Abstract
The kynurenine pathway (KP) is a major degradative pathway of tryptophan ultimately leading to the production of nicotinamide adenine dinucleotide (NAD+) and is also one of the major regulatory mechanisms of the immune response. The KP is known to be involved in several neuroinflammatory disorders including Alzheimer’s disease, amyotrophic lateral sclerosis, AIDS dementia complex, Parkinson’s disease, schizophrenia, Huntington’s disease and brain tumours. However, the KP remains a relatively new topic for the field of multiple sclerosis (MS). Over the last 2–3 years, some evidence has progressively emerged suggesting that the KP is likely to be involved in the pathogenesis of autoimmune diseases especially MS. Some KP modulators are already in clinical trials for other inflammatory diseases and would potentially provide a new and important therapeutic strategy for MS patients. This review summarizes the known relationships between the KP and MS.
Keywords: kynurenine pathway, KP, multiple sclerosis, MS, pathogenesis
Introduction
Multiple Sclerosis
Multiple sclerosis (MS) is characterized by the formation of sclerotic plaques in various areas of the central nervous system (CNS). These plaques are the result of an inflammatory response most likely caused by activation of autoimmune Th-1 cell targeting oligodendrocytes and the myelin sheath together with activated monocytic cells. These highly immunologically active areas subsequently progress to form “scarring” plaques.1 Symptoms and signs vary depending on the location and severity of the plaques.2 MS is a complex disease that can exist in various clinical subtypes but generally has a relapsing-remitting (RRMS) course often developing into secondary progressive MS (SPMS). A less common subtype, primary progressive MS (PPMS) has no remissions from onset. It has been suggested that the adaptive immune system drives the early stages of MS and the innate immunity drives the progressive stage.3
In the next few sections, we will discuss how the KP interplays with both the adaptive (T-cells) and innate immunity (mainly macrophages and microglia) that have been associated with the detrimental effects in MS progression.
The kynurenine pathway (KP)
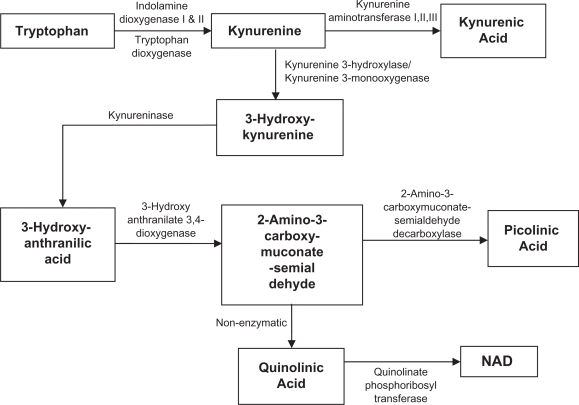
The KP is a metabolic pathway leading to the production of NAD+ from the degradation of the essential amino acid tryptophan (TRP).4 Over the last two decades the KP has been studied across various species and has been associated pathologically with several neurological diseases. Paradoxically, KP metabolites can exhibit both neuroprotective and neurotoxic properties.5,6 In this section we will briefly highlight some key metabolites and enzymes in the KP.
Indoleamine 2,3 Dioxygenase
Activation of the KP can be achieved through one of its first rate-limiting enzymes, indoleamine 2,3 dioxygenase (IDO)-1, tryptophan dioxygenase (TDO)7,8 and the recently discovered IDO-2.9 While little is known of the significance of IDO-2, extensive studies have been performed in understanding the role of IDO-1 in neuroinflammation.
IDO-1 is a key regulator of the immune response.10 Munn and co-workers showed that in the placenta, activation of IDO-1 was important in preventing rejection of the allogenic foetus.11 The mechanism is most likely related to IDO-1 mediated catabolism of TRP leading to depletion of local TRP needed for adjacent maternal T-cell proliferation thereby causing T cell apoptosis.12–15 Our studies in conjunction with others have shown that human IDO-1 expression can be up regulated by many factors such as the interferons, tumour necrosis factor (TNF)-α, platelet activating factor (PAF), amyloid beta peptide 1–42, as well as the HIV-1 proteins Nef and Tat.16–20 More recently, studies using animal models have shown that the combination of TNF-α and IFN-γ play a key role in up regulation of IDO-1 in response to bacterial infection.21
Kynurenic acid (KYNA)
In the CNS, KYNA is synthesised from kynurenine by the enzymes kynurenine aminotransferases (KAT) I and II. Rodents have an additional enzyme KAT III, which has a similar gene structure to KAT I, and which can function as a compensatory enzyme in KAT II knockout mice.22,23 KAT III has not been detected in humans. KYNA is one of the early metabolites of the KP and is generally considered as a neuroprotective compound. KYNA is capable of blocking glutamate-induced excitotoxicity via its antagonistic effects on several subtypes of ionotropic glutamate receptors24 such as N-methyl-D-aspartate (NMDA), kainate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. KYNA has a higher affinity for the glycine site of the NMDA receptors than glutamate.25 More importantly in the context of the KP, it can also effectively attenuate the excitotoxicity of the downstream KP metabolite, quinolinic acid (QUIN).26 Despite the antagonistic properties of KYNA towards QUIN, during disease the concentrations of KYNA are usually too low to make this clinically significant. Lekieffre and co-workers demonstrated that it required three times more KYNA to antagonize the excitotoxic effect exerted by the same amount of QUIN.27 Furthermore, it has been repeatedly shown that levels of KYNA are decreased in several major neurological diseases including amyotrophic lateral sclerosis (ALS), HIV-dementia, chronic brain injury and even MS28 suggesting that the neuroprotective role of KYNA had been compromised in these diseases. Ironically, KYNA in some circumstances may be detrimental. High levels of KYNA have been associated with other disorders such as schizophrenia29 and epilepsy.30 Depending of its relative concentrations, KYNA seems to have a Janus’s face within the CNS that still needs to be studied and clarified.
