Abstract
Immune escape is a fundamental trait of cancer in which the Th1-type cytokine interferon- γ (IFN-γ) seems to play a key role. Among other tumoricidal biochemical pathways, IFN-γ induces the tryptophan-degrading enzyme indoleamine 2,3-dioxygenase (IDO) in a variety of cells including macrophages, dendritic cells (DCs) and tumor cells. IDO activity has been shown to reflect the extent and the course in a plethora of malignancies including prostate, colorectal, pancreatic, cervical, endometrial, gastric, lung, bladder, ovarian, esophageal and renal cell carcinomas, glioblastomas, mesotheliomas, and melanomas. Furthermore IDO activity during malignant tumor diseases seems to be part of the tumoricidal immune defense strategy, which in the long run is detrimental to the host, when tryptophan deprivation and production of pro-apoptotic tryptophan catabolites counteract T-cell responsiveness.
Keywords: IDO, Tryptophan, Malignant tumor disease
Introduction
In vitro, Th1-type cytokine interferon-γ (IFN-γ) induces the tryptophan-degrading enzyme indoleamine 2,3-dioxygenase (IDO) in monocyte-derived macrophages, dendritic cells and in a variety of other cell types including tumor cells. In vivo, accelerated tryptophan degradation can be detected in body fluids by measurements of kynurenine and tryptophan concentrations. Calculating the kynurenine to tryptophan ratio (kyn/trp) serves as an estimate of IDO functional activity. A large proportion of patients with a variety of disorders like viral or bacterial infections, autoimmune syndromes or during allograft rejection presents with increased kyn/trp in serum/plasma or cerebrospinal fluid. Tryptophan degradation in these individuals reflects the extent, activity and the course of disease. In patients with malignant tumors, tryptophan degradation parallels disease progression, loss of immunocompetence and the development of cachexia and anemia. In addition, higher kyn/trp and lower tryptophan concentrations predict shorter overall survival.
The rate of tryptophan degradation in malignant cell lines is usually low but can be significantly enhanced by pro-inflammatory stimuli such as IFN-γ. Data suggest that tryptophan degradation in patients with cancer predominantly relates to enhanced IDO activity, which is stimulated during Th1-type immune response. This conclusion is further supported by the observation that kyn/trp in humans usually correlates closely with neopterin concentrations, which is released by human monocyte-derived macrophages and DCs particularly upon stimulation with IFN-γ, but not by stimulated tumor cells themselves. Degradation of essential amino acid tryptophan represents an effective anti-proliferative strategy, which is established during immune response. It is directed to stop malignant proliferation as is to halt growth of pathogens in infected cells.
In 1996 we demonstrated accelerated tryptophan degradation correlating to neopterin levels in normal human pregnancy, and 1998 Munn et al revealed IDO activity to be critical for the induction of maternal immunotolerance. Subsequent studies were able to substantiate the central role of IDO activation in the development of immunodeficiency and immunotolerance. Thereby IDO expression in DC is crucial in the control of regulatory T-cells.
Degradation of tryptophan in cancer patients suggests that IDO activity may be involved in several typical symptoms of chronic inflammatory conditions. IDO is also related to impaired quality of life, and this correlation is obviously due to the close association between tryptophan and the biosynthesis of neurotransmitter serotonin. Therefore, tryptophan deficiency could represent an important aspect in the pathogenesis of cognitive impairment and depression in patients with immunopathologic clinical conditions.
Data on IDO thereby provide a basis to better understand the complex interplay between immune activation cascades and the development of immunodeficiency, or in other words, of the pro- and anti-inflammatory consequences of IFN-γ, among which IDO is a central component. IFN-γ-induced IDO activity during malignant tumor diseases is part of the physiologic tumoricidal immune defense strategy, which in the long run might be detrimental to the host, when tryptophan deprivation and production of pro-apoptotic tryptophan catabolites, counteracts T-cell responsiveness.
