Abstract
Introduction:
While central nervous system serotonin has been implicated in a variety of problematic impulsive behaviors, biological manipulation of brain serotonin using acute tryptophan depletion for studying changes in impulsive behavior has received little attention.
Methods:
Using identical treatment conditions, we examined the effects of reduced serotonin synthesis for each of three matched groups using acute tryptophan depletion. Thirty healthy men and women (ages 18–45) were assigned to perform one of three tasks assessing different types of behavioral impulsivity: response initiation, response inhibition, and consequence sensitivity (N = 90). Participants completed two experimental days during which each consumed either a tryptophan-depletion or balanced-placebo amino-acid formulation and completed 5 sessions of their respective tasks at 0.25 h before and 1.5, 4.0, 5.0, and 6.0 h after beverage consumption.
Results:
During peak effectiveness (5.0 h to 6.0 h following amino-acid consumption), depletion produced selective differences dependent on the type of impulsivity being tested. Specifically, relative to baseline testing (pre-depletion), response initiation impulsivity was significantly increased during the peak effects of depletion. And, when compared to placebo control, both response initiation and consequence sensitivity impulsivity were increased during the peak effects of depletion.
Conclusion:
Though response initiation and consequence sensitivity impulsivity were affected by tryptophan depletion, response inhibition impulsivity was not, suggesting that other biological processes may underlie this specific component of impulsivity. Future research in other populations or using different pharmacological agents is warranted to further examine the biological processes underlying these components of impulsivity.
Keywords: tryptophan, impulsivity, behavior, task comparison, humans, adults
1. Introduction
Substantial evidence from both human and animal studies highlights the role that serotonin plays in the expression of impulsive behaviors. Indeed, researchers have found that reduced central serotonin function in humans is associated with a wide variety of problematic conditions involving impulsive behaviors, including drug and alcohol misuse,1–6 suicidality,7–10 aggressive and violent behavior,11–15 and borderline personality disorder.16 Despite strong evidence that markers of serotonin synthesis are correlated with impulsive behaviors, data from these kinds of associative studies cannot be used to make causal inferences. Preclinical studies have reported similarly robust associations between serotonin functioning and impulsive or impulsive-aggressive behaviors in various animal species.17,18 Unlike the associative studies in humans, preclinical studies have the ability to examine causal relationships of serotonin on impulsivity by manipulating, sometimes permanently, the serotonin system. The methods used in preclinical research have not been widely adopted in human research for various reasons including lack of effectiveness (e.g. dietary restriction19), poorly tolerated side effects (e.g. the use of parachlorophenylalanine20), or ethical concerns (e.g. gene knock outs related to serotonin). Effective and tolerable experimental methods to manipulate serotonin in humans have been available for the last 25 years, although they have only recently gained popularity. While dietary and selective inhibition methods used to reduce serotonin synthesis in humans have met with some success,19 the most effective and popular method for serotonin manipulation is acute tryptophan depletion.20–23
Acute tryptophan depletion produces a temporary reduction of the availability of serotonin’s sole precursor, the essential amino-acid tryptophan.24 Reducing brain serotonin using acute tryptophan depletion is accomplished by consumption of an amino-acid formulation containing 50 g or 100 g of 15 amino acids in a beverage that is devoid of tryptophan. This ultimately results in the reduction of brain serotonin synthesis via two primary mechanisms. Briefly, consumption of a tryptophan-free amino-acid formulation delivers a bolus dose of amino acids, which results in: 1) increased protein synthesis and incorporation of endogenous tryptophan that substantially decreases circulating plasma tryptophan;20,24–28 and 2) decreased plasma tryptophan reduces the effectiveness of competition with other large neutral amino acids (i.e. isoleucine, leucine, phenylalanine, tyrosine, and valine) for transport across the blood brain barrier.19,24,29 Together, these two mechanisms produce a significant, though transient, depletion of plasma tryptophan that reaches the maximal depletion effects (e.g. −77% to −94%25) approximately 5 to 6 hours following ingestion, which then results in a significant reduction of brain serotonin synthesis.30 Alternatively, a balanced-placebo formulation is used as control condition to maintain normal levels of circulating tryptophan (and thereby maintain normal levels of serotonin synthesis). This control condition contains the same amino acids as the depletion formulation; however tryptophan is included in a proportionate amount (1.15 g in a 50 g beverage) to the other amino acids which prevents the depletion of endogenous tryptophan. The balanced placebo also provides a control for potential effects that result simply from consumption of the aminoacid beverage (e.g. poor palatability of amino-acid beverages). These formulations, which are a safe19 and effective30 means for testing the effects of pronounced reductions in serotonin synthesis in humans, have enabled researchers to examine the causal relationships of reduced serotonin synthesis and behavioral outcomes such as impulsivity in humans.
However, human impulsivity is a complex multidimensional construct that has historically been difficult to measure. This complexity is exemplified by a definition that characterizes impulsivity as “a predisposition toward rapid, unplanned reactions to internal or external stimuli without regard to the negative consequences of these reactions to the impulsive individual or to others”31 (p. 1784). In an effort to advance the study of impulsivity, researchers have dissected this complex definition into multiple independent aspects of the impulsivity construct. For instance, Dougherty and colleagues32 have proposed that impulsivity includes at least three testable components: 1) rapid responses that occur prior to complete processing and evaluation of a stimulus (i.e. response initiation); 2) failures to inhibit an already initiated response (i.e. decreased response inhibition); and 3) persistent reward-directed choices for smaller rewards delivered sooner, despite an equal availability of larger rewards delivered later (i.e. consequence sensitivity). These components can be assessed with laboratory measures that have been developed to capture different aspects of impulsive behavior.32 Furthermore, these laboratory tasks are sensitive to state fluctuations in impulsivity that would be expected as a result of acute tryptophan depletion and are appropriate for use in healthy samples as well as those with psychiatric and behavioral problems that often feature impulsive-type symptoms (e.g. conduct disorder, bipolar disorder, suicidality, substance abuse, aggressive behaviors, criminality).25,32–50
Few studies have examined the effects of tryptophan depletion on impulsive responding in healthy controls, and findings have generally been inconsistent. Experimental manipulation of serotonin in healthy individuals is beneficial in that it permits examination of the impact of reduced serotonin on the expression of impulsive behaviors without potential confounds (e.g. psychopathology) that may affect interpretation of study findings in impaired populations. Based on the preliminary studies that have used tryptophan depletion to test impulsivity among healthy individuals, there is some evidence that tryptophan depletion results in increased response initiation impulsivity,40,51–53 response inhibition impulsivity (i.e. decreased response inhibition),54,55 and consequence sensitivity impulsivity.56 However, increased impulsive responding has not been a universal finding,54,57 and results have often varied according to differences in sample characteristics (e.g. gender53). Furthermore, previous studies have typically included only one measure of impulsivity; consequently, the various components of this complex multidimensional construct have not been adequately assessed in prior research studies. In general, the methodologies used and the populations tested have varied considerably, making it difficult to compare across these studies or draw broad conclusions about the effects of tryptophan depletion on different components of impulsivity.
