Abstract
The endogenous neuroinhibitory amino acid receptor antagonist kynurenic acid (KYNA) has been hypothetically linked to physiological processes and to the pathogenesis of several brain disorders. The aim of this study was to search KYNA metabolism i.e. KYNA levels and enzymes synthesising KYNA kynurenine aminotransferase I and II (KAT I and II) in the central nervous system (CNS) and in the peripheral nervous system. Within the investigated species we found a remarkably low KYNA content (3.4 nM) in piglet’s serum compared to rat and human serum. Furthermore, in contrast to high KAT activity present in rat and human livers, a lack of KAT I and KAT II activity was found in piglet liver and other piglet peripheral organs. Therefore we attempted to find a reason for the absence of KYNA formation in piglet peripheral tissue and we researched to find if KYNA formation in rat liver homogenate (measured under standard assay conditions for KAT activity) can be influenced by the application of piglet tissue homogenates and other body fluids. KYNA formation in rat liver homogenate was investigated in the presence of piglet liver, piglet brain, rat brain and human brain homogenates, and also in the presence of cerebrospinal fluid (CSF) of the control and of Multiple Sclerosis patients. We found a significant and dose dependent reduction of rat liver KAT I and KAT II activities in the presence of piglet brain, piglet liver, and human brain, but not in the presence of rat brain homogenate. Interestingly, CSF of the human control subjects significantly lowered rat liver KAT I activity. Furthermore, the inhibitory effect of CSF of Multiple Sclerosis (MS) patients was significantly weaker when compared to the CSF of control subjects. Our data, for the first time, indicated the presence of active component(s)—depressing factor—in the body, which was able to block KYNA formation. Reduced KAT inhibitory effect by CSF of MS patients would suggest a lowered “depressing factor” level in CSF of MS patients and is possibly responsible for an enhancement of KYNA formation and for glia activation and gliosis in the CNS. Subsequently, two fractions obtained after centrifugation of CSF from patients with Neuroborreliosis showed a significantly different ability to block KAT I activity. The CSF-sediment fraction exerts a stronger inhibitory activity than the CSF-supernatant fraction, supporting further the presence of a depressing factor. For the first time, data revealed and demonstrated the ability of endogenous components to block KYNA’s synthesis. We propose that a glia depressing factor (GDF), which is abundantly present in the body, might simultaneously control glia cell’s KAT activity, respectively KYNA synthesis and also glia proliferation. The mechanism(s) of action, the composition and structure of this factor needs to be further elaborated.
Keywords: kynurenine aminotransferases, kynurenic acid, brain, liver, cerebrospinal fluid, cerebrolysin, glia depressing factor
Introduction
In the last thirty years particular attention was paid to tryptophan research and findings related to the role of kynurenine metabolites in neurodegenerative and neuroinflammatory processes revealed remarkable evidence and importance for its functional involvement. Kynurenic acid (KYNA), an endogenous metabolite of the kynurenine pathway of tryptophan degradation, is a well known endogenous antagonist of three glutamate ionotropic excitatory amino acid receptors, i.e. N-methyl-D-aspartate (NMDA), alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) and kainate1,2 and of the nicotine cholinergic subtype alpha-7 receptors.3 Suggested KYNA’s anticonvulsive and neuroprotective properties have been demonstrated by various pharmacological approaches1,4 and there is evidence that KYNA improves cognition and memory.5 Conversely, it has also been demonstrated that KYNA interferes with the working memory6 and an impairment of both cholinergic and glutamatergic neurotransmission due to KYNA actions has been proposed in neuro-psychiatric and neurological diseases.3,7
Interestingly, it has also been shown that within investigated tryptophan metabolites KYNA enhances the oxygen consumption in rat heart mitochondria, in an in vitro study,8 and therefore an essential role of KYNA for the cell function of the myocardium can not be excluded.
An enhancement of KYNA levels in the brain and/or serum has been found under various experimental pathological conditions, as demonstrated in the animal models of asphyxia,9 dystonia,10 or epilepsy.11 KYNA’s involvement in human neurodegenerative and neuroinflammatory processes has been well documented,1,12 thus the increased KYNA metabolism is seen in Alzheimer’s patients,13 in patients with subcortical sclerotic encephalopathy,14 in patients infected with HIV-1 virus,15,16 in patients with Schizophrenia17 and also in elderly human subjects.18 KYNA is synthesized by irreversible transamination of L-kynurenine.20,21 In organs of mammalians several aminotransferases convert L-kynurenine into KYNA.21,22 In peripheral tissues of rats there are at least four types of proteins which are capable of catalysing the kynurenine-2-oxoacids transamination reaction to produce KYNA.21,22 In human and rat brain tissues kynurenine aminotransferase I, II and III (KAT I KAT II and KAT III) were described, and significant differences in respect to the regional brain distribution were revealed.13,23–25 KAT I, KAT II and KAT III are capable of synthesizing KYNA, and show different catalytic characteristics,23–29 which suggests that substantially KAT II and probably KAT III act under physiological conditions, whereas KAT I may have a particular importance in pathological conditions, like in microglia activation. There is also data indicating that human KAT I is a multifunctional enzyme and might play a role in KYNA synthesis even under physiological conditions.30
Research on KAT cellular localisation indicates that in the rat brain KAT II has a preferential astrocytic and microglia localisation.31 Furthermore, Guillemin and co-workers demonstrated the presence of KAT(s) mRNA in human astrocytes.32 Studies using in situ hybridisation have shown that KAT I mRNA activity is expressed in mitochondria of neurons and glial cells and also in the cytosol of choroid plexus epithelial cells.33
The aim of this study was to investigate the probability that endogenous compounds, e.g. proteins/cells, present in the tissues or body fluids might exert the ability to influence KYNA synthesis. Since rat liver exerts a very dominant capacity to synthesize KYNA we used the measurement of KAT activity in the liver as a fundament to search the effect of these endogenous compounds. For this purpose KYNA formation in the reaction mixture, which contained rat liver homogenate and homogenate of piglet tissue or rat tissue or human tissue or human CSF, was investigated.