3-Hydroxykynurenine (3-HK)
3-HK is increasingly being considered for its role in neurotoxicity. Elevations in 3-HK levels have been found to be associated with numerous neurological diseases including Huntington disease,31 HIV dementia32 and Parkinson’s disease33 The pathogenetic nature of this association was initially suggested by 3-HK’s ability to mediate cytotoxicity in a neuronal cell line.34,35 However, the concentrations were supraphysiological leading to its dismissal as a neurotoxin.36 Nonetheless, it is now known that 3-HK can generate ROS leading to neuronal cell death at pathophysiological concentrations.37 Furthermore, 3-HK acts synergistically with QUIN to mediate excitotoxic damage.38
Quinolinic Acid (QUIN)
QUIN is likely to be one of the most significant KP metabolites in terms of biological activity. It is an agonist of the NMDA receptor and as such is considered an endogenous excitotoxin.39 In physiological conditions, QUIN is a crucial substrate for production of a key molecule for cell survival, NAD+. We showed that at physiological concentrations (50 nM), QUIN facilitates the production of NAD+ in both human primary astrocytes and neurons which is crucial for energy homeostasis and cellular repair.40 However, elevated QUIN concentrations have been found in several major neuroinflammatory diseases such as Alzheimer’s disease, Parkinson’s disease, HIV dementia and more recently in ALS.41–43 The exact roles of QUIN in these various neurological diseases have not been fully defined but several mechanisms for QUIN-induced cell toxicity have been identified:44 1) QUIN can activate NMDA receptors in pathophysiological concentrations that results in excitotoxicity;45 2) QUIN is capable of increasing the release of glutamate by neurons and decreasing glutamate uptake by astrocytes and can also inhibit astroglial glutamine synthetase46 consequently leading to the accumulation of glutamate in the microenvironment and excitotoxicity;47,48 3) QUIN can kill astrocytes resulting in neurotoxicity because of the loss of astrocytic detoxifying function 4) QUIN is associated with lipid peroxidation. Some studies have also shown that QUIN can potentiate oxidative stress through generating and working synergistically with ROS in the mitochondria.49 This leads to lipid peroxidation and energy depletion that ultimately results in cell death.49–51 5) More recently, we have demonstrated that QUIN can induce tau hyperphosphorylation in human neurons that is likely to contribute to microtubular dysfunction with consequent neuronal dysfunction.52
Direct Evidence of KP Involvement in MS
Tryptophan degradation and KP activation in MS
The first evidence dated back to the early eighties, when Manaco A. reported a decrease in TRP in the plasma and CSF of MS patients.53 Then Fuchs and co-workers54 suggested that the KP may be involved in MS based on the correlation of the adaptive immune system and KP activation. This was later not supported by a study showing no significant difference in TRP concentration in serum and CSF of MS patients compared to controls.55 However, with a better understanding of the KP and the development of more efficient methods to quantify the KP metabolites and KP enzyme expression, its involvement in MS has been re-evaluated. More recent studies have shown that patients with chronic MS have low TRP concentrations in serum and CSF suggesting activation of the KP.56,57
Abnormality in KP metabolism in MS
Other evidence comes from analysis of human MS ex vivo samples. Rejdak et al showed that the levels of KYNA are decreased in the CSF of patients with MS.58 These results are supported by another study by Baran et al showing that the expression of KAT I and II (enzymes responsible for the production of KYNA) is decreased in post-mortem MS brain sections.59 Kepplinger et al60 however, found the opposite, that there is an increased in the KYNA levels in CSF samples of MS patients. This was supported by Hartai et al61 who found that KAT I and II activity was increased in red blood cells and plasma from MS patients. These contradictory results probably relate to the differences in the time of collection of the samples during the course of the disease. Indeed, when Rejdak et al. quantified KYNA in active MS patients, their results are in agreement with the data from Hartai et al The conflict between the earlier and later studies of Rejdak et al (2002) and the later study (2007) were likely resolved because the samples obtained from MS patients in the earlier study were in the chronic inactive phase of the disease while the later study used MS patients with an exacerbation.62 Thus the KP is induced during the active phase of MS leading to increased production of KYNA but as the disease progresses the KP profile changes resulting in decreased KYNA levels implicating the shift of the KP towards neurotoxicity.
Indirect Evidence for KP Involvement in MS using in Vitro Models
Microglia and macrophages
Previous studies have shown that only monocytic cells including activated microglia and macrophages are capable of producing QUIN in excitotoxic concentrations within the CNS.17,63 However, the amount of QUIN produced by microglia is significantly lower (20–40 times less) compared to macrophages.63,64 We previously showed that this is likely to be due to different levels of expression of three of the KP enzymes (See Table 1) between human microglia and macrophages.65 It is very likely that KP activation in both perivascular macrophages and microglia may contribute to the neuropathology of MS.64,66 Indeed, one of the hallmarks of MS lesions is the presence of activated microglia and perivascular macrophages along the boundary of the lesion site.67 The presence of these activated cells is likely to be associated with the production of QUIN in concentrations sufficient to induce brain cell death at the site of lesion. These monocytic cells will also release some pro-inflammatory cytokines that can activate the KP resulting in an amplifying feedback mechanism leading to further production of QUIN and exacerbation of neuro- and gliotoxicity (discussed in section I-B.4). In addition, these same cytokines will also activate inducible nitric oxide synthase (iNOS) leading to an increased production of reactive oxygen species that would further exacerbate the neurodegenerative mechanisms involved in MS. Although speculative, we believe that both oxidative stress and the KP are playing a crucial role in MS pathology, which is further discussed in section V.
Table I.
Summary of KP enzymes expression in human neurons and glial cells.
| KP enzymes | Macrophages + IFN-γ (Guillemin et al 2001a) | Microglia + IFN-γ (Espey et al 1997) | Astrocytes + IFN-γ (Guillemin et al 2001b) | Neurons + IFN-γ (Guillemin et al 2007) | Oligodendrocytes + IFN-γ (Lim et al 2007) |
|---|---|---|---|---|---|
| IDO | + | + | + | + | − |
| TDO | + | + | + | + | − |
| KAT-I | + | + | + | + | + |
| KAT-II | + | + | + | − | − |
| KMO | + | + | − | + | + |
| KYNU | + | + | + | + | + |
| 3-HAO | + | + | + | + | + |
| QPRTase | + | + | + | + | + |
+, Enzyme expressed;
−, Enzyme not expressed.
