Abstract
Kynurenic acid (KYNA) is an endogenous metabolite of tryptophan. Studies have revealed increased brain KYNA levels in patients with schizophrenia. Prepulse inhibition (PPI) is a behavioral model for sensorimotor gating and found to be reduced in schizophrenia. Previous studies have shown that pharmacologically elevated brain KYNA levels disrupt PPI in the rat. The aim of the present study was to investigate the receptor(s) involved in this effect. Rats were treated with different drugs selectively blocking each of the sites that KYNA antagonizes, namely the glutamate recognition site of the N-methyl-D-aspartate receptor (NMDAR), the α7* nicotinic acetylcholine receptor (α7nAChR) and the glycine site of the NMDAR. Kynurenine (200 mg/kg) was given to replicate the effects of increased levels of KYNA on PPI. In order to block the glutamate recognition site of the NMDAR, CGS 19755 (10 mg/kg) or SDZ 220–581 (2.5 mg/kg) were administered and to antagonize the α7nAChR methyllycaconitine (MLA; 6 mg/kg) was given. L-701,324 (1 and 4 mg/kg) or 4-Chloro-kynurenine (4-Cl-KYN; 25, 50 and 100 mg/kg), a drug in situ converted to 7-Chloro-kynurenic acid, were used to block the glycine-site of the NMDAR. Administration of SDZ 220-581 or CGS 19755 was associated with a robust reduction in PPI, whereas L-701,324, 4-Cl-KYN or MLA failed to alter PPI. Kynurenine increased brain KYNA levels 5-fold and tended to decrease PPI. The present study suggests that neither antagonism of the glycine-site of the NMDA receptor nor antagonism of the α7nAChR disrupts PPI, rather with regard to the effects of KYNA, blockade of the glutamate recognition-site is necessary to reduce PPI.
Keywords: kynurenic acid, kynurenine, sensorimotor gating, α7* nicotinic acetylcholine receptor, NMDA/glycine-site
Introduction
Kynurenic acid is an endogenous tryptophan metabolite, primarily synthesized in and released from astrocytes.1–3 During the last decade, several studies implicate KYNA in the pathophysiology of various psychiatric conditions.4,5 For example, studies of patients with schizophrenia have revealed elevated levels of KYNA in both the cerebrospinal fluid (CSF) and in the post mortem prefrontal cortex.6,7 In addition, suicidal attempters with major depressive disorder8 as well as patients with bipolar disorder9 display elevated levels of KYNA in the CSF.
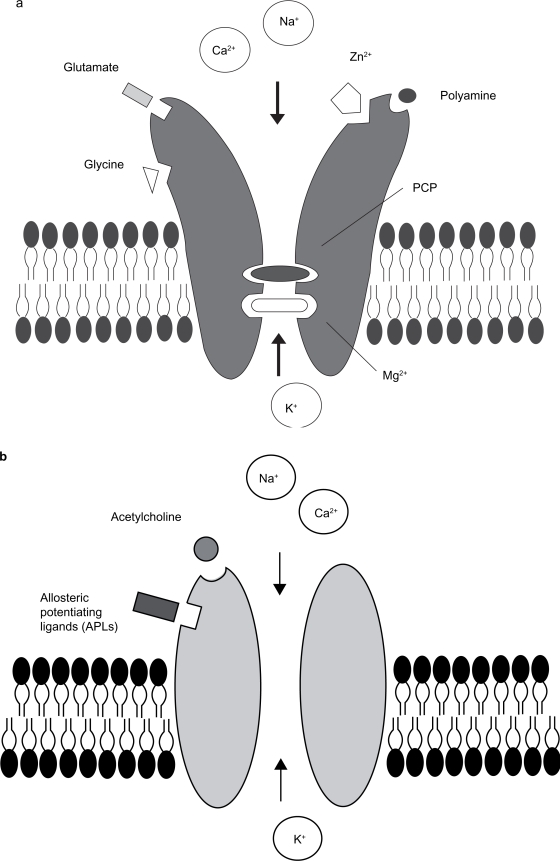
Kynurenic acid (KYNA, Fig. 1a) is an antagonist at glutamatergic and cholinergic receptors. In particular, low concentrations of KYNA block the glycine co-agonist site of the N-methyl-D-aspartate receptor (NMDAR, Fig. 2a; IC50 = 8–15 μM10,11) and the α7 nicotinic acetylcholine receptor (α7nAChR, Fig. 2b; IC50 = 7 μM12). At higher concentrations, KYNA also blocks the glutamate recognition site of the NMDA receptor (IC50 = 200–500 μM10) and the AMPA/kainate receptors (IC50 in the millimolar range13). In addition, KYNA was recently found to stimulate the previously orphan G-protein coupled receptor GPR35 in the rat (EC50 = 7 μM14).
