.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 117.Pandi-Perumal SR, Seils LK, Kayumov L, et al. Senescence, sleep, and circadian rhythms. Ageing Res Rev. 2002;1:559–604. doi: 10.1016/s1568-1637(02)00014-4. [DOI] [PubMed] [Google Scholar]
- 118.Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 119.Pandi-Perumal SR, Zisapel N, Srinivasan V, et al. Melatonin and sleep in aging population. Exp Gerontol. 2005;40:911–25. doi: 10.1016/j.exger.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 120.Blanco M, Kriber N, Cardinali DP. [A survey of sleeping difficulties in an urban Latin American population] Rev Neurol. 2004;39:115–19. [PubMed] [Google Scholar]
- 121.Blanco M, Kriguer N, Lloret SP, et al. Attitudes towards treatment among patients suffering from sleep disorders. A Latin American survey. BMC Fam Pract. 2003;4:17. doi: 10.1186/1471-2296-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Duffy JF, Czeisler CA. Age-related change in the relationship between circadian period, circadian phase, and diurnal preference in humans. Neurosci Lett. 2002;318:117–20. doi: 10.1016/s0304-3940(01)02427-2. [DOI] [PubMed] [Google Scholar]
- 123.Daan S, Beersma DG, Borbely AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–83. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 124.Swaab DF, Dubelaar EJ, Hofman MA, et al. Brain aging and Alzheimer’s disease; use it or lose it. Prog Brain Res. 2002;138:343–73. doi: 10.1016/S0079-6123(02)38086-5. [DOI] [PubMed] [Google Scholar]
- 125.Ginaldi L, De Martinis M, D’Ostilio A, et al. The immune system in the elderly: I. Specific humoral immunity. Immunol Res. 1999a;20:101–8. doi: 10.1007/BF02786466. [DOI] [PubMed] [Google Scholar]
- 126.Rea IM, Stewart M, Campbell P, et al. Changes in lymphocyte subsets, interleukin 2, and soluble interleukin 2 receptor in old and very old age. Gerontology. 1996;42:69–78. doi: 10.1159/000213775. [DOI] [PubMed] [Google Scholar]
- 127.Pawelec G. Immunosenescence: impact in the young as well as the old. Mech Ageing Dev. 1999;108:1–7. doi: 10.1016/s0047-6374(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 128.McLeod JD. Apoptotic capability in ageing T cells. Mech Ageing Dev. 2000;121:151–9. doi: 10.1016/s0047-6374(00)00206-2. [DOI] [PubMed] [Google Scholar]
- 129.Richter M, Jodouin CA. The delay in the synthesis and secretion of immunoglobulins by the B cells of healthy ambulatory elderly is due to subtle defects in the null cells and the B cells. Aging Immunol Infect Dis. 1993;4:1–16. [Google Scholar]
- 130.Song H, Price PW, Cerny J. Age-related changes in antibody repertoire: contribution from T cells. Immunol Rev. 1997;160:55–62. doi: 10.1111/j.1600-065x.1997.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 131.McArthur WP. Effect of aging on immunocompetent and inflammatory cells. Periodontol. 2000;1998;16:53–79. doi: 10.1111/j.1600-0757.1998.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 132.Pierpaoli W, Lesnikov V. Theoretical considerations on the nature of the pineal ‘ageing clock.’. Gerontology. 1997;43:20–5. doi: 10.1159/000213833. [DOI] [PubMed] [Google Scholar]
- 133.Reiter RJ, Tan D, Kim SJ, et al. Augmentation of indices of oxidative damage in life-long melatonin-deficient rats. Mech Ageing Dev. 1999;110:157–73. doi: 10.1016/s0047-6374(99)00058-5. [DOI] [PubMed] [Google Scholar]
- 134.Reiter RJ. Melatonin: Lowering the high price of free radicals. News Physiol Sci. 2000a;15:246–250. doi: 10.1152/physiologyonline.2000.15.5.246. [DOI] [PubMed] [Google Scholar]
- 135.Reiter RJ. Melatonin and aging. In: Mosley JE, Armbrecht HJ, Coe RM, Vellas B, editors. The Science of Geriatrics. Vol I. New York: Springer; 2000b. pp. 232–333. [Google Scholar]
- 136.Tan DX, Manchester LC, Terron MP, et al. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and reactive nitrogen species. J Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 137.Tan DX, Reiter RJ, Manchester LC, et al. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2:181–97. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- 138.Reiter RJ, Tan DX, Jou MJ, et al. Biogenic amines in the reduction of oxidative stress: Melatonin and its metabolites. Biogenic Amines. 2008;22:1–15. [PubMed] [Google Scholar]
- 139.Rodriguez C, Mayo JC, Sainz RM, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 140.Tomas-Zapico C, Coto-Montes A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J Pineal Res. 2005;39:99–104. doi: 10.1111/j.1600-079X.2005.00248.x. [DOI] [PubMed] [Google Scholar]