Material and Methods
Chemicals
L-kynurenine, KYNA and pyridoxal-5’-phosphate were purchased from Sigma. [3H]L-kynurenine (specific activity, 41 Ci/mmole) was purchased from Amersham, England. All other chemicals used were of the highest purity that was commercially available.
Biological materials
Piglets
Piglets (Veterinary Medical University of Vienna, Vienna, Austria) weighing 23 kilograms to 27 kilograms of body weight were used. Blood samples were collected in the morning and frozen at −60 °C until analysis. Piglets were sacrificed, and the peripheral organs the liver, heart and lung were dissected, the brain was removed and the frontal cortex was extracted and samples were frozen at −60 °C until analysis. The number of piglets used was n = 6. Fresh liver tissue of adult pigs from a commercial slaughter-house was obtained and stored at −60 °C until analysis, the number of samples was n = 3.
Rats
Male Sprague-Dawley rats (Research Institute for Animal Breeding, Himberg, Austria) weighing 200 grams to 220 grams of body weight were used. The animals were housed in groups of four to five per cage, in a room with controlled light/dark cycle (12 h light/12 h dark), and were given free access to laboratory food and top water. Rats were sacrificed in the morning, the liver was dissected, the brain immediately removed and the frontal cortex extracted and samples were frozen at −60 °C until analysis. Blood samples were collected and frozen at −60 °C until analysis. The number of rats used was n = 10.
Human material
Post mortem human samples of frontal cortices of normal subjects, aged between 40 and 45 years, were received frozen from the Institute of Neurology, Medical University Vienna, Vienna, Austria and stored at −43 °C until analysis. The number of human samples used was n = 10. Post mortem human heart samples (ventricular tissue) of normal subjects (n = 5), aged between 30 and 38 years, were received frozen from the Department of Pathology, Medical University Vienna, Vienna, Austria and stored at −43 °C until analysis.
Human CSF and serum samples
CSF and serum samples of normal human subjects were obtained from the Medical Laboratory of the Neuropsychiatric Hospital Mauer, Amstetten/Mauer, Austria. Twenty five individuals of a larger series of patients with severe headache who underwent lumbar puncture to exclude subarachnoidal haemorrhage or meningitis were selected as normal subjects for this study after CSF investigation, neuroimaging and clinical follow up. The number of serum used was n = 8 of 25. Twenty three CSF samples were from patients diagnosed as relapsing remitting Multiple Sclerosis (MS) according to international accepted MS criteria and 5 CSF samples were from patients diagnosed as Neuroborreliosis. All samples were provided from the Neurological Department of the Neuropsychiatric Hospital Landesklinikum Mauer-Amstetten, Austria. Ages ranged between 25 years and 54 years for control subjects and 23 years and 48 years for MS patients and 45 years and 59 years for patients with Neuroborreliosis, respectively. Samples of CSF and serum were coded and the study was carried out according to Lower Austrian Ethical Regulations.
Methods
Clinical routine investigations
Measurement of protein content, albumin, IgG, IgM and cell count was carried out using routine laboratory methods. The ratio of CSF: serum IgG and ratio CSF: serum albumin and IgG index were calculated.34 For determination of oligoclonal IgG bands, aragose iso-electric focusing was performed, followed by transfer to cellulose nitrate membrane and double antibody avidin-biotin-peroxidase labelling.35
Neuroradiological investigations
Magnetic resonance tomography (MRT) was carried out in all patients with suspected MS and Neuroborreliosis and computed tomography (CT) and/or MRT was performed in headache patients. No patients with strokes were included in this study.
Preparation of samples
Sample collection
Samples of human CSF and human serum of control subjects and samples of CSF of MS and Neuroborreliosis subjects were collected in 1 mL aliquots and stored at −43 °C until analysed.
CSF centrifugation
A sample of 900 μl of CSF from patients with Neuroborreliosis were centrifuged at 1000 rpm for 5 min, the supernatant of 870 μl was carefully removed, labeled as CSF supernatant fraction (CSF-Sup), and the rest of 30 μl with sediment was resuspended into 100 μl of homogenization buffer (i.e. the fraction of 30 μl was 4.3 times diluted) and labeled as CSF-sediment fraction (CSF-Sed). The obtained two fractions were immediately tested for the ability to block rat liver KAT I activity.
Preparation of homogenate
The organ samples were homogenised in an ice bath in 10 volumes (wt/vol) of 5 mM Tris-acetate buffer pH 8.0 containing 50 μM pyridoxal-5’-phosphate and 10 mM mercaptoethanol (homogenisation buffer) and the homogenates obtained were immediately used for KAT I and II activities measurement.
Measurement of KYNA
The measurement of KYNA was performed according to Shibata et al36 and Swartz et al37 with modification as described by Baran et al13 Briefly, the tissues (liver, heart, lung, and brain frontal cortex) were homogenised in an ice bath in 10 volumes (wt/vol) with H2O and immediately mixed with 0.2 M HCl (vol/vol), whereas serum samples were mixed with 0.2 M HCl (vol/vol) and centrifuged for 20 min, at 14,000 rpm. The supernatant obtained was applied to a Dowex 50 W cation exchange column pre-washed with 0.1 M HCl. Subsequently, the column was washed with 1 ml 0.1 M HCl and 1 ml distilled water, and KYNA was eluted with 2 ml distilled water38 and was quantitated by a high performance liquid chromatography (HPLC) system coupled with fluorescence detection.