For these reasons, the goal of the current study was to examine the effects of tryptophan depletion on components of behavioral impulsivity in three groups of healthy individuals matched for gender, age, ethnicity, education, and intelligence. Participants were assigned to one of three task groups that corresponded with each of the three components of impulsivity outlined previously by Dougherty and colleagues:32 increased response initiation, decreased response inhibition, and decreased consequence sensitivity. All groups underwent identical tryptophan depletion procedures, thereby allowing for parallel comparison of the effects of tryptophan depletion on each of the components of impulsivity. The primary aims were: 1) to determine the extent to which tryptophan depletion would produce changes in impulsivity relative to baseline performance (prior to experimental manipulation); and 2) to determine whether tryptophan depletion would produce differential effects on the individual components of impulsivity being tested. While results from previous literature have been somewhat inconsistent, we hypothesized that tryptophan depletion would increase impulsivity relative to both baseline and the balanced-placebo formulation for all three types of impulsivity measured by the tasks.
2. Methods
2.1. Participants
A total of 90 healthy adults (45 men and 45 women) were recruited through community advertisements and thoroughly screened prior to being offered participation in the study. Volunteers calling in response to advertisements targeted at healthy adults were initially screened using a brief telephone interview, and those appearing to meet study criteria were invited to the laboratory to complete an in-depth screening interview. Onsite screening included a health history, physical exam, and a psychiatric screening using the Structured Clinical Interview for DSM-IV psychiatric disorders (SCID-IV58). Volunteers had to meet the following inclusion criteria: (a) being from 18 through 45 years old; (b) having an IQ ≥ 80 (estimated using the Wechsler Abbreviated Scale of Intelligence59); (c) reporting the absence of any DSM-IV Axis I psychiatric disorder; (d) using no prescribed medications within the previous three months; (e) reporting no regular use of over-the-counter medications; (f) smoking ≤ one pack of cigarettes per day; and (g) having good physical health.
Volunteers meeting inclusion criteria were offered study participation and assigned to one of three experimental task groups (n = 30 each) including the Immediate Memory Task (IMT), GoStop Impulsivity Paradigm (GoStop), and Single Key Impulsivity Paradigm (SKIP). Groups were closely matched for age, gender, education, and race. Prior to study participation, volunteers provided written informed consent and all study procedures were reviewed and approved by our Institutional Review Board. This study was conducted in accordance with the Declaration of Helsinki and FDA guidelines for Good Clinical Practice.
2.2. Experimental design and procedure
The study required four days of participation: two performance stabilization days followed by two experimental days. The performance stabilization days allowed participants to become familiar with the study procedures and environment and provided time for the participants’ task performance to stabilize. On these days, participants completed five sessions of their assigned behavioral impulsivity task across the day in a manner similar to experimental days. On experimental days, participants consumed one of two different types of amino-acid beverages, either tryptophan depletion or balanced-placebo control (see Section 2.3). All participants experienced the two amino-acid beverage conditions on two different experimental days, and the order of the two beverages was counterbalanced. A minimum of 48 hours separated experimental days to avoid potential carry over effects of the aminoacid manipulations.40,60,61 The daily testing schedule on experimental days included a baseline pre-drink testing session (8:15 am) followed by administration of the amino-acid beverage and 4 subsequent testing sessions at 1.5 h (10:30 am), 4.0 h (1:00 pm), 5.0 h (2:00 pm), and 6.0 h (3:00 pm) after consuming the amino-acid beverage. The schedule of behavioral testing was selected to be consistent with the identified time-course changes in plasma tryptophan following the 50 g tryptophan depletion administration.25 The behavioral testing sessions were conducted in a sound-attenuated chamber equipped with a 17-in computer monitor and a computer mouse. Between testing sessions, participants were permitted to read, watch television, or relax (but not sleep) in an assigned waiting lounge. Participants were monitored by research staff using closed-circuit television monitors.
All testing days began at 8:00 am and ended at 5:00 pm. Upon arrival, the experimenters administered a breathalyzer test (AlcoTest® 7110 MKIII C, Draeger Safety Inc., Durango, CO) and obtained a urine sample for drug screening (THC, cocaine, benzodiazepines, opiates, and amphetamines; Panel/Dip Drugs of Abuse Testing Device, Redwood Biotech, Santa Rosa, CA). For women, the urine sample was also used to conduct a pregnancy test each day of participation. Based on findings that nicotine withdrawal affects laboratory-measured behavior,62 one smoking break was provided at noon during which participants were permitted one cigarette.
2.3. Amino-acid administration procedure
Standardized procedures were used to administer the tryptophan depletion and balanced-placebo beverages. Amino-acid formulations contained approximately 50 g of 15 amino acids;22,25 the only difference between the two formulations was the amount of tryptophan in the drink (depletion = 0.0 g, balanced placebo = 1.15 g; see Table 1). Consistent with previous studies, these amino acids were combined with 240 ml of water and flavored with one packet of saccharin sweetener and powdered raspberry-lemonade flavoring.25 To increase the palatability of these amino-acid beverages, L-cysteine and L-methionine were administered in gelatin capsules. The drinks were consumed between 8:35 and 9:00 am, and were administered in a double-blind fashion. This timing allowed for their peak effects to be achieved by the afternoon testing sessions (Sessions 4 and 5). Lastly, participants were instructed to fast after midnight the night before each of the experimental days to avoid potential dietary interference with the amino-acid manipulation. Participants were provided a meal after completing their behavioral testing sessions.
Table 1.
Amino acids contained in the 50 g tryptophan depletion and balanced-placebo formulations.
| L-tryptophan formulations | |
| L-tryptophan depletion | 0.00 |
| L-tryptophan balanced placebo | 1.15 |
| 15 amino acids | |
| L-alanine | 2.75 |
| L-arginine | 2.45 |
| L-cysteine | 1.35 |
| Glycine hydromonochloride | 1.60 |
| L-histidine | 1.60 |
| L-isoleucine | 4.00 |
| L-leucine | 6.75 |
| L-lysine | 4.45 |
| L-methionine | 1.50 |
| L-phenylalanine | 2.85 |
| L-proline | 6.10 |
| L-serine | 3.45 |
| L-threonine | 3.25 |
| L-tyrosine | 3.45 |
| L-valine | 4.45 |
| Depletion, total grams | 50.00 |
| Balanced-Placebo, total grams | 51.15 |
2.4. Measures of impulsivity
As indicated above, study participants were assigned to one of the three types of behavioral impulsivity testing paradigms, and each were read a set of standardized instructions prior to their first stabilization session.33,36,37 The paradigms are described below.
2.4.1. Immediate Memory Task (IMT)
The IMT is a continuous performance test that can be used to measure response initiation impulsivity.63 In this 10-min task, a series of 5-digit numbers are displayed on a monitor screen in black and centered on a white background. The numbers (2.3 cm in height) are randomly generated and appear for 500 msec at a rate of one per second. The participant is instructed to respond (mouse click) when the 5-digit number they see is identical to the one that preceded it. While this task yields a wide variety of data, there are three main dependent variables: 1) correct detections, where the participant correctly responds to a 5-digit number that is identical to the preceding number; 2) commission errors, where the participant incorrectly responds to a 5-digit number that differs from the preceding number by only one digit (its position and value determined randomly); and 3) response latencies, which is the time in milliseconds between stimulus presentations and the participant’s recorded responses. The IMT Ratio is the primary dependent measure of impulsivity for this task (i.e. the proportion of commission errors relative to the correct detections32,63).