We are only beginning to understand the complexity of pathophysiological cascades in the tumor microenvironment, where particular immune cells play important roles in terms of tumor development and progression. The Th1-type cytokine IFN-γ has been shown to be crucial in this process via serving as a stimulus for anti-proliferative biochemical pathways. These include the kynurenine pathway in which the immunomodulatory enzyme IDO and its recently discovered relative IDO2 initiate the first step in the degradation of the essential amino acid L-tryptophan to ultimately form a plethora of immunomodulatory metabolites. Encoded by the INDO gene at human chromosome 8p12, IDO is an isoenzyme of hepatic tryptophan 2,3-dioxygenase (tryptophan pyrrolase, TDO) which was first described and isolated in 1963.1,2 Over decades it has been reported that IDO plays an immunosuppressive role in a variety of chronic infections, including viral, parasitic and bacterial infections such as human immunodeficiency virus (HIV), malaria, hepatitis C, Toxoplasma gondii and Chlamydia.1–3 IDO is widely expressed in human tissues and cell subsets and its immunoregulatory properties have been shown to play a dominant role in immune responses and regulation towards self and foreign antigens. A better understanding of these mechanisms will not only allow us to unveil the pathogenesis of many diseases but also discover new therapeutic approaches in terms of prevailing tumor escape and inducing immune tolerance.4 Maternal tolerance towards the semi-allogeneic fetus is only one example in which IDO has been shown to mediate immune privilege by preventing T-cell driven rejection.5 This ground-breaking discovery paved the way for further research addressing the immunoregulatory potential of IDO including a series of studies focusing on the role of IDO-mediated tryptophan metabolites in the immune escape of tumors.6 This review highlights the experimental and clinical findings of IDO-induced tryptophan degradation in the pathogenesis of malignant tumor disease and discusses potential novel anti cancer-therapeutic strategies by targeting IDO.
IDO Mediated Tryptophan Metabolism
The two intracellular enzymes IDO and TDO initiate the rate limiting step in L-tryptophan degradation along the kynurenine pathway and ultimately form immunomodulatory metabolites like L-kynurenine, 3-hydroxykynurenine, 3-Hydroxyanthranilic acid and picolinic acid. In case the immune system gets activated, high amounts of IFN-γ are released mainly by leukocytes. This results in sustained IDO activation most prominently in antigen presenting cells (APCs) like DCs, which leads to the afore-mentioned accumulation of downstream tryptophan products. Besides that, a small amount of tryptophan is used for melatonin synthesis in the pineal gland or metabolized to serotonin in the nervous system. Leucocytes are furthermore the cell source to produce redox active compounds like superoxide (OH*), which generates Fe2+ from Fe3+ within the heme-prosthetic group of IDO allowing it to proceed tryptophan metabolism (Fig. 1). This mechanism suggests that IDO activity is restricted to sites of infection or inflammation.7–9 Furthermore the redox potential of the microenvironment seems to influence the function of tryptophan metabolites as well.9
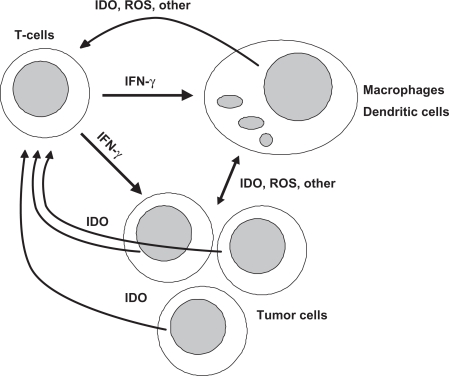
Figure 1.
The developing malignant process is recognized by immunocompetent cells and a broad response of several immunocompartments is mounted against the foreign surface structures of tumor cells. Among the immunoregulatory cytokines released during innate and adaptive immune responses, Th1-type cytokine interferon-γ (IFN-γ) stimulates several antiproliferative and tumoricidal biochemical pathways such as the formation of reactive oxygen species (ROS) and the degradation of tryptophan by indoleamine 2,3-dioxygenase (IDO) in macrophages, dendritic cells and tumor cells. As one important consequence, tryptophan stravation and the formation of toxic effector molecules like kynurenine derivatives and anthranilate not only affect proliferation of target tumor cells but also of T-cells and their immunoresponsiveness.