Determination of KAT I and KAT II activities
KAT I and KAT II activities were measured using a radio-enzymatic assay described by Schmidt et al27 with minor modification. In brief, the reaction mixture contained homogenate and homogenisation buffer or second homogenate, or serum or CSF, 100 μM 1.175 μCi/μmol [3H]L-kynurenine (or 100 μM L-kynurenine), 1 mM pyruvate, 70 μM pyridoxal-5’-phosphate and 150 mM 2-amino-2-methyl-l-propranol buffer pH 9.6 for KAT I or 150 mM Tris-acetate buffer pH 7.0 for KAT II, in a total volume of 200 μl. After incubation for 16 hours at 37 °C the reaction was stopped by adding 14 μl of 50% trichloroacetic acid and 1 ml of 0.1 M HCl. Denatured proteins were removed by centrifugation and the synthesised [3H]KYNA was purified on Dowex 50 W cation-exchange column38 and quantified by liquid scintillation spectrometry. Blanks were prepared by boiling samples of homogenate for 15 minutes before adding the reaction mixture. In separate experiments using 100 μM L-kynurenine the KYNA formed was quantified by HPLC system.
Linearity of assay with different times and piglet tissue amounts
The incubation time and the tissue amount with respect to linearity of KAT I and KAT II activities measurement in homogenate of rat or piglet organs were evaluated. KAT I and II activities of rat brain and liver, or piglet brain, liver and heart showed linearity between 1 mg and 20 mg for brain and heart, and between 0.125 mg and 0.75 mg for liver in the incubation mixture. KAT I and II activities measurement was linear up to 16 hours of incubation time.
Experimental design
Measurement of KYNA
The KYNA content was measured in the serum and in the brain (frontal cortex) of piglet, rat and human samples.
Measurement of KAT I and KAT II activities in piglet, rat and human
KAT I and KAT II were determined: in the homogenate of piglet, rat or human brain (frontal cortex, 75 μl of homogenate prepared in 20 volumes, wt/vol); in homogenate of rat or human heart (75 μl of homogenate prepared in 20 volumes, wt/vol); in the homogenate of rat, piglet or pig liver (25 μl of homogenate prepared in 100 volumes, wt/vol) and in the homogenate of piglet lung (25 μl of homogenate prepared in 100 volumes, wt/vol).
KAT activities in the mixture of two different homogenates
KAT I and KAT II activities were measured in the reaction mixture containing rat liver and rat brain homogenates, or rat liver and piglet brain homogenates, or rat liver and piglet liver homogenates, or rat liver and human brain homogenates.
Influence of human CSF on rat liver KAT I and II activities
Influence of human CSF of normal control subjects on the rat liver KAT I and KAT II activities was investigated. The effect of human CSF of MS patients on rat liver KAT I activity was compared to effect of human CSF of normal control subjects. Then, the effect of boiled human CSF of control subjects and boiled CSF of MS patients on KAT I activity was researched.
Influence of CSF-sediment (CSF-Sed) and CSF-supernatant (CSF-Sup) fractions on KAT I
Rat liver KAT I activity in the presence of CSF-Sed or CSF-Sup fractions, obtained after CSF centrifugation, using two doses of 35 μl and 75 μl, respectively, was researched.
Data analyses
All data are given as means ± S.E.M. For statistical analyses, the one-way ANOVA-test and a Student’s t-test were applied, respectively. Each sample was determined in duplicate or triplicate. Asterisks indicate a significant difference: *P < 0.05; **P < 0.01; ***P < 0.001 compared to the used control, respectively.
Results
KYNA level
In piglet serum the KYNA content was at a low nano-molar range, i.e. 3.39 ± 0.09 nM, and the KYNA level was approximately eight times lower than in the human serum and twenty six times lower than in the rat serum (Table 1). The KYNA levels found in rat and human serum corresponded well with previously published data.1,11,18,39
Table 1.
Kynurenic acid (KYNA) levels in the serum of different species.
| Species | KYNA in serum [fmol/μl] |
|---|---|
| Piglet | 3.39 ± 0.09 (6) |
| Rat | 89.72 ± 1.40 (10) |
| Human | 27.91 ± 0.99 (8) |
All data is given as means ± SEM. Number of samples is given in parentheses.
In the piglet frontal cortex KYNA level was found at low nanomolar concentration (4.55 ± 0.09 nM, n = 6) (data not shown).
KAT I and KAT II activities
The KAT I and KAT II activities in the piglet frontal cortex were higher than in human frontal cortex, i.e. approximately three times higher for KAT I and four times higher for KAT II, but lower than in the rat frontal cortex, i.e. five times lower for KAT I and ten times lower for KAT II (Table 2). No activities (very low or even negative values) of KAT I and KAT II were found in the piglet liver, heart and lung, whereas in the rat liver KAT I and KAT II activities were very high. In the adult pig liver, the KAT I and KAT II activities were not or only marginal detectable (data not shown).
Table 2.
