Abstract
Wide bandgap conductors such as In2O3 and ZnO are used as transparent conducting oxides (TCOs). To date, TCOs are realized using post transition metal cations with largely spread s-orbitals such as In3+, Sn4+, Zn2+ and Cd2+. On the other hand, no good electronic conductor has been realized in oxides of Al, Si and Ge. Here we report the conversion of an oxide of Ge into a good electronic conductor by employing the concept of superdegeneracy. We find that cubic SrGeO3, synthesized under high pressure, displays a direct bandgap of 3.5 eV, a carrier mobility of 12 cm2(Vs)−1, and conductivities of 3 Scm−1 (DC) and 400 Scm−1 (optical conductivity). This is the first Ge-based electronic conductive oxide, and expands the family of TCOs from ionic oxides to covalent oxides.
 Transparent conducting oxides are wide bandgap conductors that have found a range of applications in optoelectronic devices. In this study, Hosono and colleagues fabricate the first transparent conducting oxide based on germanium.
Transparent conducting oxides are wide bandgap conductors that have found a range of applications in optoelectronic devices. In this study, Hosono and colleagues fabricate the first transparent conducting oxide based on germanium.
Transparent conducting oxides (TCOs)1, which have a high electrical conductivity and a high optical transparency in visible light, are necessary for a variety of optoelectronic applications. However, high conductivity materials are generally not optically transparent. To date, metal elements such as In, Sn, Zn and Cd ('TCO-cations') are essential for TCO formation and form a frontier in the periodic table that separates themselves from the insulating elements such as Al, Si and Ge (the only exception is 12CaO·7Al2O3 with a special nanocage structure2). These TCO-cations are post-transition metal cations with electronic configurations of (n−1)d10ns0 (n is the principal quantum number and should be ≥4 to obtain good electrical conductivities)3,4,5. The spherical spread vacant s orbitals of these metal cations form highly dispersed conduction bands (CBs) with small effective electron masses, and consequently contributes to high electron mobilities. New TCO materials such as MgIn2O4 have been developed based on this guideline6,7,8.
However, it is difficult to create a TCO based on the metal cations that are located in the upper right part of the periodic table, because the spatial spread of their vacant s orbitals is small. The small CB dispersion results in a large effective mass and this raises the energy levels of the CB minimum (CBM), which makes electron doping difficult. In reality, sufficient electronic conduction has yet been obtained for the oxides of metals such as Al, Si and Ge.
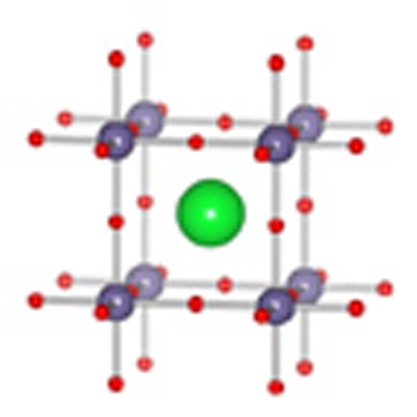
Herein, we show that cubic-SrGeO3 with the perovskite-type structure (Fig. 1a) is the first Ge-based transparent electronic conductor. This achievement proposes a new design concept for covalent TCOs, which realizes a deep CBM because of superdegeneracy that appears in high-symmetry crystals of a highly covalent nature.