Astrocytes
Astrocytes represent the most abundant cell type in the CNS. Their representation in the CNS reflects their importance in critical roles such as glutamate recycling, cellular homeostasis, providing trophic and metabolic support, participating in the blood brain barrier cohesion and immune regulation within the CNS. We previously characterized the profile of the KP in human astrocytes (See Table 1) and showed that this cell type lacks the enzyme, kynurenine hydroxylase and hence, is unable to synthesize downstream KP metabolites, particularly QUIN.68 Interestingly, primary monocultures of human astrocytes, under cytokine stimulation generate large amounts of kynurenine (KYN) and subsequently lead to the production of the neuroprotective KYNA (see Fig. 1). Furthermore, Rejdak and colleagues showed an increased activation of astrocytes based on the astrocytic marker—S100β—that correlates with increased production of KYNA in CSF of MS patients.62 This suggests that astrocytes probably play a neuroprotective role in MS. This fits well with the neuroprotective role of astrocytes under physiological condition or early pathologic stages of the disease to provide compensatory mechanisms against neurotoxicity. In pathological conditions especially where innate immunity is involved for example in MS, activated microglia and perivascular macrophages at the inflammatory site can take up and metabolise the large amounts of astrocyte-derived KYN as extra substrate to produce QUIN.68 Furthermore, we have previously demonstrated that only small amounts of QUIN can be catabolised by human astrocytes due to the low saturable activity of quinolinate phospho-ribosyltransferase responsible for QUIN degradation.68,69 Moreover, our previous studies demonstrated QUIN at pathophysiological concentrations of 500 to 1200 nM can induce apoptosis in up to 14% of human astrocytes.43 Finally, QUIN can impair the function of glutamine synthetase thereby augmenting glutamate related toxicity.46,70
Figure 1.
Simplified diagram of the kynurenine pathway.
Neuron
Apart from oligodendrocyte death and demyelination, neuronal loss is another pathological feature of MS.71,72 Although the exact mechanism for axonal injury in MS is not well understood, there have been studies supporting the idea of monocytic cells and/or glial cells playing a role in producing inflammatory mediators leading to axonal loss at the site of active lesions.73–75 Glutamate excitotoxicity is a likely candidate because glutamate antagonists reduce axonal damage, the effect is not significant: neither lesion size nor course of inflammation is affected.76 Thus an alternative toxin appears more significant. As monocytic cells produce QUIN, it is biologically plausible that it might exert independently, or synergistically act with glutamate, to alter neuronal cytoskeleton52 ultimately leading to axonal loss.
We have recently characterized the KP in human neurons (See Table 1), showing that cytokine stimulation can lead to the production of two neuroprotective KP metabolites, KYNA and picolinic acid (PIC).77 It is likely that neurons will produce neuroprotective and anti-inflammatory (for PIC) compounds in the inflammatory areas in MS brains. Similar to astrocytes, neurons can also produce KYNA at the low micro-molar range, which is unlikely to be sufficient (see B2, page 5) to completely antagonize the excitotoxic effects of QUIN produced by surrounding microglia and macrophages. Schwartz and co-workers have previously shown that acute administration of QUIN can induce axon sparing lesions at pathophysiological concentrations in a rat brain slice.78 Similarly, chronic exposure to QUIN in the rat striatum leads to cognitive deficit.79 We have previously shown that chronic exposure of human primary neurons to QUIN at nano-molar concentrations is sufficient to induce neuronal cell death.80,81 This suggests that minute amounts of QUIN present in the human CNS may be involved in the chronic progression of axonal loss in MS.
Oligodendrocytes
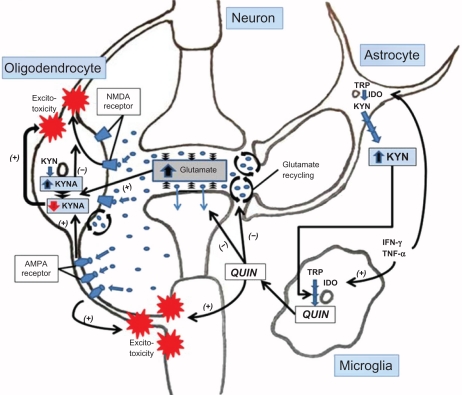
We have recently characterized the KP in purified primary cultures of human oligodendrocytes isolated from foetal brain tissue.82 Our data indicate that human foetal oligodendrocytes are among the rare cell types lacking IDO expression. This finding is potentially important for MS because the lack of IDO expression in human oligodendrocytes suggests that these cells have a lesser ability to protect themselves from activated T cell attack in the early stages of MS where adaptive immunity is important. However, this remains speculative, as IDO expression in adult oligodendrocytes is still unknown. Until recently, it was believed that oligodendrocytes only expressed AMPA/kainate receptors but not NMDA receptors.83–85 However, in 2005 Salter and Fern demonstrated that NMDA receptors are present on oligodendrocyte processes whereas AMPA/kainate receptors were localized on the cell somata.86 This further defined a new role for QUIN toxicity in MS, as QUIN, which is also an agonist of the NMDA receptor is likely to activate the oligodendrocyte NMDA receptors leading to excitotoxicity, cell death and demyelination. Furthermore, inhibition of the AMPA/kainate receptor with NBQX (2,3-dioxo-6-nitro-1, 2, 3, 4-tetrahydrobenzo [f] quinoxaline-7-sulphonamide) had no effect on the lesion size and no reduction of inflammatory response in EAE mouse brain.76 This implies that glutamate excitotoxicity may actually play only a limited role in the loss of oligodendrocytes. QUIN produced during the primary inflammation response, might lead to an even more significant excitotoxic effect on oligodendrocytes (see Fig. 2). This is further supported by data showing that complete rescue of oligodendrocyte injury requires the blocking of both AMPA/kainate and NMDA receptors.86 Furthermore, Cammer showed that in vitro, QUIN induces up to 30 and 50% apoptotic cell death of oligodendrocytes upon 48 h of 0.1 and 1mM QUIN exposure, respectively.87 This study strongly supports the potential involvement of QUIN toxicity in oligodendrocyte injury and death via apoptosis during MS. However, the concentration of QUIN used in this study was above the pathophysiological concentrations found during neuroinflammatory conditions.16,28
Figure 2.