2.4.2. GoStop impulsivity paradigm (GoStop)
The GoStop is a stop-signal task that measures response inhibition aspects of impulsivity.39 In this 12-min task, a series of 5-digit numbers are displayed for 500 msec with a 1,500 msec inter-stimulus interval. Like the IMT above, 5-digit numbers appear in series, and some of these numbers are identical to the immediately preceding 5-digit number. Participants are instructed to respond to these matching numbers (matching numbers are considered the go signal). In this task, however, some of these matching numbers are first presented in black and then turn red. This is a stop signal cue, and the participants are instructed to withhold responding to any matching numbers that turn red. The timing of these stop signals varied across the testing session (e.g. 50, 150, 250 and 350 msec). The two dependent measures of interest were: 1) correct responses, where the participant responds to a number that matches the preceding number (and the number remains black); and 2) response inhibition failures, where the participant fails to withhold responding to a matching number when a stop signal has appeared. The primary dependent measure is the GoStop Ratio,32 which is calculated as the number of response inhibition failures (i.e. incorrect responses to stop trials) relative to the number of correct responses (i.e. go trials). The GoStop Ratio has been validated as a measure of the ability to inhibit an already initiated response, and data from the 150 msec stop delay typically provides the best group discrimination.26
2.4.3. Single Key Impulsivity Paradigm (SKIP)
The SKIP37 is a consequence sensitivity measure of impulsivity that assesses an individual’s preference for rewards of different delays and magnitudes (e.g. preference for smaller-sooner over larger-later rewards). This is a free-operant task where the participant may respond as often as desired by clicking a computer mouse to accumulate points. During the session, two point counters appear on the computer monitor: one presents feedback about the delay contingency on a point counter at the bottom of the monitor in the form of a 2-sec display of the points earned for the most recent response; the other counter is a constant display at the top of the monitor showing the total accumulation of earnings throughout the session. Participants are provided general instructions that the task will last about 20 minutes, during which time they may respond as often as they wish and the longer they wait between responses, the more points that response will be worth. Participants must infer specific reward contingencies from the feedback provided for each response. Individual responses produce a reward that increases exponentially and is calculated using the number of seconds that have elapsed since the previous response. The formula used in this task was [the number of seconds elapsed + (3 × the number of seconds elapsed2)]/1000. One example using this formula is that after a 60-sec delay a response would earn 10.86 points, but after a 300-sec delay a response would earn 270.3 points. The second response was only 5 times longer than the first, but the reward was 25 times larger. While this task yields a wide variety of data, there are three primary types: 1) the average response interval gives information about the reward-delay tolerance; 2) the total number of responses is another indicator of preference for smaller-sooner rewards; and 3) the longest response delay recorded during a testing session is an indicator of a participant’s willingness to delay responses to obtain rewards of larger magnitude. The primary dependent measure used in this study was the longest delay (i.e. the longest elapsed time between two consecutive reward responses39), which reflects the maximal delay that an individual is willing to tolerate for a larger reward.
2.4.4. Barratt Impulsiveness Scale (BIS-11)
The BIS-1164 is a 30-item questionnaire where individuals rate the frequency of several common impulsive (e.g. “I do things without thinking”) or non-impulsive (“I am self-controlled”) behavioral traits on a scale from 1 (“rarely/never”) to 4 (“almost always/always”). Scores range from 30 to 120, with higher scores indicating more impulsiveness. The BIS-11, which was completed only once, was used to characterize the groups’ self-reported trait impulsivity.
2.5. Compensation
To maximize effortful performance, participants received a performance-based monetary bonus at the end of each day. Each of the paradigms generated points based on performance, and the bonus was determined by comparing the sum of each day’s points to the sum of points earned on the first day of participation (Performance Stabilization Day 1). The performance bonus was computed as a proportion of the first baseline day performance such that points earned equivalent to Day 1 resulted in a payment of $15. Points earned above Day 1’s performance resulted in earning more money (up to $20 maximum) and points earned less than Day 1’s performance resulted in earning less money (down to a $10 minimum). These earnings were added to a daily payment of $60, so that on average, participants were compensated between $70–80 per day.
2.6. Data analyses
Analyses were conducted to examine differences in both demographic and performance data between and within the three impulsivity task groups (i.e. IMT, GoStop, and SKIP). Participant characteristics (i.e. age, education, IQ, and BIS-11) were compared among the task groups using univariate analyses of variance (ANOVA), and within-group differences between men and women were tested using two-tailed independent t-tests. Chi-Square tests were used to compare the racial/ethnic distribution of men and women within each group and to compare the distribution across the three tasks. Differences in laboratory behavioral impulsivity performance were tested between men and women within each task group to examine whether the effects of tryptophan manipulations differed by gender. Results of preliminary 2 × 2 × 5 (Gender × Amino-acid Drink × Time of Testing) ANOVAs conducted separately for each task type showed there were no main effects or interactions related to Gender, therefore task data were collapsed across gender in subsequent analyses. SKIP data were transformed using a reflection strategy to correct the non-normal negative skew prior to analyses.65 As recommended when using these transformations, median scores are used in the graphical presentation of these data (Fig. 1, bottom panel). Individuals with performance data more than 2 standard deviations from the session mean at two or more testing times on performance stabilization and experimental days were considered outliers and excluded from analyses, resulting in 26 individuals in the IMT group and 27 individuals in the GoStop group. For the SKIP task, all individuals were retained for the primary analyses; however, four outliers were excluded from analyses of one of the supplementary variables (Total Responses). In all cases, removal of outliers enhanced interpretability without changing the direction of results.
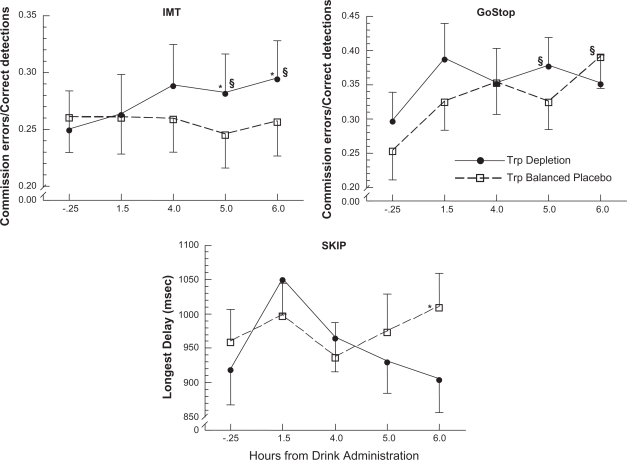
Figure 1.
The effects of two amino-acid conditions on IMT (top panel), GoStop (middle panel), and SKIP (bottom panel) performance across pre-drink baseline and post-drink testing times (mean ± SEM). For the SKIP, data were transformed using a reflection strategy to correct non-normal negative skew.
*Indicates significant differences between Trp depletion and the balanced placebo.
§Indicates a significant difference from pre-drink baseline performance.