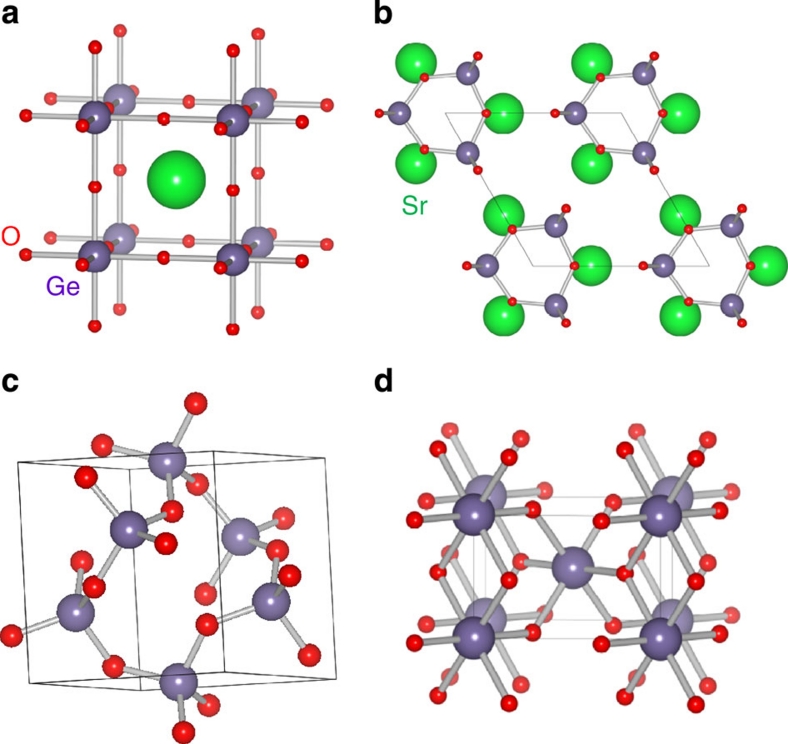
Figure 1. Crystal structures of Ge oxides.
(a) HP-SrGeO3 with cubic perovskite type. (b) AP-SrGeO3 composed of isolated rings23,24. (c) AP-GeO2 has a complex α-quartz type structure25. (d) HP-GeO2 takes rutile-type composed of GeO6 octahedra26.
Results
Sample structures
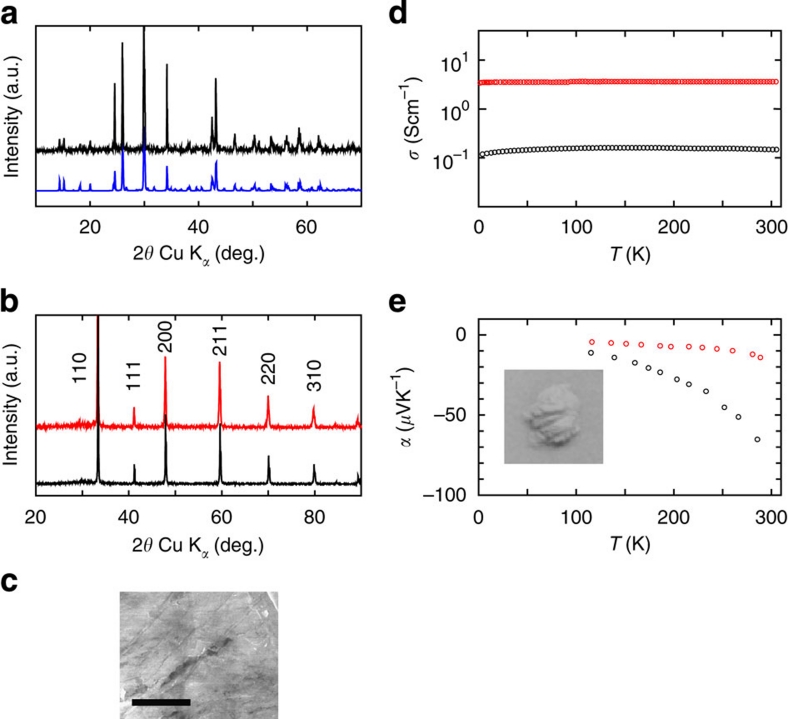
Powder samples of undoped and La-doped SrGeO3 ((Sr0.95La0.05)GeO3) were synthesized at ambient pressure (AP). These samples were identified as the AP-SrGeO3 phase by powder X-ray diffraction, as shown in Figure 2a. It has a monoclinic lattice and is composed of an isolated (GeO4) ring structure (Fig. 1b). A high-pressure (HP) treatment at 5.5 GPa and 1,100 °C converted these structures to cubic-SrGeO3 (HP-SrGeO3) with perovskite type9 (Fig. 1a), as shown in Figure 2b (a photograph of the material is shown in the inset of Fig. 2e). The observed dense microstructure of the polished surface of the pellet, shown in a scanning electron microscopic (SEM) image in Figure 2c enables us the optical reflectance measurements, as well as carrier transport experiments.
Figure 2. Powder X-ray diffraction (XRD) patterns and electrical properties.