Expression of the KP enzymes in human primary neurons, astrocytes, macrophages, microglia, and oligodendrocytes.
Indirect Evidence of KP Involvement in MS Using Experimental Autoimmune Encephalomyelitis (EAE) Animal Model
EAE is a commonly used animal model to study MS.88 Several studies with the EAE model have provided relevant information concerning the involvement of the KP in MS. Kwidzinski and co-workers demonstrated the importance of IDO-1 in the modulation of the immune response in EAE.89 The study showed that inhibition of IDO-1 activity using 1-methyl-tryptophan (1-MT) resulted in a significant exacerbation of the disease status, implying that IDO-1 can down-regulate neuroinflammation Sakurai and co-workers showed that the proliferative response of the T cells was increased when IDO-1 was inhibited by 1-MT.90 More recently, Matysiak and co-workers demonstrated that IDO-1 induction in the presence of dendritic cells led to T cell inhibition while 1-MT abolished this effect.91 Flanagan et al showed that the levels of the excitotoxin QUIN are elevated in the spinal cord of EAE rats.92 Another neurotoxic KP metabolite, 3-HK has also been found increased in EAE rat.93
Current Speculations and Hypothetical Model of KP Involvement in MS
Activation of KP in MS — Good or Bad?
It is unclear whether KP activation is detrimental or beneficial in the context of MS as both neuroprotectant and neurotoxin compounds can be produced through this pathway. Interferon-β1, a current treatment for MS, can activate the KP and possibly limit the benefit of the treatment.94 Hence, the following hypothetical model can be raised: The KP may be beneficial in the early stage of the disease because adaptive immunity is thought to be important and as previously mentioned activation of the KP via IDO-1 can down regulate T cell proliferation. However, prolonged activation of the KP may lead to chronically elevated levels of QUIN and other neurotoxins produced by perivascular macrophages. This would lead to further neurological deficits and as such would be expected to be a feature of the later stages of MS.
As mentioned above (I-B.4), QUIN facilitates glutamate release by neurons and inhibits glutamate uptake by astrocytes leading to an accumulation of glutamate in the microenvironment and excitotoxicity.47 Oligodendrocytes are constitutively involved in glutamate clearance from the white matter.95 Although speculative, it is likely that QUIN also inhibits glutamate uptake and recycling by oligodendrocytes. It is important to mention that glutamate is a potent inhibitor of KYNA synthesis in oligodendrocytes and that AMPA but not NMDA can potentiate such an inhibitory effect by decreasing KYNA production.96 Hence, during neuroinflammatory conditions such as MS, over production of QUIN by activated monocytic cells will lead to 1) an increase in glutamate levels; and 2) a decrease of neuroprotective KYNA, both phenomena in combination leading to an exacerbation of excitotoxicity (see Fig. 2). Apart from its involvement in the disturbance of the glutamine-glutamate cycle, QUIN is also likely to induce directly neurotoxicity and gliotoxicity.43,97
Based on our data,82 it is likely that the KP in oligodendrocytes will lead to production of neuroprotective molecules during MS. We have shown that human foetal oligodendrocytes can express one of the KYNA producing enzymes, KAT I, but not KAT II. This is supported by a previous study showing that the rat oligodendrocyte cell line OLN-93 expressed both KAT I and II that results in KYNA production when KYN was added to the cell culture.96 We obtained similar results showing that the human oligodendroglial cell line MO3.13 express both KAT I and II. Nonetheless, we still need to show that human primary adult oligodendrocytes also express KAT I and/or KAT II in before unequivocally concluding their neuroprotective functions.16,77,98 It is tempting to speculate that, as for neurons,77 human oligodendrocytes are more likely to synthesize KYNA as a compensatory mechanism against the excitotoxicity of QUIN production by activated monocytic cells and again similar to neurons they are able to take up and metabolise at least some of this QUIN. Braidy et al recently showed that human primary neurons and astrocytes can catabolise QUIN as substrate to produce NAD+ providing more energy to the cell and promotion of the enzymes involved in DNA repair.81 This is likely to happen in human oligodendrocytes as they also express the QUIN-catabolising enzyme quinolinate phosphoribosyltransferase (QPRT) (see Fig. 1 and Table 1). However additional experiments are needed to demonstrate that QPRT in human oligodendrocytes is fully functional.
KP and oxidative stress in MS pathology
Emerging data suggests that oxidative stress plays a major role in the pathogenesis of MS.99,100 Inflammatory cytokines inducing IDO-1 also activate iNOS, especially in monocytic lineage. INOS activation leads to production of nitric oxide (NO), which plays an important role in both oxidative stress and regulation of IDO-1 activity.101 NO has a bidirectional effect on the activity of IDO-1 with an inverse relation between NO concentration and IDO-1 activity.102 Studies had indicated that iNOS is up regulated in EAE and MS.103–105 Patients with active MS have significantly higher NOS activity and NO concentration in the CSF.106
As we mentioned previously, at the early stages of the disease, KP/IDO-1 activation is crucial for the immunosuppression of T cells and the presence of high levels of NO might suppress IDO-1 activity and thus promote MS progression. Furthermore, production of QUIN from activated macrophages, also producing ROS and NO, further potentiates oxidative stress and irreversible cell death. Then a question could be: if NO suppresses IDO-1, where is the source of QUIN? As mentioned, even if IDO-1 is down regulated, activated astrocytes are capable of producing large amounts of KYN that are taken up by macrophages as substrate for QUIN production.107 In the chronic progressive form of MS with a moderate and constant activation of the KP, production of neurotoxic KP metabolites, such as QUIN, 3HK together with ROS might further amplify the neurodegenerative processes.50 The possibility of those neurotoxic KP metabolites fuelling oxidative stress further highlights the importance of regulating the KP in MS.