Immunosuppressive Effects of IDO
The exact mechanism, how IDO acts immunosuppressive, is still under debate. Two main theories have been proposed either involving tryptophan metabolites or starving cells of tryptophan. After IDO activation has been described through the course of successful pregnancy,10 in 1998 Munn and Mellor discovered that IDO expression at the feto-maternal-interface was crucial to prevent fetal rejection in pregnant mice. Pharmacologic enzyme inhibition with 1-methyl-tryptophan (1-MT) resulted in T cell mediated rejection of allogeneic but non-syngeneic fetuses.5 This maternal tolerance concept was based on the theory that local tryptophan depletion would maintain pregnancies through suppression of T-cell driven fetal rejection. Tryptophan depletion induces cell cycle arrest in lymphocytes11 ultimately driving these cells into apoptosis.12 This is mainly regulated through the amino acid sensitive general control non- depressible 2 (GCN2) stress kinase pathway which becomes activated upon IDO-induced tryptophan degradation and production of uncharged tRNA in T cells.13 A tryptophan threshold in order to inhibit T cell proliferation could not been defined yet. Munn et al reported that tryptophan concentrations in vitro had to decrease below 0.5–1 μM to inhibit T-cell proliferation,11 others report that even completely tryptophan free cell culture medium could not sufficiently inhibit T lymphocytes from growing.14 In humans, plasma tryptophan levels range between 50–100 μM. It is currently unclear to what extent tryptophan degradation and starvation accounts for IDO mediated immunosuppression since minimum tryptophan threshold limit values have not been defined and probably can not be defined in vivo. In case of inflammation and tumor growth necrotic cells release their intracellular stocks and thereby supply an additional source of tryptophan to the particular microenvironment. Furthermore, local decrease in tryptophan can easily be replaced through diffusion from surrounding tissues. Taken all of these afore mentioned results into consideration it is currently unclear to what extent tryptophan starvation accounts for IDO-mediated immunosuppression.
The tryptophan metabolite theory is nourished by the fact that downstream metabolites of tryptophan cause cell cycle arrest and apoptosis in lymphocytes.14–16 Furthermore, they foster via to date largely unknown mechanisms the differentiation of naïve CD4+ T cells into T regulatory cells (Treg).17 However one study suggests that 3-hydroxyanthralinic acid directly blocks T cell antigen receptor-triggered nuclear factor-κB (NF-κB) activation through kinase 3-phosphoinositide-dependent protein kinase 1 (PDK1) signaling which ultimately leads to activated type 2 T helper cell death.18
In summary, both theories are not necessarily mutually exclusive but rather a concerted contribution of both mechanisms might contribute to IDO-mediated immune suppression in vivo.
Tumor Immunity Through IDO Mediated Tryptophan Catabolism
Immune escape is a fundamental trait of cancer.19 A variety of tumor escape mechanisms have been elucidated over the past.6,20 Key players in this crucial escape mechanism are Tregs and regulatory DCs, which create tolerance to the antigen they are presenting. Furthermore, IDO also seems to play a detrimental role in tumor immunity by acting in a forward feedback loop to instruct naïve CD4+ T cells to become Tregs but also by mediating Tregs to drive naive DCs into a regulatory status.21,22 By provoking a cycle of antigen tolerance IDO might switch the tumor microenvironment from hostile to supportive for tumor cells and also initiate a peripheral immune escape mechanism that facilitates progression to a more invasive tumor status.19 Supporting the fact that IDO has a fundamental role in immune control, recent evidence suggests that the immunosuppressive drug dexamethasone operates upon IDO induction in DCs by reverse signaling through the glucocorticoid induced B7 inhibitory T cell co-receptor.23 IDO does not seem to be involved in tolerizing self-antigens but rather in creating a tolerant state for non-self antigens (e.g. fetal antigens) where immune non-responsiveness is important.24 However in the eventuality of cancer, immune unresponsiveness to tumor antigens results in disease aggravation.