(a) Measured powder XRD pattern of the SrGeO3 sample synthesized at AP (black symbols) along with its simulation pattern (blue symbols). (b) Measured powder XRD patterns of the undoped (black symbols) and La-doped SrGeO3 samples (red symbols) after the HP treatment. (c) SEM image of polished surface of HP-SrGeO3 pellet. Scale bar shows 3 μm. (d) Temperature dependence of the electrical conductivities and (e) Seebeck coefficients for the undoped (black symbols) and the La-doped HP-SrGeO3 (red symbols). Inset to e shows a photograph of the undoped HP-SrGeO3 powder.
Electrical properties
Figure 2d,e show the temperature dependence of the electrical conductivities and the Seebeck coefficients for the undoped and the La-doped HP-SrGeO3 samples. The electrical conductivities are nearly temperature independent. Also, the Seebeck coefficients are negative for both the samples, which indicates that these were degenerate n-type conductors. The DC conductivity, σDC, at 300 K for the La-doped sample was 3 Scm−1, being consistent with the smaller Seebeck coefficients and the smaller temperature dependence than those of the undoped HP-SrGeO3.
Optical properties and optical conductivity
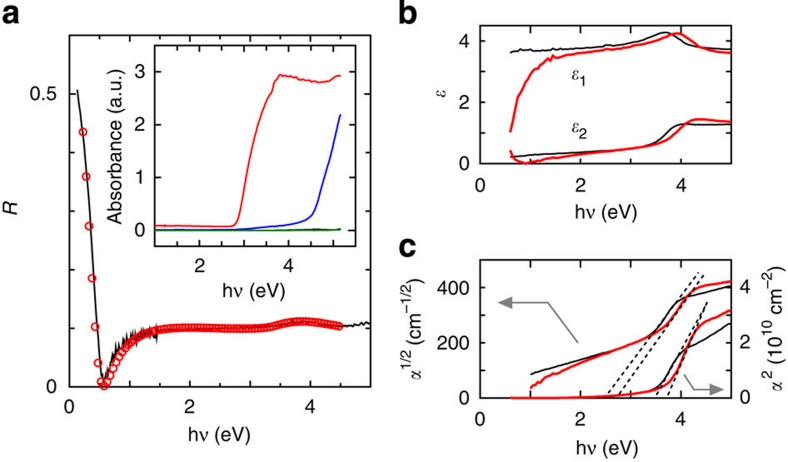
Figure 3 shows optical reflectance spectra, complex dielectric functions (ɛ*(ω)=ɛ1(ω)+jɛ2(ω), where j denotes the imaginary unit), and absorption spectra of the Ge oxides discussed in this paper. From the absorbance spectra shown in the inset of Figure 3a, it is evident that HP-GeO2 and HP-SrGeO3 have smaller bandgaps than the AP phases. The sharp increase in reflectance at <0.5 eV can be reproduced using the Drude model with a plasma frequency of 1.0 eV and a momentum relaxation time of 2.0 fs, which substantiates that HP-SrGeO3 has high-density free electrons in the CB. The optical conductivity (σopt(ω)≡ωɛ2(ω)) at the DC limit, σopt(ω=0), of 410 Scm−1 was obtained from this fitting. The carrier density (Nopt) and mobility (μopt) can be determined from these results by assuming a reasonable electron effective mass, me*. In many TCOs, the effective electron masses range between 0.25–0.35 me (me denotes the rest mass of the electron)10. Assuming me*=0.3 me, we obtained Nopt=2.2×1020 cm−3 and μopt=11.6 cm2(Vs)−1. We needed to incorporate a 75 nm thick surface insulating layer to reproduce the dip structure that occurs at 0.55 eV, which suggests that the mobile electrons are annihilated in the thin surface region.
Figure 3. Bandgaps of HP-SrGeO3 and related germanium oxides.
(a) Reflectance spectra of La-doped HP-SrGeO3. The solid line shows the measured data and the red circles show the simulated result using the Drude–Tauc–Lorentz combined model. The inset shows the diffuse reflectance spectra of the powder samples of undoped AP-GeO2 (the green line), HP-GeO2 (blue), AP-SrGeO3 (completely overlapped with the data of AP-GeO2, the green line) and HP-SrGeO3 (red). (b) Dielectric functions and (c) absorption spectra for undoped (the black lines) and La-doped HP-SrGeO3 (the red lines) obtained by spectroscopic ellipsometry. The absorption spectra are plotted for the direct and indirect transition-type semiconductors.