Omnibus analyses were conducted separately for each of the three primary behavioral task variables (i.e. IMT Ratio, GoStop 150 msec Ratio, and SKIP Longest Delay) using a 2 × 5 (Amino-acid Drink × Time of Testing) repeated-measures ANOVA. Within-group planned comparisons of the behavior observed at 5.0 h and 6.0 h following amino-acid administration (i.e. peak depletion effects) were conducted with two-tailed paired t-tests to determine the extent of behavioral changes: 1) resulting from each of the amino-acid formulations when compared to their respective baseline pre-drink performance; and 2) resulting from tryptophan depletion relative to the balanced placebo. Analysis of pre-drink baseline testing between tryptophan depletion and the balanced placebo showed no performance differences for any of the task groups (P > 0.05). PASW© version 17.0.2 (SPSS, Inc., Chicago, IL) was used for all data analyses, and the significance criterion for all comparisons was set at P ≤ 0.05.
3. Results
3.1. Participant characteristics
Characteristics of participants in each of the three impulsivity task groups (i.e. IMT, GoStop, and SKIP) are presented in Table 2. Participants in the three impulsivity task groups were similar (p’s > 0.05) in their demographic characteristics (i.e. age, education, IQ) and a self-report measure of trait impulsivity (Barratt Impulsiveness Scale-11). Within each task group, there were no significant gender differences on any of these variables (P’s > 0.05).
Table 2.
Demographic characteristics and trait impulsivity between and within task groups.
| Characteristics |
IMT |
GoStop |
SKIP |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (yrs) | 27.3 | 6.0 | 26.9 | 8.1 | 32.5 | 9.6 | 28.9 | 10.4 | 29.5 | 8.9 | 27.2 | 7.5 |
| Education (yrs) | 14.9 | 2.4 | 14.7 | 2.3 | 14.4 | 1.7 | 15.2 | 2.5 | 13.8 | 1.8 | 14.7 | 2.0 |
| WASI (Total) | 102.5 | 10.9 | 102.8 | 6.1 | 103.4 | 10.7 | 102.4 | 14.9 | 98.7 | 11.0 | 104.7 | 12.6 |
| BIS-11 (Total) | 62.1 | 8.9 | 56.7 | 6.5 | 59.9 | 8.9 | 54.9 | 5.5 | 57.7 | 9.3 | 59.7 | 8.4 |
| Cigarettes/Day | 1.7 | 5.5 | 0 | 0 | 1.9 | 3.8 | 0.3 | 1.3 | 2.9 | 5.9 | 0.7 | 2.6 |
| Ethnicity | n | n | n | n | n | n | ||||||
| AA/Cauc/Hisp | 6/7/0 | 8/5/0 | 8/6/0 | 7/5/1 | 8/6/1 | 8/6/1 | ||||||
Notes: AA, African American; Cauc, Caucasian; Hisp, Hispanic; BIS-11, Barratt Impulsiveness Scale (Version 11); WASI, Wechsler Abbreviated Scale of Intelligence.
3.2. The effects of amino-acid manipulations on primary measures of behavioral impulsivity
The effects of the amino-acid manipulations on behavioral impulsivity tasks were of specific interest in this study. Consistent with previous studies,25,40 we examined the data in two ways. First, we examined changes in behavioral impulsivity over time from the baseline pre-drink testing session to later testing sessions when the tryptophan manipulation was at the peak effects (5.0 h and 6.0 h following amino-acid drink administration25). Second, we examined differences between the two amino-acid drink conditions at the later testing sessions. The results of analyses for the primary impulsivity variable from each task are described below.
3.2.1. Immediate Memory Task (IMT)
Impulsivity increased during the peak effects of tryptophan depletion. This was shown by a significant interaction between the amino-acid condition and time of testing (F4,100 = 3.05, P = 0.020). As expected, when comparing changes in behavioral impulsivity from the baseline testing session to later testing sessions, there was a significant increase in impulsive responding at both 5.0 h (Session 4) and 6.0 h (Session 5) post-drink testing relative to baseline performance for the tryptophan depletion condition (Session 4: t25 = 2.62, P = 0.015; Session 5 t25 = 3.02, P = 0.006; Fig. 1, top panel). However, no performance differences were observed between the baseline testing session and later testing sessions for the balanced-placebo condition (P > 0.05). When comparing depletion effects to placebo, impulsivity was significantly greater following depletion. This was indicated by differences in impulsivity between the two amino-acid drink conditions at the two later testing sessions (Session 4: t25 = 2.13, P = 0.044; and Session 5: t25 = 2.51, P = 0.019).
3.2.2. GoStop
In contrast to IMT performance differences described above, impulsivity (i.e. GoStop Ratio) increased over the course of the day regardless of the drink condition. This was indicated by a significant main effect for time of testing (F4,104 = 4.39, P = 0.003). As might be expected from the results of the omnibus analysis, planned comparisons of the behavioral changes from the baseline testing session to later sessions showed that impulsive performance was elevated at the later sessions for both the depletion and balanced-placebo conditions (middle panel). Specifically, impulsivity was elevated at 5.0 h post-drink testing (Session 4) following tryptophan depletion (t26 = 2.22, P = 0.036) and, contrary to our hypothesis, impulsivity was also elevated at 6.0 post-drink testing (Session 5) for the balanced-placebo condition (t26 = 2.82, p = 0.009). There were no significant differences in impulsivity between the two drink conditions at either 5.0 h or 6.0 h following drink administration (P > 0.05).
3.2.3. Single Key Impulsivity Paradigm (SKIP)
Similar to the IMT, tryptophan depletion increased impulsivity on the SKIP. This was indicated by a main effect for amino-acid drink (F1,116 = 4.32, P = 0.047). There were no significant differences in impulsivity between the baseline testing session and later testing sessions for either drink condition (P > 0.05). Although impulsivity did not differ between the two drink conditions at 5.0 h post-drink testing (P > 0.05), there was a significant difference between the two drink conditions at 6.0 h post-drink testing (Session 5: t29 = 2.44, P = 0.021; Fig. 1, bottom panel). That is, participants in the tryptophan depletion condition showed significantly less tolerance for delaying responses (i.e. Longest Delay between consecutive responses became shorter) relative to the balanced placebo.
3.3. The effects of amino-acid manipulations on additional dependent measures
In addition to analyses of the primary variables of interest (presented above), we examined the peak effects of the amino-acid manipulations on all of the main outcome variables for each of the three impulsivity tasks. Specifically, paired t-tests were used to compare behavioral responses in the tryptophan depletion and the balanced-placebo conditions at 5.0 h (Session 4) and 6.0 h (Session 5) post-drink administration. Parallel to the findings reported above, there was a significant difference between the two amino-acid drink conditions on indices of behavioral impulsivity, but not on dependent variables reflecting attention (e.g. correct detections) and reaction speed (e.g. response latencies). The results of these analyses are presented in Table 3.
Table 3.