Conclusion
In this review we have tried to combine and discuss known and potential links between the KP metabolites and the neuropathogenesis of MS. While it is still unclear whether KP activation is beneficial or detrimental, it seems likely that the KP would be more beneficial in the short term and become detrimental in the long term. Further studies are necessary to characterise KP components at different stages of MS to determine whether the hypotheses raised in this manuscript are valid.
Acknowledgments
This work was supported by the Multiple Sclerosis Research Association (MSRA) and the University of New South Wales.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–8. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalb RC. Multiple Sclerosis: The Questions You Have—The Answers You Need. 4th ed., Fourth Edition edition. Demos Medical Publishing; 2008. [Google Scholar]
- 3.Weiner HL. The challenge of multiple sclerosis: how do we cure a chronic heterogeneous disease. Ann Neurol. 2009;65:239–48. doi: 10.1002/ana.21640. [DOI] [PubMed] [Google Scholar]
- 4.Moroni F. Tryptophan metabolism and brain function: focus on kynurenine and other indole metabolites. Eur J Pharmacol. 1999;375:87–100. doi: 10.1016/s0014-2999(99)00196-x. [DOI] [PubMed] [Google Scholar]
- 5.Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–79. [PubMed] [Google Scholar]
- 6.Kwidzinski E, Bechmann I. IDO expression in the brain: a double-edged sword. J Mol Med. 2007;85:1351–9. doi: 10.1007/s00109-007-0229-7. [DOI] [PubMed] [Google Scholar]
- 7.Rafice SA, Chauhan N, Efimov I, Basran J, Raven EL. Oxidation of L-tryptophan in biology: a comparison between tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase. Biochem Soc Trans. 2009;37:408–12. doi: 10.1042/BST0370408. [DOI] [PubMed] [Google Scholar]
- 8.Takikawa O, Yoshida R, Kido R, Hayaishi O. Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J Biol Chem. 1986;261:3648–53. [PubMed] [Google Scholar]
- 9.Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol. 2008 doi: 10.1016/j.biocel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Zelante T, Fallarino F, Bistoni F, Puccetti P, Romani L. Indoleamine 2,3-dioxygenase in infection: the paradox of an evasive strategy that benefits the host. Microbes Infect. 2009;11:133–41. doi: 10.1016/j.micinf.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 12.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–72. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terness P, Bauer TM, Rose L, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–57. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002;107:452–60. doi: 10.1046/j.1365-2567.2002.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–77. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 16.Heyes MP, Chen CY, Major EO, Saito K. Different kynurenine pathway enzymes limit quinolinic acid formation by various human cell types. Biochem J. 1997;326(Pt 2):351–6. doi: 10.1042/bj3260351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pemberton LA, Kerr SJ, Smythe G, Brew BJ. Quinolinic acid production by macrophages stimulated with IFN-gamma, TNF-alpha, and IFN-alpha. J Interferon Cytokine Res. 1997;17:589–95. doi: 10.1089/jir.1997.17.589. [DOI] [PubMed] [Google Scholar]
- 18.Smith DG, Guillemin GJ, Pemberton L, et al. Quinolinic acid is produced by macrophages stimulated by platelet activating factor, Nef and Tat. J Neurovirol. 2001;7:56–60. doi: 10.1080/135502801300069692. [DOI] [PubMed] [Google Scholar]
- 19.Takikawa O, Habara-Ohkubo A, Yoshida R. IFN-gamma is the inducer of indoleamine 2,3-dioxygenase in allografted tumor cells undergoing rejection. J Immunol. 1990;145:1246–50. [PubMed] [Google Scholar]
- 20.Guillemin GJ, Smythe GA, Veas LA, Takikawa O, Brew BJ. A beta 1–42 induces production of quinolinic acid by human macrophages and microglia. Neuroreport. 2003;14:2311–5. doi: 10.1097/00001756-200312190-00005. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor JC, Andre C, Wang Y, et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009;29:4200–9. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu P, Di Prospero NA, Sapko MT, et al. Biochemical and phenotypic abnormalities in kynurenine aminotransferase II-deficient mice. Mol Cell Biol. 2004;24:6919–30. doi: 10.1128/MCB.24.16.6919-6930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu P, Li Z, Zhang L, Tagle DA, Cai T. Characterization of kynurenine aminotransferase III, a novel member of a phylogenetically conserved KAT family. Gene. 2006;365:111–8. doi: 10.1016/j.gene.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 24.Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–7. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- 25.Birch PJ, Grossman CJ, Hayes AG. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988;154:85–7. doi: 10.1016/0014-2999(88)90367-6. [DOI] [PubMed] [Google Scholar]
- 26.Foster AC, Vezzani A, French ED, Schwarcz R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci Lett. 1984;48:273–8. doi: 10.1016/0304-3940(84)90050-8. [DOI] [PubMed] [Google Scholar]
- 27.Lekieffre D, Plotkine M, Allix M, Boulu RG. Kynurenic acid antagonizes hippocampal quinolinic acid neurotoxicity: behavioral and histological evaluation. Neurosci Lett. 1990;120:31–3. doi: 10.1016/0304-3940(90)90160-b. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Guillemin GJ. Kynurenine Pathway Metabolites in Humans: Disease and Healthy States. International Journal of Tryptophan Research. 2009;2:1–19. doi: 10.4137/ijtr.s2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96–8. doi: 10.1016/s0304-3940(01)02242-x. [DOI] [PubMed] [Google Scholar]