Activities of kynurenine aminotransferase I and II (KAT I and KAT II) in piglet, rat and human tissues.
| Species/Organ | KAT I [pmol/mg wet weight tissue/h] | KAT II [pmol/mg wet weight tissue/h] | |
|---|---|---|---|
| Piglet | Brain | 2.54 ± 0.36 (6) | 2.34 ± 0.42 (6) |
| Liver | nd (5) | nd (5) | |
| Heart | nd (5) | nd (5) | |
| Lung | nd (5) | nd (5) | |
| Rat | Brain | 12.72 ± 0.78 (8) | 22.71 ± 1.27 (8) |
| Liver | 369.49 ± 30.18 (10) | 2,109.4 ± 141.4 (10) | |
| Human | Brain | 0.919 ± 0.021 (5) | 0.538 ± 0.020 (5) |
| Heart | 0.732 ± 0.030 (5) | 0.451 ± 0.021 (5) | |
All data is given as means ± SEM. As a region of the brain the frontal cortex was used. Number of animals and number of human subjects are given in parentheses; nd not detectable and/or even negative value.
KAT I and KAT II activities in mixtures of two different homogenates
KAT I and KAT II activities of rat liver or rat brain homogenate or a mixture of the two different homogenates are shown in Table 3. The activity of KAT I and KAT II in the reaction mixture containing rat liver and brain homogenates was comparable to the total activity of both homogenates if incubated separately. In contrast, the piglet brain, human brain or piglet liver homogenate significantly lowered the rat liver KAT I and KAT II activities. Piglet brain homogenate reduced rat liver KAT I by 54.5% of the control (P < 0.001) and KAT II by 76.0% of the control (P < 0.01), and human brain homogenate reduced the rat liver KAT I by 70% of the control (P < 0.01) and KAT II by 72% of the control (P < 0.01). Piglet liver homogenate reduced the rat liver KAT I by 50.8% of the control (P < 0.001) and KAT II by 69.1% of the control (P < 0.01). Boiled piglet liver homogenate lost the ability to block the rat liver KAT activities, thus, KAT I was 98.6% and KAT II 99.1% of the control, respectively. The reduction of rat liver KAT I and II activities was more pronounced for KAT I than for KAT II and in the presence of piglet brain homogenate than of human brain homogenate, and being dependent on the amount of homogenate added (data not shown).
Table 3.
Kynurenine aminotransferase I and II (KAT I and KAT II) activities in rat liver homogenate: influence of rat brain, piglet brain or human brain.
| Homogenate | KAT I [dpm/reaction mixture] [% of control] | KAT II [dpm/reaction mixture] [% of control] |
|---|---|---|
| Rat liver (Control, CO) | 2966.6 ± 181.5 (5) 100% |
19929.2 ± 1036.2 (5) 100% |
| Rat brain | 2240.0 ± 89.5 (5) | 3654.6 ± 123.9 (5) |
| Rat liver + rat brain | 4931.6 ± 191.4 (5) | 23212.06 ± 544.1 (5) |
| Rat liver + piglet brain | 1617.8 ± 55.4*** (5) 54.5% of CO |
15150.8 ± 856.9** (5) 76.0% of CO |
| Rat liver + piglet liver | 1506.36 ± 47.6*** (5) 50.8% of CO |
13762.0 ± 814.0** (5) 69.1% of CO |
| Rat liver + piglet liver boiled | 2925.4 ± 214.5 (5) 98.6% of CO |
19755.5 ± 930.9 (5) 99.1% of CO |
| Rat liver + human brain | 2077.27 ± 52.7** (5) 70% of CO |
14346.0 ± 669.5** (5) 72% of CO |
All data is given as means ± SEM. KAT I and KAT II activities were assayed as described in Material and Methods; Number of independent measurements are given in parentheses. KAT I and KAT II activities expressed in [pmol/mg wet tissue weight/h] are: for rat liver KAT I is 337.3 ± 25.5 (5) and KAT II is 2292.1 ± 119.2 (5), and for rat brain KAT I is 17.17 ± 0.69 (5) and KAT II is 28.0 ± 0.95 (5), respectively. Significance of differences:
P < 0.05;
P < 0.01;
P < 0.001 vs. corresponding control.
Abbreviations: nd, not detectable; CO, control.
Effect of human CSF on rat liver KAT activities
CSF of human control subjects reduced rat liver KAT I to 20.5% of control (P < 0.001) and KAT II was 103% of control (Table 4). The reduction of rat liver KAT I by human CSF was dependent on the amount of CSF added. Measurement of KAT I and KAT II in CSF samples revealed only marginal activity, which is comparable to our previously published data.18,41
Table 4.
Alterations of rat liver kynurenine aminotransferase I and II (KAT I and KAT II) activities in the presence of cerebrospinal fluid (CSF) of normal human subjects.
| Homogenate | KAT I [pmol/mg wet tissue weight/h] [% of control] | KAT II [pmol/mg wet tissue weight/h] [% of control] |
|---|---|---|
| Rat liver (Control, CO) | 311.6 ± 39.6 (4) 100% |
1914.8 ± 134.8 (4) 100% |
| Rat liver + CSF 75 μl | 63.9 ± 21.6 (4) 20.5% of CO** |
1946.9 ± 154.1 (4) 103% of CO |
| Rat liver + CSF 30 μl | 112.4 ± 7.0 (3) 36.1% of CO* |
nd |
| Rat liver + CSF 10 μl | 233.4 ± 12.8 (3) 74.9% of CO |
nd |
All data is given as means ± SEM. KAT I and KAT II activities were assayed as described in Material and Methods; Number of independent measurements are given in parentheses. KAT I and KAT II activities of CSF were: 121.3 ± 27.5 (4) and 15.1 ± 4.3 (4) [fmol/μl/h], respectively. Significance of differences:
P < 0.05;
P < 0.01; vs. corresponding control.
Abbreviations: nd, not determined.