In addition, T cells also appear to be predominantly sensitive to IDO activation, since upon tryptophan starvation they cannot proliferate and become activated by means of antigen presentation and they rather translate into an anergic state. Tryptophan starvation furthermore triggers a Gcn2-dependent stress-signaling pathway that ultimately leads to cell growth arrest through phosphorylation and translational initiation at the ribosome.13 Furthermore T cells seem to be preferentially vulnerable to kynurenine derivatives and other catabolites generated by the IDO pathway25 which along with tryptophan restriction appears to be important for induction of Tregs and immune suppression.22,26
Clinical Significance of IDO Expression in Human Malignancies
IDO expression can be detected in most human cancers including prostate, colorectal, pancreatic, cervical, endometrial, gastric, lung, bladder, ovarian, esophageal and renal cell carcinomas, glioblastomas, mesotheliomas, and melanomas.27–33 Clinical studies stress that high IDO expression of tumor cells correlates with outcome (Table 1). In endometrial cancer, ovarian cancer and high-grade osteosarcomas, respectively, IDO expression correlates with poor overall survival.34–36 Furthermore, our own group was able to show that IDO had a significant prognostic value in terms of liver metastasis in patients with colorectal cancer. IDO expression goes along with reduced CD3+ lymphocyte infiltration suggesting a suppressive function on tumor reactive T-cells.37 In patients with hepatocellular carcinoma similar findings were reported.38 Furthermore a significant relationship was found between the amount of IDO positive tumor infiltrating cells and overall survival in non small cell lung cancer.39 In addition the presence of metastatic infiltrating IDO positive cells into the lymph node at initial diagnosis correlated with a significantly worse clinical outcome in malignant lymphoma.40 In contrast IDO expression in tumor endothelial cells in patients with renal cell carcinoma correlates with long-term survival.41 Apart from IDO, pyrazinopyrimidine compound neopterin has been shown to be a reliable prognostic marker for human malignancies.42 Neopterin is released as an inflammatory marker in large amounts from human monocyte-derived macrophages and DCs preferentially following stimulation with the pro-inflammatory cytokine IFN-γ, thus reflecting the immune activation status. Increased neopterin levels in patients with melanomas, breast cancers, squameous cell carcinomas, gynecological tumors, and colorectal carcinomas have been shown to go along with poor prognostic outcomes.32,43–46
Table 1.
| Tumor entity | Overall survival of patients with increased IDO expression | Progression-free survival of patients with increased IDO expression | References |
|---|---|---|---|
| Malignant melanoma | Reduced | Reduced | 31,32 |
| Acute myeloid leukemia | Reduced | Reduced | 33 |
| Ovarian serous carcinoma | Reduced | Not evaluated | 34 |
| Ovarian clear cell carcinoma | No correlation | Not evaluated | 34 |
| Endometrial carcinoma | Reduced | Reduced | 35 |
| Osteosarcoma | Reduced | Reduced | 36 |
| Colorectal carcinoma | No correlation | Not evaluated | 37 |
| Hepatocellular carcinoma | Not evaluated | Prolonged | 38 |
| Renal cell carcinoma | Prolonged | Not evaluated | 41 |
| Esophageal squammous cell carcinoma | Not evaluated | Not evaluated | 29 |
| Lung cancer | Not evaluated | Not evaluated | 30 |
Since IFN-γ is one of the strongest inducers of IDO expression47 and due to its sustained effects on tumor cell proliferation the cross-linked interplay of IFN-γ and IDO seems to be of great importance. This hypothesis is additionally supported by in vivo studies in an ovarian carcinoma mouse model where IL-12, a cytokine with anti-tumor activity, induced complete regression of fibrosarcomas and ovarial carcinomas due to IFN-γ induced IDO activity.48
Besides its anti-proliferative effects on transformed cells49 IFN-γ seems to exert even stronger inhibitory effects on human cancer cell proliferation upon IDO expression. Almost two decades ago it has been shown in vitro that IFN-γ induced IDO expression leads to tryptophan degradation in cell culture medium resulting in cell proliferation arrest. However tryptophan supplementation of growth medium reversed the anti-proliferative effects of IFN-γ.50
In conclusion, IDO induction seems to be one mechanism by which IFN-γ inhibits malignant cell outgrow thereby being a decisive mechanism of tumor attack.
IDO Inhibition, a Potential Mechanism of Cancer Therapy in the Future?