- 30.Heyes MP, Saito K, Devinsky O, Nadi NS. Kynurenine pathway metabolites in cerebrospinal fluid and serum in complex partial seizures. Epilepsia. 1994;35:251–7. doi: 10.1111/j.1528-1157.1994.tb02428.x. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds GP, Pearson SJ. Increased brain 3-hydroxykynurenine in Huntington’s disease. Lancet. 1989;2:979–80. doi: 10.1016/s0140-6736(89)90987-2. [DOI] [PubMed] [Google Scholar]
- 32.Sardar AM, Bell JE, Reynolds GP. Increased concentrations of the neurotoxin 3-hydroxykynurenine in the frontal cortex of HIV-1-positive patients. J Neurochem. 1995;64:932–5. doi: 10.1046/j.1471-4159.1995.64020932.x. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa T, Matson WR, Beal MF, et al. Kynurenine pathway abnormalities in Parkinson’s disease. Neurology. 1992;42:1702–6. doi: 10.1212/wnl.42.9.1702. [DOI] [PubMed] [Google Scholar]
- 34.Eastman CL, Guilarte TR. Cytotoxicity of 3-hydroxykynurenine in a neuronal hybrid cell line. Brain Res. 1989;495:225–31. doi: 10.1016/0006-8993(89)90216-3. [DOI] [PubMed] [Google Scholar]
- 35.Eastman CL, Guilarte TR. The role of hydrogen peroxide in the in vitro cytotoxicity of 3-hydroxykynurenine. Neurochem Res. 1990;15:1101–7. doi: 10.1007/BF01101711. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds GP, Pearson SJ. Neurochemical-clinical correlates in Huntington’s disease – applications of brain banking techniques. J Neural Transm Suppl. 1993;39:207–14. [PubMed] [Google Scholar]
- 37.Okuda S, Nishiyama N, Saito H, Katsuki H. Hydrogen peroxide-mediated neuronal cell death induced by an endogenous neurotoxin, 3-hydroxykynurenine. Proc Natl Acad Sci U S A. 1996;93:12553–8. doi: 10.1073/pnas.93.22.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guidetti P, Schwarcz R. 3-Hydroxykynurenine potentiates quinolinate but not NMDA toxicity in the rat striatum. Eur J Neurosci. 1999;11:3857–63. doi: 10.1046/j.1460-9568.1999.00806.x. [DOI] [PubMed] [Google Scholar]
- 39.Stone TW, Perkins MN. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur J Pharmacol. 1981;72:411–2. doi: 10.1016/0014-2999(81)90587-2. [DOI] [PubMed] [Google Scholar]
- 40.Braidy N, Grant R, Adams S, Brew BJ, Guillemin GJ. Mechanism for quinolinic Acid cytotoxicity in human astrocytes and neurons. Neurotox Res. 2009;16:77–86. doi: 10.1007/s12640-009-9051-z. [DOI] [PubMed] [Google Scholar]
- 41.Heyes MP. The kynurenine pathway and neurologic disease. Therapeutic strategies. Adv Exp Med Biol. 1996;398:125–9. doi: 10.1007/978-1-4613-0381-7_20. [DOI] [PubMed] [Google Scholar]
- 42.Guillemin GJ, Meininger V, Brew BJ. Implications for the kynurenine pathway and quinolinic acid in amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:166–76. doi: 10.1159/000089622. [DOI] [PubMed] [Google Scholar]
- 43.Guillemin GJ, Wang L, Brew BJ. Quinolinic acid selectively induces apoptosis of human astrocytes: potential role in AIDS dementia complex. J Neuroinflammation. 2005;2:16. doi: 10.1186/1742-2094-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guillemin GJ, Kerr SJ, Brew BJ. Involvement of quinolinic acid in AIDS dementia complex. Neurotox Res. 2005;7:103–23. doi: 10.1007/BF03033781. [DOI] [PubMed] [Google Scholar]
- 45.Monaghan DT, Beaton JA. Quinolinate differentiates between forebrain and cerebellar NMDA receptors. Eur J Pharmacol. 1991;194:123–5. doi: 10.1016/0014-2999(91)90134-c. [DOI] [PubMed] [Google Scholar]
- 46.Ting k, Brew BJ, Guillemin GJ. Effect of Quinolinic acid on human astrocytes: Implications in Alzheimer’s disease. J Neuroinflammation. 2009. in press. [DOI] [PMC free article] [PubMed]
- 47.Tavares RG, Tasca CI, Santos CE, et al. Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem Int. 2002;40:621–7. doi: 10.1016/s0197-0186(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 48.Tavares RG, Tasca CI, Santos CE, Wajner M, Souza DO, Dutra-Filho CS. Quinolinic acid inhibits glutamate uptake into synaptic vesicles from rat brain. Neuroreport. 2000;11:249–53. doi: 10.1097/00001756-200002070-00005. [DOI] [PubMed] [Google Scholar]
- 49.Sas K, Robotka H, Toldi J, Vecsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci. 2007;257:221–39. doi: 10.1016/j.jns.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 50.Rajda C, Bergquist J, Vecsei L. Kynurenines, redox disturbances and neurodegeneration in multiple sclerosis. J Neural Transm Suppl. 2007:323–9. doi: 10.1007/978-3-211-73574-9_40. [DOI] [PubMed] [Google Scholar]
- 51.Kalman B, Laitinen K, Komoly S. The involvement of mitochondria in the pathogenesis of multiple sclerosis. J Neuroimmunol. 2007;188:1–12. doi: 10.1016/j.jneuroim.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 52.Rahman A, Ting K, Cullen KM, Braidy N, Brew BJ, Guillemin GJ. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS ONE. 2009;4:e6344. doi: 10.1371/journal.pone.0006344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monaco F, Fumero S, Mondino A, Mutani R. Plasma and cerebrospinal fluid tryptophan in multiple sclerosis and degenerative diseases. J Neurol Neurosurg Psychiatry. 1979;42:640–1. doi: 10.1136/jnnp.42.7.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fuchs D, Reibnegger G, Werner ER, Wachter H. Immune-mediated mechanisms in multiple sclerosis. J Neurol. 1990;237:125. doi: 10.1007/BF00314679. [DOI] [PubMed] [Google Scholar]
- 55.Ott M, Demisch L, Engelhardt W, Fischer PA. Interleukin-2, soluble interleukin-2-receptor, neopterin, L-tryptophan and beta 2-microglobulin levels in CSF and serum of patients with relapsing-remitting or chronic-progressive multiple sclerosis. J Neurol. 1993;241:108–14. doi: 10.1007/BF00869773. [DOI] [PubMed] [Google Scholar]