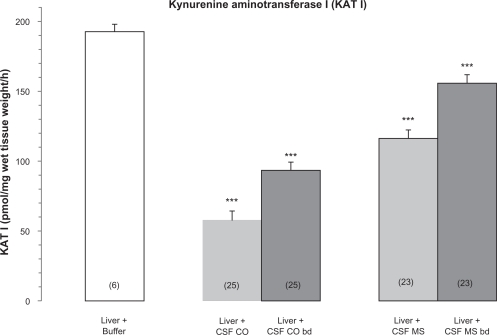
The effect of CSF of MS patients on rat liver KAT I activity is shown in Figure 1. The reduction of rat liver KAT I activity was more pronounced by CSF of the control subjects than by the CSF of the MS patients. Boiled CSF of MS patients and control subjects in part lost the ability to block the rat liver KAT I activity.
Figure 1.
Effect of cerebrospinal fluid (CSF) of Multiple Sclerosis (MS) and of control subjects (CO) on rat liver kynurenine aminotransferase I (KAT I) activity. KAT I activity was assayed as described in Material and Methods. Data is expressed in [pmol/mg wet tissue weight/h] and represent means ± SEM; CSF CO bd and CSF MS bd—CSF samples after 15 min of boiling. Number of independent measurements are given in parentheses. Significance of differences: ***P < 0.001 vs. the corresponding control rat liver KAT I activity.
Influence of human CSF-supernatant (CSF-Sup) and CSF-sediment (CSF-Sed) fractions on rat liver KAT I activity
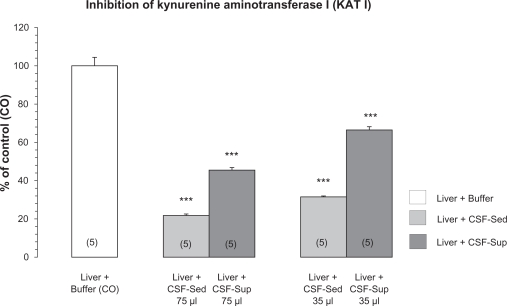
The effect of two different CSF fractions obtained due to centrifugation of CSF of patients with Neuroborreliosis is shown in Figure 2. Both CSF-Sup (75 μl) and CSF-Sed fractions (75 μl) significantly lowered rat liver KAT I by 46.81% and 21.64% of control (P < 0.001), respectively. The inhibitory effect of CSF-Sed fraction was markedly stronger compared to CSF-Sup fraction since CSF-Sed fraction was 4.3 times diluted, due to the fraction preparation procedure. The application of a lower dose of 35 μl of both fractions revealed a weaker effect and it was 67.5% and 31.5% of the control, respectively.
Figure 2.
Effect of different fractions of cerebrospinal fluid (CSF) of patients with Neuroborreliosis on rat liver kynurenine aminotransferase I (KAT I) activity. KAT I activity was assayed as described in Material and Methods. Fractions of CSF-sediment (CSF-Sed) and CSF-supernatant (CSF-Sup) obtained after CSF centrifugation were prepared as described in Material and Methods. Data is expressed in % of control and represent means ± SEM. Number of independent measurements are given in parentheses. Significance of differences: ***P < 0.001 vs. the corresponding control rat liver KAT I activity (CO) and between both application of 35 and 75 μl.
Discussion
From previous studies it is known that in rats and humans KAT activity is predominantly present in the liver—other organs exerting only moderate activity.24,40 For the first time, our data demonstrated no KAT activity in porcine liver and also in other piglet peripheral organs. We also found very low KYNA levels in piglet serum (3.4 nM). Kynurenine metabolism in piglet frontal cortex was easily detectable and KAT activities and KYNA content measured in rat and human control subjects correlated well with earlier published data.1,11,18,39 Lack of KAT activity in piglet liver could indicate an absence of the biochemical machinery to synthesise KYNA. An alternative explanation is the presence of unknown yet endogenous components which interfere with KYNA formation.
The significant observation in our present study was that KYNA formation in rat liver, measured under standard assay condition for KAT activity, was indeed altered in the presence of porcine liver homogenate. In fact, not only the extract of piglet liver but also piglet brain lowered significantly rat liver KAT I and KAT II activities. Interestingly, no inhibition of rat liver KAT was observed in the presence of rat brain homogenate indicating a lack or very low level of inhibitory component(s). This finding does not rule out the possibility of interference because it may critically depend on the species and age. The inhibition of rat liver KAT was also seen in the presence of human brain and CSF, and serum (Baran personal information), as well. The inhibitory effect was dose dependent, it was more pronounced for KAT I than for KAT II, and was heat sensitive indicating an involvement of thermosensitive protein-like compound(s).
The coherence between a high inhibitory capacity and low KAT activity (and likely low KYNA formation) of investigated biological materials is significant. It is possible that low KAT activity measured in tissue is due to the presence of this endogenous KAT inhibiting compound(s).
The most interesting observation in our study was that the inhibitory effect of CSF of MS patients was significantly weaker compared to CSF of the human control subjects, suggesting a clinically important effect. This effect of suppressed inhibition may cause a higher KYNA synthesis and activation of glia in the CNS of MS patients. This hypothesis is supported by the fact that indeed enhanced KYNA levels in the CSF42 and the presence of gliosis and plaque formation in the brain in the acute stage of MS patients has been reported.49
The inhibitory capacity of human CSF to block KAT I could play a particularly important role since CSF is produced by choroid plexus epithelial cells and KAT I mRNA activity is expressed in the cytosol of the choroid plexus epithelial cells.33 It is questionable if choroid plexus epithelial cells or other cells like lymphocytes or even neurons are involved in the formation of those inhibitors. In that respect, we found that the inhibitory effect was notably present by using CSF-Sed fraction and this data strongly indicated the involvement of proteins/cells of CSF.