Since IDO plays a major role in the escape of malignant cells from immunological attack it is obvious that blocking its activity should increase the anti-tumoral response and halt tumor progression. In vitro studies showed that 1-MT treatment delayed outgrowth of mouse melanoma cells that have been engrafted into syngeneic hosts.51 Furthermore over-expression of IDO promotes tumor growth, which again could be partially reversed by pharmacologic inhibition.28 However, in a murine breast cancer model it has been shown recently that 1-MT, although present in sufficient concentrations, only slightly retarded autochthonous breast cancer. Because 1-MT only seems to be able to slow down and not to prevent tumor growth it was combined with several chemotherapeutic drugs or radiotherapy resulting in even more pronounced anti-tumoral effects.52,53 The molecular mechanism by which IDO inhibition contributes to the beneficial partnership with chemotherapy still remains to be elucidated. 1-MT exists in two isoforms, L-1-MT and D-1-MT which both inhibit IDO and its relative IDO2, respectively. In subsequent murine tumor models it turned out that only D-1-MT significantly prolonged survival when combined with conventional chemotherapeutics.53 However, IDO has a tenfold higher affinity for the L-isomer than the D-isomer, indicating that D-1-MT is less efficient in blocking IDO enzymatic activity. Interestingly D-1-MT did not block IFN-γ treated tumor cell lines or IDO transfected cell lines.53 However, in some other experiments it was able to block tryptophan degradation of IDO expressing DCs that were used as stimulator cells in allogeneic mixed lymphocyte culture and accordingly increased T-cell proliferation in the co culture system. The question why D-1-MT inhibits IDO activity in only some cells and does not inhibit the activity of the purified IDO enzyme was answered when another IDO isoform was discovered. The gene IDO2 encodes an IDO-like protein and has recently been detected on the human chromosome 8. IDO2 is inhibited by D-1-MT but unaffected by L-1-MT54 and is constitutively expressed in several tissues.55 When compared to IDO, IDO2 has only 3%–5% of enzymatic activity, however this activity can be increased through modified assay conditions.4,55 Although D-1-MT has recently entered clinical trials (NCT00567931; New link genetics corporation; Patent Storm) therapeutic effects in humans still have to be figured out. Current data have revealed that only the Levo-isoform of 1-MT has IDO blocking capacities in human malignancies where only IDO but not IDO2 is expressedv.56 Furthermore, even if IDO2 would be active, only a subset of patients would benefit from therapy with D-1-MT as 50% of Caucasians lack functional IDO2 alleles.4,54
Conclusion
Summarizing the experimental and clinical data above, there is clear evidence that IDO interferes with immune regulation. IDO has evolved from a simple tryptophan catabolizing enzyme into an important immune regulator and an important player in tumor immunosurveillance. There is a great body of evidence that IDO is expressed by tumor cells, tumor infiltrating immune cells and in tumor draining lymph nodes and that the expression of IDO contributes to the ability of tumors to evade the immune system. Taken together these facts offer a rationale for the clinical investigation of the capacity of IDO inhibitors to increase the efficacy of anticancer immunotherapy, in addition to conventional tumor radiation and chemotherapeutic agents. In particular, combining chemotherapy with pharmacologic IDO inhibition seems to be key to success in preventing IDO mediated immunologic tolerance when chemotherapy has already destroyed tumor cells and released tumor antigens.
Studying IFN-γ-induced IDO activity in tumors may also lead to a better general understanding of immunoregulation in cancer and foster novel therapeutic strategies for modern cancer warfare.
Acknowledgments
Parts of this work were supported by Österreichische Krebshilfe—Krebsgesellschaft Tirol, Austria.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors report no conflicts of interest.
References
- 1.Thomas SM, Garrity LF, Brandt CR, Schobert CS, Feng GS, Taylor MW, et al. IFN-gamma-mediated antimicrobial response. Indoleamine 2,3-dioxygenase-deficient mutant host cells no longer inhibit intracellular Chlamydia spp. or Toxoplasma growth. J Immunol. 1993 Jun 15;150(12):5529–34. [PubMed] [Google Scholar]
- 2.Fujigaki S, Saito K, Takemura M, Maekawa N, Yamada Y, Wada H, et al. L-tryptophan-L-kynurenine pathway metabolism accelerated by Toxoplasma gondii infection is abolished in gamma interferon-gene-deficient mice: cross-regulation between inducible nitric oxide synthase and indoleamine-2,3-dioxygenase. Infect Immun. 2002 Feb;70(2):779–86. doi: 10.1128/iai.70.2.779-786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen AM, Ball HJ, Mitchell AJ, Miu J, Takikawa O, Hunt NH. Increased expression of indoleamine 2,3-dioxygenase in murine malaria infection is predominantly localised to the vascular endothelium. Int J Parasitol. 2004 Nov;34(12):1309–19. doi: 10.1016/j.ijpara.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Lob S, Konigsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer. 2009 Jun;9(6):445–52. doi: 10.1038/nrc2639. [DOI] [PubMed] [Google Scholar]