- 56.Sandyk R. Tryptophan availability and the susceptibility to stress in multiple sclerosis: a hypothesis. Int J Neurosci. 1996;86:47–53. doi: 10.3109/00207459608986697. [DOI] [PubMed] [Google Scholar]
- 57.Rudzite V, Berzinsh J, Grivane I, Fuchs D, Baier-Bitterlich G, Wachter H. Serum tryptophan, kynurenine, and neopterin in patients with Guillain-Barre-syndrome (GBS) and multiple sclerosis (MS) Adv Exp Med Biol. 1996;398:183–7. doi: 10.1007/978-1-4613-0381-7_30. [DOI] [PubMed] [Google Scholar]
- 58.Rejdak K, Bartosik-Psujek H, et al. Decreased level of kynurenic acid in cerebrospinal fluid of relapsing-onset multiple sclerosis patients. Neurosci Lett. 2002;331:63–5. doi: 10.1016/s0304-3940(02)00710-3. [DOI] [PubMed] [Google Scholar]
- 59.Baran H, Kepplinger B, Newcombe J, Stolze K, Kainz A, Nohl H. Lowered kynurenine aminotransferase activities in CNS of MS patients. Society for Neuroscience 30th Annual Meeting Abstracts; 2000. [Google Scholar]
- 60.Kepplinger B, Baran H, Kainz A, Ferraz-Leite H, Newcombe J, Kalina P. Age-related increase of kynurenic acid in human cerebrospinal fluid—IgG and beta2-microglobulin changes. Neurosignals. 2005;14:126–35. doi: 10.1159/000086295. [DOI] [PubMed] [Google Scholar]
- 61.Hartai Z, Klivenyi P, Janaky T, Penke B, Dux L, Vecsei L. Kynurenine metabolism in multiple sclerosis. Acta Neurol Scand. 2005;112:93–6. doi: 10.1111/j.1600-0404.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 62.Rejdak K, Petzold A, Kocki T, et al. Astrocytic activation in relation to inflammatory markers during clinical exacerbation of relapsing-remitting multiple sclerosis. J Neural Transm. 2007;114:1011–5. doi: 10.1007/s00702-007-0667-y. [DOI] [PubMed] [Google Scholar]
- 63.Espey MG, Chernyshev ON, Reinhard JF, Jr, Namboodiri MA, Colton CA. Activated human microglia produce the excitotoxin quinolinic acid. Neuroreport. 1997;8:431–4. doi: 10.1097/00001756-199701200-00011. [DOI] [PubMed] [Google Scholar]
- 64.Heyes MP, Saito K, Markey SP. Human macrophages convert L-tryptophan into the neurotoxin quinolinic acid. Biochem J. 1992;283(Pt 3):633–5. doi: 10.1042/bj2830633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guillemin GJ, Smith DG, Smythe GA, Armati PJ, Brew BJ. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv Exp Med Biol. 2003;527:105–12. doi: 10.1007/978-1-4615-0135-0_12. [DOI] [PubMed] [Google Scholar]
- 66.Heyes MP, Achim CL, Wiley CA, Major EO, Saito K, Markey SP. Human microglia convert l-tryptophan into the neurotoxin quinolinic acid. Biochem J. 1996;320(Pt 2):595–7. doi: 10.1042/bj3200595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benveniste EN. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med. 1997;75:165–73. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- 68.Guillemin GJ, Kerr SJ, Smythe GA, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem. 2001;78:842–53. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 69.Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49:15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- 70.Ting KK, Brew BJ, Guillemin GJ. Effect of quinolinic acid on gene expression in human astrocytes: implications for Alzheimer’s disease. International Congress Series. 2007;1304:384–8. [Google Scholar]
- 71.Perry VH, Anthony DC. Axon damage and repair in multiple sclerosis. Philos Trans R Soc Lond B Biol Sci. 1999;354:1641–7. doi: 10.1098/rstb.1999.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huizinga R, Gerritsen W, Heijmans N, Amor S. Axonal loss and gray matter pathology as a direct result of autoimmunity to neurofilaments. Neurobiol Dis. 2008;32:461–70. doi: 10.1016/j.nbd.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 73.Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120(Pt 3):393–9. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- 74.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–85. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 75.Bjartmar C, Wujek JR, Trapp BD. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci. 2003;206:165–71. doi: 10.1016/s0022-510x(02)00069-2. [DOI] [PubMed] [Google Scholar]
- 76.Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med. 2000;6:67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- 77.Guillemin GJ, Cullen KM, Lim CK, et al. Characterization of the kynurenine pathway in human neurons. J Neurosci. 2007;27:12884–92. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwarcz R, Whetsell WO, Jr, Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983;219:316–8. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- 79.Shear DA, Dong J, Haik-Creguer KL, Bazzett TJ, Albin RL, Dunbar GL. Chronic administration of quinolinic acid in the rat striatum causes spatial learning deficits in a radial arm water maze task. Exp Neurol. 1998;150:305–11. doi: 10.1006/exnr.1998.6767. [DOI] [PubMed] [Google Scholar]
- 80.Kerr SJ, Armati PJ, Guillemin GJ, Brew BJ. Chronic exposure of human neurons to quinolinic acid results in neuronal changes consistent with AIDS dementia complex. Aids. 1998;12:355–63. doi: 10.1097/00002030-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 81.Braidy N, Grant R, Brew BJ, Adams S, Jayasena T, Guillemin GJ. Effects of Kynurenine Pathway Metabolites on Intracellular NAD+ Synthesis and Cell Death in Human Primary Astrocytes and Neurons. International Journal of Tryptophan Research. 2009;2:61–9. doi: 10.4137/ijtr.s2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lim CK, Smythe GA, Stocker R, Brew BJ, Guillemin GJ. International Congress Series. Elsevier B.V.; Nov 1, 2007. Characterization of the kynurenine pathway in human oligodendrocytes; pp. 213–7. [Google Scholar]