Performance means and standard deviations of the primary dependent measures for each task following each of the two amino-acid formulations at the time points of peak effectiveness.
| 5.0 h | 6.0 h | |||||||
|---|---|---|---|---|---|---|---|---|
|
Tryptophan depletion |
Balanced placebo |
Tryptophan depletion |
Balanced placebo |
|||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| IMT | ||||||||
| Correct detections | 96.41 | 3.72 | 96.49 | 4.02 | 96.19 | 4.67 | 96.48 | 4.51 |
| Response latency | 421.21 | 51.44 | 419.22 | 43.51 | 418.33 | 48.88 | 416.48 | 43.01 |
| Commission errors | 27.09 | 16.51 | 23.69 | 15.04* | 28.24 | 16.02 | 24.75 | 15.31* |
| Response latency: FA | 429.52 | 60.54 | 432.30 | 57.58 | 430.57 | 58.08 | 428.81 | 48.57 |
| Ratio | 0.28 | 0.17 | 0.25 | 0.15* | 0.26 | 0.16 | 0.30 | 0.17* |
| GoStop (150 ms) | ||||||||
| Correct detections | 7.41 | 4.12 | 6.44 | 4.28 | 6.89 | 4.10 | 7.74 | 4.85 |
| Response inhibition failures | 78.11 | 2.44 | 78.30 | 1.77 | 78..41 | 1.72 | 78.30 | 2.45 |
| Ratio | 0.38 | 0.21 | 0.33 | 0.22 | 0.35 | 0.21 | 0.39 | 0.25 |
| SKIP | ||||||||
| Total responses | 3.5 | 2.5 | 2.73 | 1.85* | 3.50 | 3.3 | 3.15 | 2.01 |
| Average response interval | 704.51 | 367.04 | 679.56 | 364.77 | 739.19 | 312.21 | 728.64 | 319.09 |
| Longest delay | 931.93 | 259.31 | 974.84 | 294.37 | 905.36 | 270.95 | 1010.49 | 264.66* |
Note: FA, False Alarms.
Indicates significant difference between the Trp depletion (T–) and balanced placebo (TB) conditions at P < 0.05.
4. Discussion
The purpose of this study was to determine how changes in the underlying biological state of healthy adults affect performance on behavioral measures of impulsivity. One important aspect of this study was to test how serotonergic dysregulation would affect impulsivity in matched samples of healthy adults with low/normal trait impulsivity. A second important aspect was the use of multiple measures of behavioral impulsivity to test the effects of acute tryptophan depletion across multiple time points under identical treatment conditions. These two points address a number of methodological limitations of previous studies that have tested the relationship between serotonin and impulsive behaviors.
While numerous studies have examined the relationships of serotonergic deficits among clinical samples with impulsive characteristics,3–10,12,14 far fewer studies have been conducted that experimentally examine the effects of tryptophan depletion on behavioral impulsivity measured in the laboratory. Furthermore, comparisons across studies are difficult because of differences in methodologies and important sample characteristics, such as age, gender, and pre-existing psychopathology. For instance, prior studies have examined the effect of tryptophan depletion among men at risk for development of alcohol or substance use disorders,56,57 aggressive adolescent boys,66 boys with attention-deficit/hyperactivity disorder,67 and aggressive adult psychiatric patients.68 In these cases, the effects of tryptophan depletion on impulsive behavior are confounded by the influence of the sample characteristics. Collectively, given the variety of clinically and behaviorally impaired samples that have been tested, reaching a consensus regarding the possible underlying behavioral and biological mechanisms responsible for the different aspects of impulsivity has not yet been possible. This underscores the importance of the present study in which we tested matched samples of normal healthy adults with a low-normal range of self-reported impulsivity. Differences in impulsivity found in this study of healthy participants can be more confidently attributed to the effects of tryptophan depletion and changes in brain serotonin.
Although some prior studies of the effects of tryptophan depletion on impulsivity have controlled sample characteristics and limited testing to healthy adult men and women,40,52,56 methodological constraints among these studies prevent clear understanding of the effects of serotonin manipulations on impulsivity. One of the most prominent methodological limitations in studies of impulsive behavior has been the restriction of testing to a single measure of impulsivity. One criticism of impulsivity assessment is that researchers have frequently relied on single methodologies used in isolation, and often among different clinical or high-risk populations.31,69,70 Studies using isolated measures have produced conflicting results, which can lead to inaccurate conclusions about the role of serotonin in impulsive behavior. Because of the different outcomes and conclusions, there is often a failure to replicate results and little consensus for the most accurate or appropriate measures of impulsivity. Assessments of multiple components of impulse control are necessary to ultimately understand the relationships of different components of impulse control among samples characterized by impulsive behavior (e.g. suicidal and drug-use behaviors69,70). To overcome this limitation, in the present study we incorporated key features of current theoretical models and systematically studied the effects of tryptophan depletion using three laboratory measures of impulsivity representative of the three-component model.32,38 Given that the differences in the timing and magnitude of the behavioral changes observed were not uniform across the three measures, the outcomes of this study support the need for using multiple measures and have important implications for identifying the role of serotonin dysregulation in these different component processes of impulsive behavior.
As described in the introduction, impulsivity is a complex multi-faceted construct, the components of which may be differentially affected by pharmacological manipulations such as tryptophan depletion. The theoretical models of impulsivity propose that deficits in response initiation are reflected by a response that is made prior to complete information processing of a stimulus, whereas deficits in response inhibition are reflected by an inability to withhold a response in the face of new information. And, deficits in consequence sensitivity are reflected by a predominant preference for a smaller-sooner rather than a larger-later reward, which theorists suggest is an inability to tolerate the delay necessary for obtaining the larger reward. Several theoretical models32,71 suggest that different behavioral mechanisms of impulsivity may be associated with distinct underlying biological differences. Consistent with these theoretical suppositions, the results of the present study illustrate that brain serotonin may play a greater role in mechanisms underlying response initiation impulsivity and sensitivity to reward, but a lesser role in mechanisms governing response inhibition. One possible explanation for this unexpected finding is that the response inhibition function (i.e. GoStop) may be more relevant to underlying deficits in noradrenergic and dopaminergic systems, which would be consistent with findings from other studies.72 Alternatively, it may be simply that the low-impulsive healthy adults, such as those tested in this study, are not sensitive to changes in response inhibition impulsivity as a result of serotonergic manipulations and that differences would be more distinct when testing other populations.
The findings of this current study are generally consistent with the few studies that have examined the effect of tryptophan depletion on impulsivity among healthy adults, and provide important extensions of that work. While two of those studies reported increased response initiation impulsivity following tryptophan depletion among healthy individuals, those studies focused predominantly on testing samples of men.51,52 The present findings indicate that the effect of tryptophan depletion on response initiation impulsivity is evident in both men and women. Increases in impulsivity also have been found when testing response inhibition impulsivity following tryptophan depletion in samples of aggressive adolescents66 and young men at risk for alcoholism;54,55 however, consistent with the present study, others have also failed to find similar decreases in response inhibition in healthy participants.54 Finally, data from this study also indicate that the effects of tryptophan depletion increase consequence sensitivity impulsivity among healthy adults, contributing to a small literature that has been inconsistent and has focused almost exclusively on individuals with substance use problems or individuals at risk for developing substance-related problems. Only three studies have examined the effects of tryptophan depletion on consequence sensitivity impulsivity (e.g. delay-discounting procedures) among healthy controls. One study using a questionnaire-based delay-discounting procedure found that tryptophan depletion had no effect on impulsivity,54 but this method of testing has drawn criticism because it requires participants to think about, rather than experience, the consequences of each choice.73 Of the two prior studies that used experiential behavioral testing, one found tryptophan depletion had a significant effect on consequence sensitivity impulsivity56 while the other did not.57 Notably, the latter study only included twelve participants and may not have had sufficient power to detect the relatively subtle behavioral effects of tryptophan manipulation in healthy individuals; however, the former tested a sample of healthy men only and did not specify the age range of the participants. Thus, the present study adds important information to this limited body of literature and provides further support for the role of serotonin in consequence sensitivity impulsivity.