- 5.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998 Aug 21;281(5380):1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 6.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005 Apr;5(4):263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 7.Hirata F, Hayaishi O. Studies on indoleamine 2,3-dioxygenase. I. Superoxide anion as substrate. J Biol Chem. 1975 Aug 10;250(15):5960–6. [PubMed] [Google Scholar]
- 8.Hayaishi O. Utilization of superoxide anion by indoleamine oxygenase-catalyzed tryptophan and indoleamine oxidation. Adv Exp Med Biol. 1996;398:285–9. doi: 10.1007/978-1-4613-0381-7_45. [DOI] [PubMed] [Google Scholar]
- 9.Thomas SR, Stocker R. Redox reactions related to indoleamine 2, 3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Rep. 1999;4(5):199–220. doi: 10.1179/135100099101534927. [DOI] [PubMed] [Google Scholar]
- 10.Schrocksnadel H, Baier-Bitterlich G, Dapunt O, Wachter H, Fuchs D. Decreased plasma tryptophan in pregnancy. Obstet Gynecol. 1996 Jul;88(1):47–50. doi: 10.1016/0029-7844(96)00084-1. [DOI] [PubMed] [Google Scholar]
- 11.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999 May 3;189(9):1363–72. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002 Dec;107(4):452–60. doi: 10.1046/j.1365-2567.2002.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005 May;22(5):633–42. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002 Aug 19;196(4):459–68. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fallarino F, Grohmann U, Vacca C, Orabona C, Spreca A, Fioretti MC, et al. T cell apoptosis by kynurenines. Adv Exp Med Biol. 2003;527:183–90. doi: 10.1007/978-1-4615-0135-0_21. [DOI] [PubMed] [Google Scholar]
- 16.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002 Aug 19;196(4):447–57. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol. 2009 Mar;41(3):467–71. doi: 10.1016/j.biocel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi T, Mo JH, Gong X, Rossetto C, Jang A, Beck L, et al. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Natl Acad Sci U S A. 2007 Nov 20;104(47):18619–24. doi: 10.1073/pnas.0709261104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prendergast GC. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. 2008 Jun 26;27(28):3889–900. doi: 10.1038/onc.2008.35. [DOI] [PubMed] [Google Scholar]
- 20.Gabrilovich DI. Molecular mechanisms and therapeutic reversal of immune suppression in cancer. Curr Cancer Drug Targets. 2007 Feb;7(1):1. [PubMed] [Google Scholar]
- 21.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003 Dec;4(12):1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 22.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006 Jun 1;176(11):6752–61. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 23.Grohmann U, Volpi C, Fallarino F, Bozza S, Bianchi R, Vacca C, et al. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007 May;13(5):579–86. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 24.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008 Jan;8(1):74–80. doi: 10.1038/nri2233. [DOI] [PubMed] [Google Scholar]
- 25.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002 Oct;9(10):1069–77. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 26.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007 May;117(5):1147–54. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lob S, Konigsrainer A, Zieker D, Brucher BL, Rammensee HG, Opelz G, et al. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. 2009 Jan;58(1):153–7. doi: 10.1007/s00262-008-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003 Oct;9(10):1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Lu G, Tang F, Liu Y, Cui G. Localization of indoleamine 2,3-dioxygenase in human esophageal squamous cell carcinomas. Virchows Arch. 2009 Oct 21; doi: 10.1007/s00428-009-0846-3. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki Y, Suda T, Furuhashi K, Suzuki M, Fujie M, Hahimoto D, et al. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer. 2009 May 30; doi: 10.1016/j.lungcan.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Brody JR, Costantino CL, Berger AC, Sato T, Lisanti MP, Yeo CJ, et al. Expression of indoleamine 2,3-dioxygenase in metastatic malignant melanoma recruits regulatory T cells to avoid immune detection and affects survival. Cell Cycle. 2009 Jun 15;8(12):1930–4. doi: 10.4161/cc.8.12.8745. [DOI] [PubMed] [Google Scholar]
- 32.Weinlich G, Murr C, Richardsen L, Winkler C, Fuchs D. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatology. 2007;214(1):8–14. doi: 10.1159/000096906. [DOI] [PubMed] [Google Scholar]