Electronic structure
The bandgap values were obtained from an analysis of the spectroscopic ellipsometry data shown in Figure 3b,c. The ɛ2(ω) values show clear absorption structures at >3 eV for the undoped and the La-doped SrGeO3. For undoped and La-doped SrGeO3, the indirect bandgaps (Eg,indir), which were estimated from the α0.5–hν plots, were 2.70 and 2.80 eV, respectively. The direct bandgaps (Eg,dir) estimated from the α2–hν plots were 3.45 and 3.70 eV, respectively.
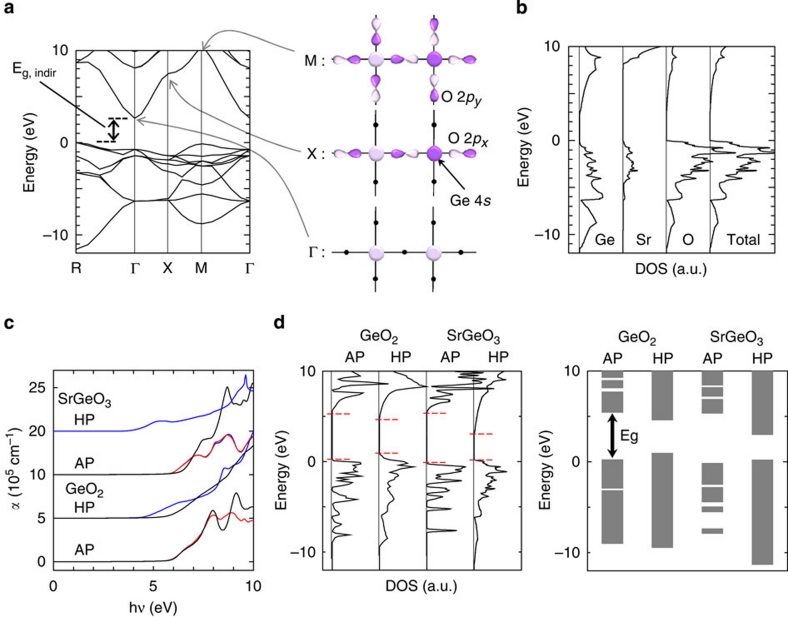
We used density functional theory to explain the high electrical conductivity of HP-SrGeO3. Figure 4a shows the band structure of HP-SrGeO3 that indicates that HP-SrGeO3 is a semiconductor with an indirect bandgap of 2.7 eV. Figure 4b shows the projected density of states (DOS) for HP-SrGeO3. Vacant Sr orbitals do not contribute to the bandgap because their contribution appears only at >8 eV, which is much higher than the CBM (located at +2.7 eV). It is thus obvious that HP-SrGeO3 is a Ge-based electronic conductor. The calculated optical absorption spectra and the DOSs of Ge oxides are shown in Figure 4c,d, respectively. The calculated absorption edge energies agree well with the experimental absorbance spectra shown in the inset of Figure 3a. However, a large discrepancy between the calculated absorption edge (3.3 eV) and bandgap (2.7 eV, extracted from the band structure in Fig. 4a) is found for HP-SrGeO3.
Figure 4. Calculated electronic structures of germanium oxides.
(a) Band structure and illustrations of the corresponding chemical bonds for HP-SrGeO3. The light and dark purple areas indicate positive and negative phases of the wave functions, respectively. The dots indicate atoms that do not contribute to the chemical bonds. (b) Total and projected DOSs (O, Sr and Ge) for HP-SrGeO3. Those of Sr and Ge are expanded by a factor of 5. (c) Calculated optical absorption spectra for AP-GeO2, HP-GeO2, AP-SrGeO3 and HP-SrGeO3. αxx, αyy and αzz are represented by the blue, red and black lines, respectively. Each spectrum is shifted by 5×105 cm−1 for clarity. (d) Total DOSs and simplified band alignment for the four Ge-based oxides. The energy is aligned by the O 2 s levels (−17 to −21 eV below their respective EVBM's). The DOSs of the unoccupied states of HP-SrGeO3 are magnified by a factor of 5. The band edges are shown by the red dashed lines.