In conclusion, under carefully controlled conditions, this study demonstrated that acute tryptophan depletion among healthy adults produced selective differences in impulsive responding that were dependent on the type of impulsivity being tested. Having identified these effects in a sample of healthy controls, this study lays the groundwork for future avenues of research involving other populations or other pharmacological agents. For example, because alcohol consumption has been shown to affect a number of different types of impulsivity among normal healthy volunteers,74,75 one logical extension of the current study would be to investigate the effect of tryptophan depletion on alcohol-induced impulsivity in a sample of healthy controls. Testing how alcohol-induced impulsivity might differ as a function of an individual’s underlying serotonergic state among healthy individuals, rather than a clinical sample, would provide important information about the serotonin-alcohol relationship without the inherent interference of underlying differences related to clinical state. This type of study would provide insight into the role that serotonin plays in either mediating or moderating the behavioral effects of alcohol. Alternatively, two recent studies have used tryptophan depletion to test behavior in clinical samples of boys with ADHD and adults with self-injurious behavior.67,68 Extending these studies to test changes in laboratory-measured behavioral impulsivity following tryptophan depletion among various clinical populations characterized by problems with impulsivity (e.g. individuals with alcohol or substance use disorders, bipolar disorder, or conduct disorder) could provide valuable insight into specific behavioral deficits of these samples.
Acknowledgments
This research was sponsored by a National Institutes of Health grant from the National Institute of Alcohol Abuse and Alcoholism (R01-AA014988). Dr. Dougherty gratefully acknowledges support from a research endowment, the William and Marguerite Wurzbach Distinguished Professorship. Lastly, the authors would like to express their appreciation to Marla Ray, B.A., Samantha John, B.S. and Sharon Cates, B.S. for their dedicated efforts as research assistants on this project.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Badawy AA, Morgan CJ, Lovett JW, Bradley DM, Thomas R. Decrease in circulating tryptophan availability to the brain after acute ethanol consumption by normal volunteers: Implications for alcohol-induced aggressive behaviour and depression. Pharmacopsychiatry. 1995;28:93–7. doi: 10.1055/s-2007-979626. [DOI] [PubMed] [Google Scholar]
- 2.Ballenger JC, Goodwin FK, Major LF, Brown GL. Alcohol and central serotonin metabolism in man. Arch Gen Psychiatry. 1979;36:224–7. doi: 10.1001/archpsyc.1979.01780020114013. [DOI] [PubMed] [Google Scholar]
- 3.Banki CM. Factors influencing monoamine metabolites and tryptophan in patients with alcohol dependence. J Neural Transm. 1981;50:89–101. doi: 10.1007/BF01249132. [DOI] [PubMed] [Google Scholar]
- 4.Borg S, Kvande H, Liljeberg P, Mossberg D, Valverius P. 5-Hydroxyindoleacetic acid in cerebrospinal fluid in alcoholic patients under different clinical conditions. Alcohol. 1985;2:415–8. doi: 10.1016/0741-8329(85)90106-5. [DOI] [PubMed] [Google Scholar]
- 5.Moss HB. Serotonergic activity and disinhibitory psychopathy in alcoholism. Medical Hypotheses. 1987;23:353–61. doi: 10.1016/0306-9877(87)90055-7. [DOI] [PubMed] [Google Scholar]
- 6.Roy A, Linnoila M. CSF studies on alcoholism and related behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:505–11. doi: 10.1016/0278-5846(89)90138-3. [DOI] [PubMed] [Google Scholar]
- 7.Asberg M, Nordstrom P, Traskman-Bendz L. Cerebrospinal fluid studies in suicide: An overview. Ann N Y Acad Sci. 1986;487:243–55. doi: 10.1111/j.1749-6632.1986.tb27903.x. [DOI] [PubMed] [Google Scholar]
- 8.Asberg M, Traskman L, Thoren P. 5-HIAA in the cerebrospinal fluid: A biochemical suicide predictor. Arch Gen Psychiatry. 1976;33:1193–7. doi: 10.1001/archpsyc.1976.01770100055005. [DOI] [PubMed] [Google Scholar]
- 9.Banki CM, Arato M. Amine metabolites, neuroendocrine findings, and personality dimensions as correlates of suicidal behavior. Psychiatric Res. 1983;10:253–61. doi: 10.1016/0165-1781(83)90072-0. [DOI] [PubMed] [Google Scholar]
- 10.Verona E, Patrick CJ. Suicide risk in externalizing syndromes: Temperamental and neurobiological underpinnings. In: Joiner TE, Rudd D, editors. Suicide Science: Expanding the boundaries. Boston: Kluwer Academic Publishers; 2000. pp. 137–73. [Google Scholar]
- 11.Brown GL, Ballanger JC, Minichiello MD, Goodwin FK. Human aggression and its relationship to cerebrospinal fluid 5-hydroxyindoleacetic acid, 3-methoxy-4-hydroxyphenylglycol, and homovanillic acid. In: Sandler M, editor. Psychopharmacology of Aggression. New York: Raven Press; 1979. pp. 131–48. [Google Scholar]
- 12.Brown GL, Ebert MH, Goyer PF, et al. Aggression, suicide, and serotonin: Relationships to CSF amine metabolites. Am J Psychiatry. 1982;139:741–6. doi: 10.1176/ajp.139.6.741. [DOI] [PubMed] [Google Scholar]
- 13.Limson R, Goldman D, Roy A, et al. Personality and cerebrospinal fluid monoamine metabolites in alcoholics and controls. Arch Gen Psychiatry. 1991;48:437–41. doi: 10.1001/archpsyc.1991.01810290049010. [DOI] [PubMed] [Google Scholar]
- 14.Virkkunen M, Kallio E, Rawlings R, et al. Personality profiles and state aggressiveness in Finnish alcoholic, violent offenders, fire setters, and healthy volunteers. Arch Gen Psychiatry. 1994;51:28–33. doi: 10.1001/archpsyc.1994.03950010028004. [DOI] [PubMed] [Google Scholar]
- 15.Virkkunen M, Rawlings R, Tokola, et al. CSF biochemistries, glucose metabolism, and diurnal activity rhythms in alcoholic, violent offenders, fire setters, and healthy volunteers. Arch Gen Psychiatry. 1994;51:20–7. doi: 10.1001/archpsyc.1994.03950010020003. [DOI] [PubMed] [Google Scholar]
- 16.Oquendo MA, Mann JJ. The biology of impulsivity and suicidality. Psychiatr Clin North Am. 2000;23:11–27. doi: 10.1016/s0193-953x(05)70140-4. [DOI] [PubMed] [Google Scholar]
- 17.Carrillo M, Ricci LA, Coppersmith GA, Melloni RH. The effect of increased serotonergic neurotransmission on aggression: A critical meta-analytical review of preclinical studies. Psychopharmacol (Berl) 2009;205:349–68. doi: 10.1007/s00213-009-1543-2. [DOI] [PubMed] [Google Scholar]