- 83.Matute C, Sanchez-Gomez MV, Martinez-Millan L, Miledi R. Glutamate receptor-mediated toxicity in optic nerve oligodendrocytes. Proc Natl Acad Sci U S A. 1997;94:8830–5. doi: 10.1073/pnas.94.16.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McDonald JW, Althomsons SP, Hyrc KL, Choi DW, Goldberg MP. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med. 1998;4:291–7. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- 85.Steinhauser C, Gallo V. News on glutamate receptors in glial cells. Trends Neurosci. 1996;19:339–45. doi: 10.1016/0166-2236(96)10043-6. [DOI] [PubMed] [Google Scholar]
- 86.Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–71. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 87.Cammer W. Oligodendrocyte killing by quinolinic acid in vitro. Brain Res. 2001;896:157–60. doi: 10.1016/s0006-8993(01)02017-0. [DOI] [PubMed] [Google Scholar]
- 88.Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–71. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- 89.Kwidzinski E, Bunse J, Aktas O, et al. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. Faseb J. 2005;19:1347–9. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- 90.Sakurai K, Zou JP, Tschetter JR, Ward JM, Shearer GM. Effect of indole-amine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;129:186–96. doi: 10.1016/s0165-5728(02)00176-5. [DOI] [PubMed] [Google Scholar]
- 91.Matysiak M, Stasiolek M, Orlowski W, et al. Stem cells ameliorate EAE via an indoleamine 2,3-dioxygenase (IDO) mechanism. J Neuroimmunol. 2008;193:12–23. doi: 10.1016/j.jneuroim.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Flanagan EM, Erickson JB, Viveros OH, Chang SY, Reinhard JF., Jr Neurotoxin quinolinic acid is selectively elevated in spinal cords of rats with experimental allergic encephalomyelitis. J Neurochem. 1995;64:1192–6. doi: 10.1046/j.1471-4159.1995.64031192.x. [DOI] [PubMed] [Google Scholar]
- 93.Chiarugi A, Cozzi A, Ballerini C, Massacesi L, Moroni F. Kynurenine 3-mono-oxygenase activity and neurotoxic kynurenine metabolites increase in the spinal cord of rats with experimental allergic encephalomyelitis. Neuroscience. 2001;102:687–95. doi: 10.1016/s0306-4522(00)00504-2. [DOI] [PubMed] [Google Scholar]
- 94.Amirkhani A, Rajda C, Arvidsson B, et al. Interferon-beta affects the tryptophan metabolism in multiple sclerosis patients. Eur J Neurol. 2005;12:625–31. doi: 10.1111/j.1468-1331.2005.01041.x. [DOI] [PubMed] [Google Scholar]
- 95.Pitt D, Nagelmeier IE, Wilson HC, Raine CS. Glutamate uptake by oligodendrocytes: Implications for excitotoxicity in multiple sclerosis. Neurology. 2003;61:1113–20. doi: 10.1212/01.wnl.0000090564.88719.37. [DOI] [PubMed] [Google Scholar]
- 96.Wejksza K, Rzeski W, Okuno E, Kandefer-Szerszen M, Albrecht J, Turski WA. Demonstration of Kynurenine Aminotransferases I and II and Characterization of Kynurenic Acid Synthesis in Oligodendrocyte Cell Line (OLN-93) Neurochem Res. 2005;30:963–8. doi: 10.1007/s11064-005-6178-z. [DOI] [PubMed] [Google Scholar]
- 97.Foster AC, Collins JF, Schwarcz R. On the excitotoxic properties of quinolinic acid, 2,3-piperidine dicarboxylic acids and structurally related compounds. Neuropharmacology. 1983;22:1331–42. doi: 10.1016/0028-3908(83)90221-6. [DOI] [PubMed] [Google Scholar]
- 98.Allegri G, Bertazzo A, Biasiolo M, Costa CV, Ragazzi E. Kynurenine pathway enzymes in different species of animals. Adv Exp Med Biol. 2003;527:455–63. doi: 10.1007/978-1-4615-0135-0_53. [DOI] [PubMed] [Google Scholar]
- 99.Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol. 2004;251:261–8. doi: 10.1007/s00415-004-0348-9. [DOI] [PubMed] [Google Scholar]
- 100.Liu Y, Zhu B, Wang X, Luo L, Li P, Paty DW, Cynader MS. Bilirubin as a potent antioxidant suppresses experimental autoimmune encephalomyelitis: implications for the role of oxidative stress in the development of multiple sclerosis. J Neuroimmunol. 2003;139:27–35. doi: 10.1016/s0165-5728(03)00132-2. [DOI] [PubMed] [Google Scholar]
- 101.Thomas SR, Mohr D, Stocker R. Nitric oxide inhibits indoleamine 2,3-dioxygenase activity in interferon-gamma primed mononuclear phagocytes. J Biol Chem. 1994;269:14457–64. [PubMed] [Google Scholar]
- 102.Lopez AS, Alegre E, Diaz A, Mugueta C, Gonzalez A. Bimodal effect of nitric oxide in the enzymatic activity of indoleamine 2,3-dioxygenase in human monocytic cells. Immunol Lett. 2006;106:163–71. doi: 10.1016/j.imlet.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 103.Bagasra O, Michaels FH, Zheng YM, et al. Activation of the inducible form of nitric oxide synthase in the brains of patients with multiple sclerosis. Proc Natl Acad Sci U S A. 1995;92:12041–5. doi: 10.1073/pnas.92.26.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu JS, Zhao ML, Brosnan CF, Lee SC. Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am J Pathol. 2001;158:2057–66. doi: 10.1016/S0002-9440(10)64677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim S, Moon C, Wie MB, et al. Enhanced expression of constitutive and inducible forms of nitric oxide synthase in autoimmune encephalomyelitis. J Vet Sci. 2000;1:11–7. [PubMed] [Google Scholar]
- 106.Danilov AI, Andersson M, Bavand N, Wiklund NP, Olsson T, Brundin L. Nitric oxide metabolite determinations reveal continuous inflammation in multiple sclerosis. J Neuroimmunol. 2003;136:112–8. doi: 10.1016/s0165-5728(02)00464-2. [DOI] [PubMed] [Google Scholar]
- 107.Guillemin GJ, Kerr SJ, Smythe GA, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem. 2001;78:1–13. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]