Discussion
HP-SrGeO3 is the first Ge-based transparent electronic conductor with a bandgap of 3.5 eV, although Ge oxides are typical insulators with wide bandgaps (>5 eV). The HP-SrGeO3 is a well-known phase for geophysicists and has been investigated extensively from geophysical interests11; however, no cultivation of electronic functions has, to our knowledge, been examined so far.
To understand the peculiar properties of HP-SrGeO3, we, first, survey the characteristic features of the electronic structures of the Ge oxides. Ge ions in most of the Ge oxides synthesized at AP take tetrahedral GeO4 sites as shown in Figure 1b,c, and have large bandgaps (the inset to Fig. 3a). The insulating nature of AP-GeO2 with the α-quartz type structure (Fig. 1c) originates from the large bandgap (5.1 eV)12 and its structural flexibility. In this structure, two (GeO4) tetrahedra are connected through a twofold coordinated O ion. The tetrahedral GeO4 is composed of highly covalent Ge–O bonds, and this twofold oxygen bridging structure, (GeO4)–(GeO4), is flexible, which leads to the large bandgap and difficulty in carrier doping because of the ease of structural relaxation on impurity doping. The coordination number of Ge4+ ion increases to six in the HP phases (Fig. 1a,d), and the decrease of the bandgap is clearly observed as seen in the inset of Figure 3a.
The right-hand panel of Figure 4d shows the band alignment of these materials, which was compiled from the calculated DOSs in the left figure of Figure 4d. The CBMs of these oxides are composed of Ge 4 s and O 2p orbitals, and the valence band maximums (VBMs) are usually composed of O 2p orbitals. The differences in the VBM energies are as small as 1 eV, and therefore, the bandgap differences among these Ge oxides are caused primarily by the difference in the CBM energies.
Next, we describe the origin of the small bandgap of HP-SrGeO3 based on the concept of superdegeneracy that prohibits hybridization of relevant orbitals at the Γ point due to the symmetry and the periodicity in a crystal; that is, the transfer and overlap integrals between an s orbital and two sandwiching px orbitals are exactly zero, which leaves the s-px bonds nonbonding states at the Γ point13,14,15. The CBMs of conventional Ge-based oxides are high, because the Ge 4 s and O 2p orbitals form covalent chemical bonds and the CBM levels are raised by their resultant anti-bonding nature. However, in HP-SrGeO3 with cubic perovskite type, the CBMs belong to superdegenerate systems14,15 where the translational symmetry strictly prohibits hybridization between the Ge 4s and O 2p orbitals at the Γ point. This is illustrated in the right-bottom figure of Figure 4a. Consequently, the CBM corresponds to a nonbonding state that is exclusively composed of Ge 4s orbitals and its energy level remains unchanged. Regarding the CBM dispersion, the energy level at the X point (the right-middle figure) is significantly raised, because a Ge 4s orbital hybridizes with a lobe of an aligned O 2p orbital forming an anti-bonding orbital. Therefore, HP-SrGeO3 has a dispersed CB with a width of 7 eV, which results in a deep CBM energy and the small bandgap of 2.7 eV, despite having the largest Ge–Ge interionic distance (0.380 nm) among the four Ge-oxide crystals. The low energy and large dispersion of the CBM in the HP-SrGeO3 phase makes it possible to realize high-density electron doping and a small effective electron mass in this material.
Finally, we describe the large discrepancy between the calculated absorption edge (3.3 eV) and bandgap (2.7 eV) for HP-SrGeO3. This discrepancy originates from the fact that HP-SrGeO3 is an indirect transition-type semiconductor, which is indicated by the band structure shown in Figure 4a. The results obtained in Figures 2d,e and 3 show that the sample has a high density of doped electrons and a high electrical conductivity in addition to transparency (that is, the white colour of the sample seen in the inset of Fig. 2e). In HP-SrGeO3, the high conductivity is caused by the deep CBM, which originates from the small indirect bandgap, and the visible transparency is caused by the large direct bandgap (>3 eV).
In summary, we have found that a Ge oxide, cubic SrGeO3, is converted to a good electronic conductor by keeping visible transparency. A low CBM energy and a large CB dispersion originating from superdegeneracy in the high symmetry of the cubic perovskite structure make it possible for SrGeO3 to have both the high conductivity and the high transparency. On the basis of the present finding, we propose that wide-gap oxides with high-symmetry crystal structures and high covalency are potential candidates for new TCOs irrespective of the absence of a large overlap between the vacant s-orbitals of neighbouring metal cations. We expect this to serve as a new design concept for frontier cultivation of TCOs, and it is comparable to a design concept of 'cage conduction band'10,16, which is derived from the discovery of transparent conductive 12CaO·7Al2O3 (ref. 17).