Our recently published data has demonstrated that Cerebrolysin, which contains an extract of porcine peptides, has the ability to block not only rat liver KAT activities but also KAT activities of the rat and human brain.25 In this study we proposed that Cerebrolysin induced KAT inhibition might affect glia proliferation, too.25 In line with our suggestion a study on transgenic mouse model of Alzheimer’s disease showed that Cerebrolysin treatment significantly ameliorated cerebrovascular amyloidosis, perivascular and interstitial microgliosis, and furthermore astrogliosis was markedly reduced after Cerebrolysin treatment, as well.43 Álvarez et al44 demonstrated that Cerebrolysin reduced amoeboid microglia activity indicating that this porcine peptide extract has the ability to attenuate microglia activation. Although the therapeutic efficacy of Cerebrolysin has been proposed due to a neurotrophic activity,45 we suggested that KAT inhibition might contribute to an attenuation of microglia proliferation.25
In this study, for the first time, our accumulated findings demonstrate the differences in the capability of endogenous components of various species and various physiological and pathological conditions to block KAT activity and we propose the presence of glia depressing factor (GDF). The presence of this factor is abundant, since an inhibitory effect on KAT was observed in several homogenates of different organs, by CSF, and also in human serum. In the investigated species, significantly different inhibitory capacities of brain homogenate have been found suggesting species dependent different distribution i.e. very high in pigs, lower in humans and very moderate in rat brain homogenate.
A remarkable enhancement of rat brain KYNA metabolism i.e. enhancement of KAT activity and an increased glia proliferation during the aging process24,31 could be due to the lowering of GDF levels with aging and this needs to be proved through further investigation. On the other hand high GDF levels and low KAT activity (due to KAT blockade) may lead to a “re-direction” of the kynurenine pathway and synthesis of quinolinic acid, which acting as an endogenous agonist of NMDA receptors, is significantly involved in the synaptogenesis during brain development.46 However, under certain conditions, the “re-direction” of the kynurenine pathway might be associated with overproduction of quinolinic acid and induction of neurodegenerative processes and/or epileptic activities.1,19
Morgan et al showed that food restriction decreased the transcription of GFAP in ageing rats and lowered microglia activation during ageing,47 therefore selected food consumption might have a significant impact on many physiological and pathological processes in humans.
In summary, a remarkably low KYNA metabolism was found in piglet periphery and CNS, comparing to rat and human organs. For the first time we demonstrated that porcine tissues extract and human CSF, serum or brain extract shows the ability to block significantly KAT I and partly KAT II activities. We propose the presence of a glia depressing factor (GDF), which might have a significant impact not only on regulation of KYNA metabolism but also on regulation of glia/astroglia activity respectively and glia proliferation. Apart from the first contribution to understand the mechanism(s) involved in the regulation of KYNA metabolism, our observations might have potential diagnostic implications.48 The composition and structure of GDF and the mechanism(s) of action in mammalians, especially the role of GDF during development, aging and pathological conditions needs to be elaborated.
Acknowledgments
This work was supported by the Austrian Science Research Project FWF P15371 (to H. Baran), Oester-reichische Nationalbank Jubiläumsfonds P12316 (to H. Baran) and by the Multiple Sklerose Forschun-gsgesellschaft Wien (to B. Kepplinger and H. Baran).
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors report no conflicts of interest.
References
- 1.Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–79. [PubMed] [Google Scholar]
- 2.Birch PJ, Grossman CJ, Hayes AG. Kynurenic acid antagonizes responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988;154:85–7. doi: 10.1016/0014-2999(88)90367-6. [DOI] [PubMed] [Google Scholar]
- 3.Hilmas C, Pereira EFR, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non-α7 nicotinic receptor expression. Physiopathological implications. J Neurosci. 2001;21:7463–73. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster AC, Vezzani A, French ED, Schwarcz R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci Lett. 1984;48:273–8. doi: 10.1016/0304-3940(84)90050-8. [DOI] [PubMed] [Google Scholar]
- 5.Hlinak Z, Krejci I. Kynurenic acid and 5,7-dichlorokynurenic acids improve social and object recognition in male rats. Psychopharmacology. 1995;120:463–9. doi: 10.1007/BF02245819. [DOI] [PubMed] [Google Scholar]
- 6.Steele RJ, Stewart MG. 7-Chlorokynurenate, an antagonist of the glycine binding site on the NMDA receptor, inhibits memory formation in day-old chicks (Gallus domesticus) Behav Neural Biol. 1993;60:89–92. doi: 10.1016/0163-1047(93)90145-8. [DOI] [PubMed] [Google Scholar]