- 141.Pablos MI, Agapito MT, Guiterrez R, et al. Melatonin stimulates the activity of the detoxifying enzyme glutathione peroxidase in several tissues of chicks. J Pineal Res. 1995;19:111–5. doi: 10.1111/j.1600-079x.1995.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 142.Barlow-Walden LR, Reiter RJ, Abe M, et al. Melatonin stimulates glutathione peroxidase activity. Neurochem Int. 1995;26:497–502. doi: 10.1016/0197-0186(94)00154-m. [DOI] [PubMed] [Google Scholar]
- 143.Tan DX, Chen LD, Poeggeler B, et al. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J. 1993;1:57–60. [Google Scholar]
- 144.Tan D, Reiter RJ, Chen LD, et al. Both physiological and pharmacological levels of melatonin reduce DNA adduct formation induced by the carcinogen safrole. Carcinogenesis. 1994;15:215–8. doi: 10.1093/carcin/15.2.215. [DOI] [PubMed] [Google Scholar]
- 145.Rao KS, Loeb LA. DNA damage and repair in brain: relationship to aging. Mutat Res. 1992;275:317–29. doi: 10.1016/0921-8734(92)90035-n. [DOI] [PubMed] [Google Scholar]
- 146.Strong R, Mattamal MB, Andor AC. Free radicals, the aging brain, and age-related neurodegeerative disorders. In: Yu BP, editor. Free Radicals in Aging. Boca Raton: CRC Press; 1993. [Google Scholar]
- 147.Acuña-Castroviejo D, Coto-Montes A, Gaia Monti M, et al. Melatonin is protective against MPTP-induced striatal and hippocampal lesions. Life Sci. 1997;60:PL23–9. doi: 10.1016/s0024-3205(96)00606-6. [DOI] [PubMed] [Google Scholar]
- 148.Mayo JC, Sainz RM, Tan DX, et al. Melatonin and Parkinson’s disease. Endocrine. 2005;27:169–78. doi: 10.1385/ENDO:27:2:169. [DOI] [PubMed] [Google Scholar]
- 149.van Rensburg SJ, Daniels WM, van Zyl JM, et al. A comparative study of the effects of cholesterol, beta-sitosterol, beta-sitosterol glucoside, dehydroepiandrosterone sulphate and melatonin on in vitro lipid peroxidation. Metab Brain Dis. 2000;15:257–65. doi: 10.1023/a:1011167023695. [DOI] [PubMed] [Google Scholar]
- 150.Ferrari CK. Functional foods, herbs and nutraceuticals: towards biochemical mechanisms of healthy aging. Biogerontology. 2004;5:275–89. doi: 10.1007/s10522-004-2566-z. [DOI] [PubMed] [Google Scholar]
- 151.Bondy SC, Sharman EH. Melatonin and the aging brain. Neurochem Int. 2007;50:571–80. doi: 10.1016/j.neuint.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 152.Rodríguez AB, Marchena JM, Nogales G, et al. Correlation between the circadian rhythm of melatonin, phagocytosis, and superoxide anion levels in ring dove heterophils. J Pineal Res. 1999a;26:35–42. doi: 10.1111/j.1600-079x.1999.tb00564.x. [DOI] [PubMed] [Google Scholar]
- 153.Terron M, del P, Paredes SD, Barriga C, et al. Oral administration of melatonin to old ring doves (Streptopelia risoria) increases plasma levels of melatonin and heterophil phagocytic activity. J Gerontol A Bio Sci Med Sci. 2005a;60:44–50. doi: 10.1093/gerona/60.1.44. [DOI] [PubMed] [Google Scholar]
- 154.Paredes SD, Terrón MP, Marchena AM, et al. Effect of exogenous melatonin on viability, ingestion capacity, and free-radical scavenging in heterophils from young and old ringdoves (Streptopelia risoria) Mol Cell Biochem. 2007e;304:305–14. doi: 10.1007/s11010-007-9513-7. [DOI] [PubMed] [Google Scholar]
- 155.Terrón MP, Cubero J, Barriga C, et al. Phagocytosis of Candida albicans and superoxide anion Levels in ring dove (Streptopelia risoria) heterophils: effect of melatonin. J Neuroendocrinol. 2003;15:1111–5. doi: 10.1111/j.1365-2826.2003.01103.x. [DOI] [PubMed] [Google Scholar]
- 156.Rodríguez AB, Ortega E, Lea RW, et al. Melatonin and the phagocytic process of heterophils from the ring dove (Streptopelia risoria) Mol Cell Biochem. 1997;168:185–90. doi: 10.1023/a:1006850518225. [DOI] [PubMed] [Google Scholar]
- 157.Rodríguez AB, Nogales G, Ortega E, et al. Melatonin controls superoxide anion level: modulation of superoxide dismutase activity in ring dove heterophils. J Pineal Res. 1998;24:9–14. doi: 10.1111/j.1600-079x.1998.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 158.Rodríguez AB, Nogales G, Marchena JM, et al. Suppression of both basal and antigen-induced lipid peroxidation in ring dove heterophils by melatonin. Biochem Pharmacol. 1999b;58:1301–6. doi: 10.1016/s0006-2952(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 159.Barriga C, Marchena JM, Lea RW, et al. Effect of stress and dexamethasone treatment on circadian rhythms of melatonin and corticosterone in ring dove (Streptopelia risoria) Mol Cell Biochem. 2002b;232:27–31. doi: 10.1023/a:1014833030530. [DOI] [PubMed] [Google Scholar]