Methods
Synthesis
Polycrystalline samples of SrGeO3 were prepared using a solid-state reaction from SrCO3, GeO2 and La2O3. Stoichiometric amounts (SrGeO3 for undoped, and (Sr0.95La0.05)GeO3 for La-doped SrGeO3) of the reagents were mixed and calcined at 1,200 °C for 10 h in air under AP to synthesize the AP-phase SrGeO3. The AP-phase samples were subjected to high-pressure reactions at 5.5 GPa and 1,100 °C for 1 h, using a belt-type HP apparatus. AP- and HP-phase GeO2 (AP-GeO2 and HP-GeO2, respectively) were also synthesized for comparison.
Characterization
Powder X-ray diffraction patterns were obtained using Cu Kα radiation to determine the crystal structure. The cation stoichiometry was confirmed by X-ray energy dispersive spectroscopy with a Hitachi S-4500 SEM equipped with a Kevex Sigma analyser.
DC electrical conductivity measurements were carried out by the conventional four-probe method. Seebeck coefficient measurements were performed using the static method.
Diffuse reflectance spectra were measured on powder samples over the spectral range of 240–2,600 nm using MgO powder as a reference. The data were transformed to absorbance spectra using the Kubelka–Munk relation. Absolute reflectance spectra were measured in the range from 250–2,500 nm with the spectrophotometer (Hitachi, U-4000) equipped with an absolute specular reflection attachment at an incident angle of 5° from the normal. IR reflectance spectra were measured using mirror-polished samples and a Fourier transform IR spectrometer (Perkin Elmer, Spectrum One). The incident angle was fixed at 20° from the normal and an Al mirror was used as a 100% reflectance standard. Optical dielectric functions were measured by spectroscopic ellipsometry (Jobin-Yvon, UVISEL) at incident/reflection angles of 70° in the photon energy range 0.6–5.0 eV. These data were analysed based on the Drude and Tauc-Lorentz model.
Band structure calculation
The band structures were calculated by hybrid functional level density functional theory using a code VASP 5.2 (ref. 18). We employed PBE0 hybrid functional with a standard mixing parameter of 25% for the exact-exchange term, because we confirmed that it gave a reasonable bandgap for ZnO (3.17 eV for the calculated value and 3.37 eV for the experimental value). Additionally, it gave the closest optical properties to the observed optical properties of the SrGeO3 samples among the different functionals used, that is, PBE96, PBE0 and HSE19,20,21,22.
Author contributions
H.M. performed the sample fabrication, measurements and fundamental data analysis. T.K. performed density-functional calculations and optical analyses. S.M. performed optical measurements. H.H. provided strategy and advice for the material exploration. All the authors contributed to discussion on the results for the manuscript.
Additional information
How to cite this article: Mizoguchi, H. et al. A germanate transparent conductive oxide. Nat. Commun. 2:470 doi: 10.1038/ncomms1484 (2011).
Acknowledgments
This work was supported by the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST), Japan. We thank Drs T. Atou, O. Fukunaga and S. Fujitsu at Tokyo Institute of Technology for their experimental supports.