- 7.Albuquerque EX, Pereira EF, Alkondom M, Rogers SW. Mammalian nicotinic receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baran H, Staniek K, Kepplinger B, Stur I, Draxler M, Nohl H. Kynurenines and the respiratory parameters in rat heart mitochondria. Life Sciences. 2003;72:1103–15. doi: 10.1016/s0024-3205(02)02365-2. [DOI] [PubMed] [Google Scholar]
- 9.Baran H, Kepplinger B, Herrera-Marschitz M, Stolze K, Lubec G, Nohl H. Increased kynurenic acid in the brain after neonatal asphyxia. Life Sciences. 2001;69:1249–56. doi: 10.1016/s0024-3205(01)01215-2. [DOI] [PubMed] [Google Scholar]
- 10.Richter A, Löscher W, Baran H, Gramer M. Increased levels of kynurenic acid in brains of genetically dystonic hamster. Dev Brain Res. 1996;92:111–6. doi: 10.1016/0165-3806(96)00002-8. [DOI] [PubMed] [Google Scholar]
- 11.Baran H, Gramer M, Hönack D, Löscher W. Systemic administration of kainate induces marked increase of endogenous kynurenic acid in various brain regions and plasma of rats. Euro J Pharmacology. 1995;286:167–75. doi: 10.1016/0014-2999(95)00443-o. [DOI] [PubMed] [Google Scholar]
- 12.Stone TW. Kynurenines in the CNS: From endogenous obscurity to therapeutic importance. Prog Neurobiol. 2001;64:185–218. doi: 10.1016/s0301-0082(00)00032-0. [DOI] [PubMed] [Google Scholar]
- 13.Baran H, Jellinger K, Deecke L. Kynurenine metabolism in Alzheimer’s disease. J Neural Transm. 1999;106:165–81. doi: 10.1007/s007020050149. [DOI] [PubMed] [Google Scholar]
- 14.Kepplinger B, Baran H, Kalina P, Kainz A, Nohl H. Subcortical sclerotic Encephalopathy (SSEP) is associated with increased kynurenic acid (KYNA) levels in cerebrospinal fluid (CSF), XXIX. Slovenské a Ceské Neurovasculárne Sympósium; Bratislava. 19–20 Octóbra 2001; 2001. pp. 76–7. Abstract Book. [Google Scholar]
- 15.Heyes MP, Brew BJ, Saito K, et al. Interrelationships between quinolinic acid, neuroactive kynurenines, neopterin and beta2-microglobulin in cerebrospinal fluid and serum of HIV-1-infected patients. J Neuroimmunol. 1992;40(1):71–80. doi: 10.1016/0165-5728(92)90214-6. [DOI] [PubMed] [Google Scholar]
- 16.Baran H, Hainfellner JA, Kepplinger B, Mazal PR, Schmid H, Budka H. Kynurenic acid metabolism in the brain of HIV-1 infected patients. J Neural Transm. 2000;107:1127–38. doi: 10.1007/s007020070026. [DOI] [PubMed] [Google Scholar]
- 17.Schwarcz R, Rassoulpour A, Wu H-Q, Medoff D, Tamminga CA, Robert RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50(7):521–30. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- 18.Kepplinger B, Baran H, Kainz A, Ferraz-Leite H, Newcombe J, Kalina P. Age-related increase of kynurenic acid in human cerebrospinal fluid: Positive correlation with IgG and β2-microglobulin changes. Neurosignals. 2005;14(3):126–35. doi: 10.1159/000086295. [DOI] [PubMed] [Google Scholar]
- 19.Baran H, Kepplinger B, Newcombe J, Draxler M. Reduced kynurenine aminotransferase I and II and glutamic acid decarboxylase and choline acetyltransferase activities in the central nervous system of multiple sclerosis patients. Wiener Klinische Wochenschrift. 2008;(3):46–7. [Google Scholar]
- 20.Gal EM, Sherman AD. L-Kynurenine: Its synthesis and possible regulatory function in brain. Neurochem Res. 1980;5:223–39. doi: 10.1007/BF00964611. [DOI] [PubMed] [Google Scholar]
- 21.Kido R. Kynurenate forming enzymes in liver, kidney and brain. In: Schwarcz R, Young SN, Brown RR, editors. Kynurenine and serotonin pathways: Progress in tryptophan Research Advances in Experimental Medicine and Biology. Vol. 294. Plenum Publishing Corporation; New York: 1989. pp. 201–5. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa T, Okuno E, Tsujimoto M, Nakamura M, Kido R. Kynrenine-pyruvate aminotransferase in rat kidney and brain. In: Schwarcz R, Young SN, Brown RR, editors. Kynurenine and serotonin pathways: Progress in tryptophan Research Advances in Experimental Medicine and Biology. Vol. 294. Plenum Publishing Corporation; New York: 1989. pp. 567–72. [Google Scholar]
- 23.Guidetti P, Okuno E, Schwarcz R. Characterization of rat brain kynurenine aminotransferases I and II. J Neurosci Res. 1997;50:457–65. doi: 10.1002/(SICI)1097-4547(19971101)50:3<457::AID-JNR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Gramsbergen JBP, Schmidt W, Turski WA, Schwarcz R. Age-related changes in kynurenic acid production in rat brain. Brain Res. 1992;588:1–5. doi: 10.1016/0006-8993(92)91337-e. [DOI] [PubMed] [Google Scholar]
- 25.Baran H, Kepplinger B. Cerebrolysin lowers kynurenic acid formation—An in vitro study. Eur Neuropsychopharmacol. 2009;19:161–8. doi: 10.1016/j.euroneuro.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Okuno E, Nakamura M, Schwarcz R. Two kynurenine aminotransferases in human brain. Brain Res. 1991;542:307–12. doi: 10.1016/0006-8993(91)91583-m. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt W, Guidetti P, Okuno E, Schwarcz R. Characterization of human brain kynurenine aminotransferases using [3H]kynurenine as a substrate. Neuroscience. 1993;55:177–84. doi: 10.1016/0306-4522(93)90464-q. [DOI] [PubMed] [Google Scholar]