- 160.Paredes SD, Sánchez S, Rial RV, et al. Changes in behaviour and in the circadian rhythms of melatonin and corticosterone in rats subjected to a forced-swimming test. J Appl Biomed. 2005;3:47–56. [Google Scholar]
- 161.Paredes SD, Terrón MP, Valero V, et al. Orally administered melatonin improves nocturnal rest in young and old ringdoves (Streptopelia risoria) Basic Clin Pharmacol Toxicol. 2007f;100:258–68. doi: 10.1111/j.1742-7843.2006.00032.x. [DOI] [PubMed] [Google Scholar]
- 162.Paredes SD, Marchena AM, Bejarano I, et al. Melatonin and tryptophan affect the activity-rest rhythm, core and peripheral temperatures, and interleukin levels in the ringdove: Changes with age. J Gerontol A Biol Sci Med Sci. 2009b doi: 10.1093/gerona/gln054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Campbell SS, Murphy PJ. Relationships between sleep and body temperature in middle-aged and older subjects. J Am Geriatr Soc. 1998;46:458–62. doi: 10.1111/j.1532-5415.1998.tb02466.x. [DOI] [PubMed] [Google Scholar]
- 164.Barriga-Ibars C, Rodríguez-Moratinos AB, Esteban S, et al. [Inter-relations between sleep and the immune status] Rev Neurol. 2005;40:548–56. [PubMed] [Google Scholar]
- 165.Terrón MP, Paredes SD, Barriga C, et al. Comparative study of the heterophil phagocytic function in young and old ring doves (Streptopelia risoria) and its relationship with melatonin levels. J Comp Physiol [B] 2004;174:421–7. doi: 10.1007/s00360-004-0429-1. [DOI] [PubMed] [Google Scholar]
- 166.Terrón MP, Cubero J, Marchena JM, et al. Melatonin and aging: in vitro effect of young and mature ring dove physiological concentrations of melatonin on the phagocytic function of heterophils from old ring dove. Exp Gerontol. 2002;37:421–6. doi: 10.1016/s0531-5565(01)00209-1. [DOI] [PubMed] [Google Scholar]
- 167.Terrón MP, Paredes SD, Barriga C, et al. Melatonin, lipid peroxidation, and age in heterophils from the ring dove (Streptopelia risoria) Free Radic Res. 2005b;39:613–9. doi: 10.1080/10715760500097831. [DOI] [PubMed] [Google Scholar]
- 168.Ouichou A, Pevet P. Implication of tryptophan in the stimulatory effect of delta-sleep-inducing peptide on indole secretion from perifused rat pineal glands. Biol Signals. 1992;1:78–87. doi: 10.1159/000109313. [DOI] [PubMed] [Google Scholar]
- 169.Cubero J, Narciso D, Valero V, et al. The oral administration of tryptophan improves nocturnal rest in young animals: Correlation with melatonin. Biogenic Amines. 2006b;20:53–62. [Google Scholar]
- 170.Garau C, Aparicio S, Rial RV, et al. Age related changes in the activity-rest circadian rhythms and c-fos expression of ring doves with aging. Effects of tryptophan intake. Exp Gerontol. 2006b;41:430–38. doi: 10.1016/j.exger.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 171.Cirelli C, Tononi G. On the functional significance of c-fos induction during the sleep-waking cycle. Sleep. 2000;23:453–69. [PubMed] [Google Scholar]
- 172.Cubero J, Narciso D, Valero V, et al. Oral administration of L-tryptophan in the morning affects phagocytosis and oxidative metabolism in heterophils of Streptopelia roseogrisea. Biogenic Amines. 2005;19:209–21. [Google Scholar]
- 173.Maestroni GJ. The immunotherapeutic potential of melatonin. Expert Opin Investig Drugs. 2001;10:467–76. doi: 10.1517/13543784.10.3.467. [DOI] [PubMed] [Google Scholar]
- 174.Guerrero JM, Reiter RJ. Melatonin-immune system relationships. Curr Top Med Chem. 2002;2:167–79. doi: 10.2174/1568026023394335. [DOI] [PubMed] [Google Scholar]
- 175.Dubbels R, Reiter RJ, Klenke E, et al. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J Pineal Res. 1995;18:28–31. doi: 10.1111/j.1600-079x.1995.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 176.Hattori A, Migitaka H, Iigo M, et al. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Biol Int. 1995;35:627–34. [PubMed] [Google Scholar]
- 177.Badria FA. Melatonin, serotonin, and tryptamine in some egyptian food and medicinal plants. J Med Food. 2002;5:153–7. doi: 10.1089/10966200260398189. [DOI] [PubMed] [Google Scholar]
- 178.Paredes SD. Cáceres: Servicio de Publicaciones de la Universidad de Extremadura; 2007. [Effect of the administration of melatonin and tryptophan on the activity-rest rhythms, phagocytic function and oxidative metabolism in Streptopelia risoria Modifications with age] [Google Scholar]