References
- Ginley D. S. & Bright C. Transparent conducting oxides. MRS Bull. 25, 15–18 (2000). [Google Scholar]
- Kim S.- W. et al. Metallic state in a lime-alumina compound with nanoporous structure. Nano Lett. 7, 1138–1143 (2007). [DOI] [PubMed] [Google Scholar]
- Robertson J. Electronic structure of SnO2, GeO2, PbO2, TeO2, and MgF2. J. Phys. C 12, 4767–4776 (1979). [Google Scholar]
- Kawazoe H., Yanagi H., Ueda K. & Hosono H. Transparent p-type conducting oxides: design and fabrication of p-n heterojunctions. MRS Bull. 25, 28–36 (2000). [Google Scholar]
- Medvedeva J. E. Combining optical transparency with electrical Conductivity: challenge and prospects. in Transparent Electronics (eds Facchetti, A. & Marks, T. J.) (Wiley & Sons, 2010). [Google Scholar]
- Kawazoe H., Ueda N., Un'no H., Omata T., Hosono H. & Tanoue H. Generation of electron carriers in insulating thin film of MgIn2O4 spinel by Li+ implantation. J. Appl. Phys. 76, 7935–7941 (1994). [Google Scholar]
- Freeman A. J., Poeppelmeier K. R., Mason T. O., Chan R. P. H. & Marks T. J. Chemical and thin film strategies for new transparent conducting oxides. MRS Bull. 25, 45–51 (2000). [Google Scholar]
- Cava R. J. et al. GaInO3: a new transparent conducting oxide. Appl. Phys. Lett. 64, 2071–2072 (1994). [Google Scholar]
- Shimizu Y., Syono Y. & Akimoto S. High-pressure transformations in SrGeO3, SrSiO3, BaGeO3, and BaSiO3. High Temp. High Press. 2, 113–120 (1970). [Google Scholar]
- Medvedeva J. E. Averaging of the electron effective mass in multicomponent transparent conducting oxides. Europhys. Lett. 78, 57004-1-6 (2007). [Google Scholar]
- Grzechnik A., McMillan P. F., Chamberlin R., Hubert H. & Chizmeshya A. V. G. SrTiO3-SrGeO3 perovskites obtained at high pressure and high temperature. Eur. J. Solid State Inorg. Chem. 34, 269–281 (1997). [Google Scholar]
- Boem H. F. On the photoconductivity of the vitreous GeO2. J. Non-Cryst. Solids 7, 192–202 (1972). [Google Scholar]
- Hughbanks T. Superdegerate electronic-energy levels in extended structures. J. Am. Chem. Soc. 107, 6851–6859 (1985). [Google Scholar]
- Wheeler R. A., Whangbo M.- H., Hughbanks T., Hoffmann R., Burdett J. K. & Albright T. A. Symmetrical vs asymmetric linear M-X-M linkages in molecules, polymers, and extended networks. J. Am. Chem. Soc. 108, 2222–2236 (1986). [DOI] [PubMed] [Google Scholar]
- Mizoguchi H., Woodward P. M., Byeon S.- H. & Parise J. B. Polymorphism in NaSbO3: structure and bonding in metal oxides. J. Am. Chem. Soc. 126, 3175–3184 (2004). [DOI] [PubMed] [Google Scholar]
- Sushko P. V., Shluger A. L., Hayashi K., Hirano M. & Hosono H. Electron localization and a confined electron gas in nanoporous inorganic electrides. Phys. Rev. Lett. 91, 126401-1-4 (2003). [DOI] [PubMed] [Google Scholar]
- Hayashi K., Matsuishi S., Kamiya T., Hirano M. & Hosono H. Light-induced conversion of an insulating refractory oxide into a persistent electronic conductor. Nature 419, 462–465 (2002). [DOI] [PubMed] [Google Scholar]
- Kresse G. & Furthmuller J. Efficient iterative schemes for ab-initio total-energy calculations using a plain wave basis set. Phys. Rev. B 54, 11159–11186 (1996). [DOI] [PubMed] [Google Scholar]
- Perdew J. P., Burke K. & Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). [DOI] [PubMed] [Google Scholar]
- Perdew J. P., Ernzerhof M. & Burke K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 105, 9982–9985 (1996). [Google Scholar]
- Heyd J., Scuseria G. E. & Ernzerhof M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 118, 8207–8215 (2003). [Google Scholar]
- Krukau A. V., Vydrov O. A., Izmaylov A. F. & Scuseria G. E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 125, 224106-1-5 (2006). [DOI] [PubMed] [Google Scholar]
- Hilmer W. An X-ray investigation of the strontium germinate SrGeO3. Sov. Phys. Cryst. 7, 573–576 (1963). [Google Scholar]
- Nishi F. Strontium metagermanate, SrGeO3. Acta Cryst. C 53, 399–401 (1997). [Google Scholar]
- Jorgensen J. D. Compression mechanism in α-quartz structures-SiO2 and GeO2. J. Appl. Phys. 49, 5473–5478 (1978). [Google Scholar]
- Bolzan A. A., Fong C., Kennedy B. J. & Howard C. J. Structural studies of rutile-type metal dioxides. Acta Cryst. B 53, 373–380 (1997). [Google Scholar]