- 18.Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Clin Psych Rev. 2006;26:379–95. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsh-Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, Dougherty DM. L-tryptophan: Basic metabolic functions, behavioral research, and therapeutic indications. Intern J Tryptophan Res. 2009;2:45–60. doi: 10.4137/ijtr.s2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hood SD, Bell CJ, Nutt DJ. Acute tryptophan depletion. Part I: Rationale and methodology. Aust N Z J Psychiatry. 2005;39:558–64. doi: 10.1080/j.1440-1614.2005.01627.x. [DOI] [PubMed] [Google Scholar]
- 21.Cleare AJ, Bond AJ. The effect of tryptophan depletion and enhancement on subjective and behavioral aggression in normal male subjects. Psychopharmacol (Berl) 1995;118:72–81. doi: 10.1007/BF02245252. [DOI] [PubMed] [Google Scholar]
- 22.Young SN, Ervin FR, Pihl RO, Finn P. Biochemical aspects of tryptophan depletion in primates. Psychopharmacol (Berl) 1989;98:508–11. doi: 10.1007/BF00441950. [DOI] [PubMed] [Google Scholar]
- 23.Young SN, Smith SE, Pihl RO, Ervin FR. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacol (Berl) 1985;87:173–7. doi: 10.1007/BF00431803. [DOI] [PubMed] [Google Scholar]
- 24.Fernstrom JD. Role of precursor availability in control of monoamine biosynthesis in brain. Physiol Rev. 1983;63:484–546. doi: 10.1152/physrev.1983.63.2.484. [DOI] [PubMed] [Google Scholar]
- 25.Dougherty DM, Marsh DM, Mathias CW, et al. Comparison of 50- and 100-g L-tryptophan depletion and loading formulations for altering 5-HT synthesis: Pharmacokinetics, side effects, and mood states. Psychopharmacol (Berlin) 2008;198:431–45. doi: 10.1007/s00213-008-1163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marsh DM, Dougherty DM, Mathias CW, Moeller FG, Hicks LR. Comparison of women with high and low trait impulsivity using laboratory impulsivity models of response-disinhibition and reward-choice. Pers Individ Dif. 2002;33:1291–310. [Google Scholar]
- 27.Young SN. The use of diet and dietary components in the study of factors controlling affect in humans: A review. J Psychiatry Neurosci. 1993;18:235–44. [PMC free article] [PubMed] [Google Scholar]
- 28.Young SN, Leyton M. The role of serotonin in human mood and social interaction: Insight from altered tryptophan levels. Pharmacol Biochem Behav. 2002;71:857–65. doi: 10.1016/s0091-3057(01)00670-0. [DOI] [PubMed] [Google Scholar]
- 29.Badawy AA. Tryptophan metabolism and disposition in relation to alcohol and alcoholism. Adv Exp Med Biol. 1996;398:75–82. doi: 10.1007/978-1-4613-0381-7_10. [DOI] [PubMed] [Google Scholar]
- 30.Nishizawa S, Benkelfat C, Young SN, et al. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A. 1997;94:5308–13. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–93. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- 32.Dougherty DM, Mathias CW, Marsh-Richard DM, Nouvion SO, Dawes MA, Furr RM. Distinctions in behavioral impulsivity: Implications for substance abuse research. Addict Disord Their Treat. 2009;8(2):61–73. doi: 10.1097/ADT.0b013e318172e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dougherty DM, Marsh DM. Immediate and Delayed Memory Tasks (IMT/ DMT 2.0): A research tool for studying attention, memory, and impulsive behavior [Manual] Neurobehavioral Research Laboratory and Clinic, University of Texas Health Science Center at Houston; Houston, TX: 2003. [Google Scholar]
- 34.Dougherty DM, Bjork JM, Harper RA, et al. Behavioral impulsivity paradigms: A comparison in hospitalized adolescents with disruptive behavior disorders. J Child Psycho Psychiatry. 2003;44:1145–57. doi: 10.1111/1469-7610.00197. [DOI] [PubMed] [Google Scholar]
- 35.Dougherty DM, Bjork JM, Harper RA, et al. Validation of the Immediate and Delayed Memory Tasks in hospitalized adolescents with disruptive behavior disorders. Psychol Res. 2003;53:509–32. doi: 10.1111/1469-7610.00197. [DOI] [PubMed] [Google Scholar]
- 36.Dougherty DM, Mathias CW, Marsh DM. Neurobehavioral Research Laboratory and Clinic, University of Texas Health Science Center at Houston; Houston, TX: 2003. GoStop Impulsivity Paradigm (Version 1.0) [Manual] [Google Scholar]
- 37.Dougherty DM, Mathias CW, Papageorgiou TD, Marsh DM. Neurobehavioral Research Laboratory and Clinic, University of Texas Health Science Center at Houston; Houston, TX: 2003. Single Key Impulsivity Paradigm (Version 1.0) [Manual] [Google Scholar]
- 38.Dougherty DM, Marsh DM, Mathias CW, Swann AC. The conceptualization and role of impulsivity: Bipolar disorder and substance abuse. Psychiatric Times. 2005 Jul;:32–35. [Google Scholar]
- 39.Dougherty DM, Mathias CW, Marsh DM, Jagar AA. Laboratory behavioral measures of impulsivity. Behav Res Methods. 2005;37:82–90. doi: 10.3758/bf03206401. [DOI] [PubMed] [Google Scholar]
- 40.Dougherty DM, Marsh DM, Mathias CW, et al. The effects of alcohol on laboratory-measured impulsivity after L-tryptophan depletion or loading. Psychopharmacol (Berlin) 2007;193:137–50. doi: 10.1007/s00213-007-0763-6. [DOI] [PubMed] [Google Scholar]
- 41.Fields S, Collins C, Leraas K, Reynolds B. Dimensions of impulsivity in adolescent smokers and nonsmokers. Exp Clin Psychopharmacol. 2009;17:302–11. doi: 10.1037/a0017185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ledgerwood DM, Alessi SM, Phoenix N, Petry NM. Behavioral assessment of impulsivity in pathological gamblers with and without substance use disorder histories versus healthy controls. Drug Alcohol Depend. 2009;105:89–96. doi: 10.1016/j.drugalcdep.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Zhu Y, Wu YZ. Features of functional MRI in children with oppositional defiant disorder. Journal of Central South University (Medical Science) 2008;33:571–5. [PubMed] [Google Scholar]
- 44.Mathias CW, Marsh-Richard DM, Dougherty DM. Behavioral measures of impulsivity and the law. Behav Sci and Law. 2008;26:691–707. doi: 10.1002/bsl.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCloskey MS, New AS, Siever LJ, et al. Evaluation of behavioral impulsivity and aggression tasks as endophenotypes for Borderline Personality Disorder. J Psychiatr Res. 2009;43:1036–48. doi: 10.1016/j.jpsychires.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reynolds B, Patak M, Shroff P, et al. Laboratory and self-report assessment of impulsive behavior in adolescent daily smokers and nonsmokers. Exp Clin Psychopharmacol. 2007;15:264–71. doi: 10.1037/1064-1297.15.3.264. [DOI] [PubMed] [Google Scholar]