- 33.Chamuleau ME, van de Loosdrecht AA, Hess CJ, Janssen JJ, Zevenbergen A, Delwel R, et al. High INDO (indoleamine 2,3-dioxygenase) mRNA level in blasts of acute myeloid leukemic patients predicts poor clinical outcome. Haematologica. 2008 Dec;93(12):1894–8. doi: 10.3324/haematol.13113. [DOI] [PubMed] [Google Scholar]
- 34.Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y, et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res. 2005 Aug 15;11(16):6030–9. doi: 10.1158/1078-0432.CCR-04-2671. [DOI] [PubMed] [Google Scholar]
- 35.Ino K, Yoshida N, Kajiyama H, Shibata K, Yamamoto E, Kidokoro K, et al. Indoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer. 2006 Dec 4;95(11):1555–61. doi: 10.1038/sj.bjc.6603477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urakawa H, Nishida Y, Nakashima H, Shimoyama Y, Nakamura S, Ishiguro N. Prognostic value of indoleamine 2,3-dioxygenase expression in high grade osteosarcoma. Clin Exp Metastasis. 2009 Oct 6; doi: 10.1007/s10585-009-9290-7. [DOI] [PubMed] [Google Scholar]
- 37.Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006 Feb 15;12(4):1144–51. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 38.Pan K, Wang H, Chen MS, Zhang HK, Weng DS, Zhou J, et al. Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2008 Nov;134(11):1247–53. doi: 10.1007/s00432-008-0395-1. [DOI] [PubMed] [Google Scholar]
- 39.Astigiano S, Morandi B, Costa R, Mastracci L, D’Agostino A, Ratto GB, et al. Eosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancer. Neoplasia. 2005 Apr;7(4):390–6. doi: 10.1593/neo.04658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004 Jul;114(2):280–90. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riesenberg R, Weiler C, Spring O, Eder M, Buchner A, Popp T, et al. Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. Clin Cancer Res. 2007 Dec 1;13(23):6993–7002. doi: 10.1158/1078-0432.CCR-07-0942. [DOI] [PubMed] [Google Scholar]
- 42.Sucher R, Schroecksnadel K, Weiss G, Margreiter R, Fuchs D, Brandacher G. Neopterin, a prognostic marker in human malignancies. Cancer Lett. 2009 Jun 3; doi: 10.1016/j.canlet.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Murr C, Bergant A, Widschwendter M, Heim K, Schrocksnadel H, Fuchs D. Neopterin is an independent prognostic variable in females with breast cancer. Clin Chem. 1999 Nov;45(11):1998–2004. [PubMed] [Google Scholar]
- 44.Murr C, Berchtold J, Norer B, Waldhart E, Wachter H, Fuchs D. Neopterin as a prognostic parameter in patients with squamous-cell carcinomas of the oral cavity. Int J Cancer. 1998 Oct 23;79(5):476–80. doi: 10.1002/(sici)1097-0215(19981023)79:5<476::aid-ijc5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 45.Melichar B, Solichova D, Freedman RS. Neopterin as an indicator of immune activation and prognosis in patients with gynecological malignancies. Int J Gynecol Cancer. 2006 Jan-Feb;16(1):240–52. doi: 10.1111/j.1525-1438.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- 46.Melichar B, Solichova D, Melicharova K, Malirova E, Cermanova M, Zadak Z. Urinary neopterin in patients with advanced colorectal carcinoma. Int J Biol Markers. 2006 Jul-Sep;21(3):190–8. doi: 10.1177/172460080602100309. [DOI] [PubMed] [Google Scholar]
- 47.Takikawa O, Kuroiwa T, Yamazaki F, Kido R. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J Biol Chem. 1988 Feb 5;263(4):2041–8. [PubMed] [Google Scholar]
- 48.Yu WG, Yamamoto N, Takenaka H, Mu J, Tai XG, Zou JP, et al. Molecular mechanisms underlying IFN-gamma-mediated tumor growth inhibition induced during tumor immunotherapy with rIL-12. Int Immunol. 1996 Jun;8(6):855–65. doi: 10.1093/intimm/8.6.855. [DOI] [PubMed] [Google Scholar]
- 49.Farrar MA, Schreiber RD. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 50.Ozaki Y, Edelstein MP, Duch DS. Induction of indoleamine 2,3-dioxygenase: a mechanism of the antitumor activity of interferon gamma. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1242–6. doi: 10.1073/pnas.85.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friberg M, Jennings R, Alsarraj M, Dessureault S, Cantor A, Extermann M, et al. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int J Cancer. 2002 Sep 10;101(2):151–5. doi: 10.1002/ijc.10645. [DOI] [PubMed] [Google Scholar]
- 52.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005 Mar;11(3):312–9. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 53.Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007 Jan 15;67(2):792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 54.Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007 Aug 1;67(15):7082–7. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 55.Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007 Jul 1;396(1):203–13. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 56.Loeb S, Koenigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood. 2008 Feb 15;111(4):2152–4. doi: 10.1182/blood-2007-10-116111. [DOI] [PubMed] [Google Scholar]