- 28.Baran H, Okuno E, Kido R, Schwarcz R. Purification and characterisation of kynurenine aminotransferase I from human brain. J Neurochem. 1994;62:730–8. doi: 10.1046/j.1471-4159.1994.62020730.x. [DOI] [PubMed] [Google Scholar]
- 29.Han Q, Robinson H, Cai T, Tagle DA, Li J. Biochemical and structural properties of mouse kynurenine aminotransferase III. Mol Cell Biol. 2009;29(3):784–93. doi: 10.1128/MCB.01272-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han Q, Li J, Li J. pH dependence, substrate specificity and inhibition of human kynurenine aminotransferase I. Eur J Biochem. 2004;271:4804–14. doi: 10.1111/j.1432-1033.2004.04446.x. [DOI] [PubMed] [Google Scholar]
- 31.Roberts RC, Du F, McCarthy KE, Okuno E, Schwarcz R. Immunocyto-chemical localization of kynurenine aminotransferase in the rat striatum: A light and electron microscopic study. J Comp Neurol. 1992;326:82–90. doi: 10.1002/cne.903260107. [DOI] [PubMed] [Google Scholar]
- 32.Guillemin GJ, Kerr SJ, Smythe GA, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem. 2001;78(4):842–53. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 33.Tamburin M, Mostardini M, Benatti L. Kynurenine aminotransferase I (KAT I) isoform gene expression in the rat brain: An in situ hybridization study. Neuroreport. 1999;10:61–5. doi: 10.1097/00001756-199901180-00012. [DOI] [PubMed] [Google Scholar]
- 34.Tibbling G, Link H, Öhman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest. 1977;37:385–90. doi: 10.1080/00365517709091496. [DOI] [PubMed] [Google Scholar]
- 35.Olsson T, Kostulas V, Link H. Improved detection of oligoclonal IgG in cerebrospinal fluid by isoelectric focusing in agarose, double-antibody peroxidase labelling, and avidin-biotin amplification. Clin Chem. 1984;30(79):1246–9. [PubMed] [Google Scholar]
- 36.Shibata K. Fluorometric microdetermination of kynurenic acid, an endogenous blocker of neurotoxicity, by high performance liquid chromatography. J Chromat. 1988;430:376–80. doi: 10.1016/s0378-4347(00)83173-4. [DOI] [PubMed] [Google Scholar]
- 37.Swartz KJ, Matson WR, MacGarvey U, Ryan EA, Beal MF. Measurement of kynurenic acid in mammalian brain extracts and cerebrospinal fluid by high-performance liquid chromatography with fluorometric and coulometric electrode assay detection. Analyt Biochem. 1990;85:363–76. doi: 10.1016/0003-2697(90)90309-w. [DOI] [PubMed] [Google Scholar]
- 38.Turski WA, Gramsbergen JBP, Traitler H, Schwarcz R. Rat brain slices produce and liberate kynurenic acid upon expose to L-kynurenine. J Neurochem. 1989;52:1629–36. doi: 10.1111/j.1471-4159.1989.tb09218.x. [DOI] [PubMed] [Google Scholar]
- 39.Moroni F, Russi P, Lombardi G, Beni M, Carla V. Presence of kynurenic acid in the mammalian brain. J Neurochem. 1988;51:177–80. doi: 10.1111/j.1471-4159.1988.tb04852.x. [DOI] [PubMed] [Google Scholar]
- 40.Baran H, Amann G, Lubec B, Lubec G. Kynurenic acid and kynurenine aminotransferase in heart. Pediatr Res. 1997;41(3):404–10. doi: 10.1203/00006450-199703000-00017. [DOI] [PubMed] [Google Scholar]
- 41.Baran H, Kepplinger B, Kainz A, et al. Kynurenine aminotransferases in human cerebrospinal fluid. J Neurol. 2003;250(Sup2):II/10, 4. [Google Scholar]
- 42.Kepplinger B, Baran H, Kainz A, Newcombe J, Nohl H. Altered kynurenic acid levels in CSF and serum of patients with multiple sclerosis. Multiple Sclerosis. 2001;7(Sup1) S118:P400. [Google Scholar]
- 43.Rockenstein E, Adame A, Mante M, et al. Amelioration of the cerebrovascular amyloidosis in a transgenic model of Alzheimer’s disease with the neurotrophic compound Cerebrolysin™. J Neural Transm. 2005;112:269–82. doi: 10.1007/s00702-004-0181-4. [DOI] [PubMed] [Google Scholar]
- 44.Álvarez XA, Lombardi VRM, Fernández-Novoa L, et al. Cerebrolysin reduces microglia activation in vivo and in vitro: a potential mechanism of neuroprotection. J Neural Transm. 2000;59:281–92. doi: 10.1007/978-3-7091-6781-6_30. [DOI] [PubMed] [Google Scholar]
- 45.Veinbergs I, Mante M, Mallory M, Masliah E. Neurotrophic effects of Cerebro-lysin in animal models of excitotoxicity. J Neural Transm. 2000;59:273–80. doi: 10.1007/978-3-7091-6781-6_29. [DOI] [PubMed] [Google Scholar]
- 46.McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory aminoacids during central nervous system development. Brain Res Rev. 1990;15:41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- 47.Morgan TE, Rozovsky I, Goldsmith SK, Stone DJ, Yoshida T, Finch CE. Increased transcription of the astrocyte gene GFAP during middle-age is attenuated by food restriction: implications for the role of oxidative stress. Free Radic Biol Med. 1997;23(3):524–8. doi: 10.1016/s0891-5849(97)00120-2. [DOI] [PubMed] [Google Scholar]
- 48.Baran H, Kepplinger B. New approach to differentiate multiple sclerosis from other inflammatory CNS disorders. Euro J of Neurol. 2009;16(Sup3):259:P1629. [Google Scholar]
- 49.Larsson HB, Frederiksen J, Petersen J, et al. Assessment of demyelination, edema, and gliosis by in vivo determination of T1 and T2 in the brain of patients with acute attack of multiple sclerosis. Magn Reson Med. 1989;11(3):337–48. doi: 10.1002/mrm.1910110308. [DOI] [PubMed] [Google Scholar]