- 47.Swann AC, Anderson J, Dougherty DM, Moeller FG. Measurement of inter-episode impulsivity in bipolar disorder: A preliminary report. Psychiatry Res. 2001;101:195–7. doi: 10.1016/s0165-1781(00)00249-3. [DOI] [PubMed] [Google Scholar]
- 48.Swann AC, Lijffijt M, Lane SD, Moeller FG. Severity of bipolar disorder is associated with impairment of response inhibition. J Affect Disord. 2009;116:30–6. doi: 10.1016/j.jad.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Trait impulsivity and response inhibition in antisocial personality disorder. J Psychiatr Res. 2009;43:1057–63. doi: 10.1016/j.jpsychires.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu CS, Liao SC, Lin KM, Tseng MMC, Wu ECH, Liu SK. Multidimensional assessments of impulsivity in subjects with a history of suicide attempts. Compr Psychiatry. 2009:315–21. doi: 10.1016/j.comppsych.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Murphy FC, Smith KA, Cowen PJ, Robbins TW, Sahakian BJ. The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacol (Berlin) 2002;163:42–53. doi: 10.1007/s00213-002-1128-9. [DOI] [PubMed] [Google Scholar]
- 52.Walderhaug E, Lunde H, Nordvik JE, Landrø NI, Refsum H, Magnusson A. Lowering of serotonin by rapid tryptophan depletion increases impulsiveness in normal individuals. Psychopharmacol (Berlin) 2002;164:385–91. doi: 10.1007/s00213-002-1238-4. [DOI] [PubMed] [Google Scholar]
- 53.Walderhaug E, Magnusson A, Neumeister A, et al. Interactive effects of sex and 5-HTTLPR on mood and impulsivity during tryptophan depletion in healthy people. Biol Psychiatry. 2007;62:593–9. doi: 10.1016/j.biopsych.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Crean J, Richards JB, de Wit H. Effect of tryptophan depletion on impulsive behavior in men with or without a family history of alcoholism. Behav Brain Res. 2002;136:349–57. doi: 10.1016/s0166-4328(02)00132-8. [DOI] [PubMed] [Google Scholar]
- 55.LeMarquand DG, Benkelfat C, Pihl RO, Palmour RM, Young SN. Behavioral disinhibition induced by tryptophan depletion in nonalcoholic young men with multigenerational family histories of paternal alcoholism. Am J Psychiatry. 1999;156:1771–9. doi: 10.1176/ajp.156.11.1771. [DOI] [PubMed] [Google Scholar]
- 56.Schweighofer N, Bertin M, Shishida K, et al. Low-serotonin levels increase delayed reward discounting in humans. J Neurosci. 2008;28:4528–32. doi: 10.1523/JNEUROSCI.4982-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanaka S, Schweighofer N, Asahi S, et al. Serotonin differentially regulates short- and long-term prediction of rewards in the ventral and dorsal stratum. PLoS One. 2007;2(12):1–7. e1333. doi: 10.1371/journal.pone.0001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; NY: 2001. [Google Scholar]
- 59.Psychological Corporation . Wechsler Abbreviated Scale of Intelligence (WASI) Manual. Harcourt Brace and Company; San Antonio, TX: 1997. [Google Scholar]
- 60.Bjork JM, Dougherty DM, Moeller FG, Cherek DR, Swann AC. The effects of tryptophan depletion and loading on laboratory aggression: Time-course and food restricted control. Psychopharmacol (Berlin) 1999;142:24–30. doi: 10.1007/s002130050858. [DOI] [PubMed] [Google Scholar]
- 61.Marsh DM, Dougherty DM, Moeller FG, Swann AC, Spiga R. Laboratory-measured aggressive behavior of women: Acute tryptophan depletion and augmentation. Neuropsychopharmacol. 2002;26:660–71. doi: 10.1016/S0893-133X(01)00369-4. [DOI] [PubMed] [Google Scholar]
- 62.Cherek DR. Effects of smoking different doses of nicotine on human aggressive behavior. Psychopharmacol (Berlin) 1981;75:339–45. doi: 10.1007/BF00435849. [DOI] [PubMed] [Google Scholar]
- 63.Dougherty DM, Marsh DM, Mathias CW. Immediate and Delayed Memory Tasks: A computerized measure of memory, attention, and impulsivity. Behav Res Methods Instrum Comput. 2002;34:391–8. doi: 10.3758/bf03195467. [DOI] [PubMed] [Google Scholar]
- 64.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 65.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 2nd ed. New York: Harper Collins Publishers; 1989. [Google Scholar]
- 66.LeMarquand DG, Pihl RO, Young SN, et al. Tryptophan depletion, executive functions, and disinhibition in aggressive, adolescent males. Neuropsychopharm. 1998;19:333–41. doi: 10.1016/S0893-133X(98)00029-3. [DOI] [PubMed] [Google Scholar]
- 67.Zepf FD, Stadler C, Demisch L, Schmitt M, Landgraf M, Poustka F. Serotonergic functioning and trait impulsivity in attention-deficit/hyperactivity-disordered boys (ADHD): Influence of rapid tryptophan depletion. Hum Psychopharmacol Clin Exp. 2008;23:43–51. doi: 10.1002/hup.896. [DOI] [PubMed] [Google Scholar]
- 68.McCloskey MS, Ben-Zeev D, Lee R, Berman ME, Coccaro EF. Acute tryptophan depletion and self-injurious behavior in aggressive patients and healthy volunteers. Psychopharmacol (Berl) 2009;203:53–61. doi: 10.1007/s00213-008-1374-6. [DOI] [PubMed] [Google Scholar]
- 69.Dougherty DM, Mathias CW, Marsh DM, Moeller FG, Swann AS. Suicidal behaviors and drug abuse: Impulsivity and its assessment. Drug Alcohol Depend. 2004;76:93–105. doi: 10.1016/j.drugalcdep.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 70.Erinoff L, Anthony JC, Brown GK, et al. Overview of workshop on drug abuse and suicidal behavior. Drug Alcohol Depend. 2004;76:3–9. doi: 10.1016/j.drugalcdep.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 71.Logan GD. On the ability to inhibit thought and action: A users’ guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory Processes in Attention, Memory, and Language. San Diego, CA: Academic Press; 1994. pp. 189–239. [Google Scholar]
- 72.Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: Relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1285–92. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 73.Frederick S, Loewenstein G, O’Donoghue T. Time discounting and time preference: A critical review. J Econ Lit. 2002;40:351–401. [Google Scholar]
- 74.Dougherty DM, Marsh DM, Moeller FG, Chokshi RV, Rosen VC. The effects of moderate and high doses of alcohol on attention, impulsivity, discriminability, and response bias in Immediate and Delayed Memory Task performance. Alcohol Clin Exp Res. 2000;24:1702–11. [PubMed] [Google Scholar]
- 75.Dougherty DM, Marsh-Richard DM, Hatzis ES, Nouvion SO, Mathias CW. A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug Alcohol Depend. 2008;96:111–20. doi: 10.1016/j.drugalcdep.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]