Figures 1.
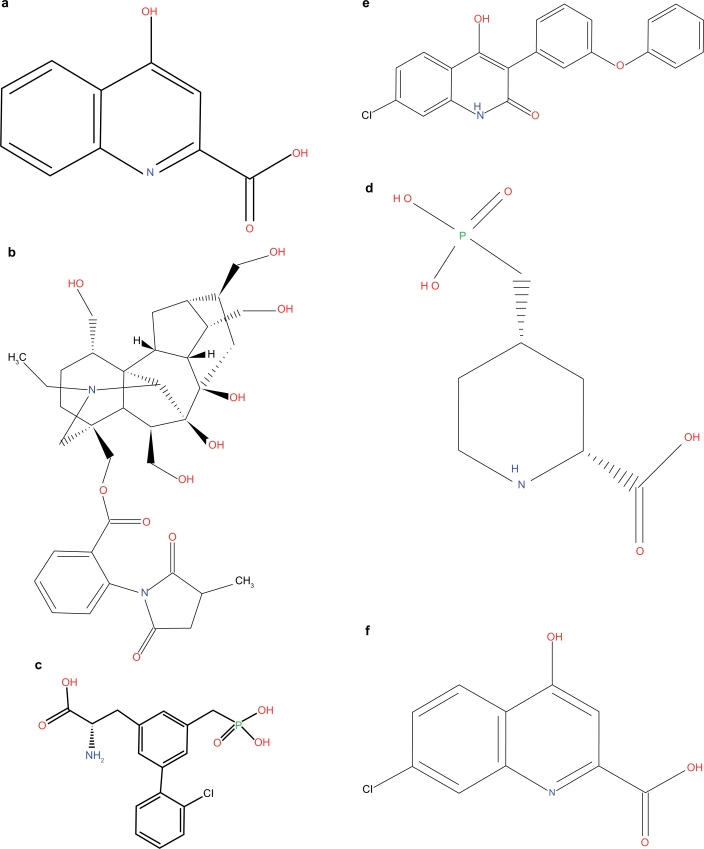
Kynurenic acid a) and selective inhibitors for α7nAChR (MLA, b), glutamate recognition-site of the NMDAR (SDZ 220–581, c; CGS 19755, d) and the glycine-site of the NMDAR (L-701,324, e; 7-Cl-KYNA, f).
Figures 2.
Scheme of the NMDAR a) and α7nAChR b) and their allosteric- and ligand binding-sites.
The physiological significance of brain KYNA has been demonstrated in a number of studies during the last decade.4 However, it is unclear which receptor(s) participate in the various effects of KYNA in the brain. Previous studies have shown that acute and chronic pharmacological elevation of brain KYNA is associated with increased firing of rat midbrain dopamine (DA) neurons,15–21 an effect recently shown to be mediated via blockade of the glycine-site of the NMDA receptor.16,18 Furthermore, local administration of KYNA in the rat striatum decreases terminal DA release via specific blockade of α7nAChR.22 A previous study has also shown that pharmacologically elevated levels of KYNA disrupts prepulse inhibition (PPI) in the rat,17,23 although the specific receptor mechanism involved was not ascertained.
PPI is defined as the attenuation of the startle response to a startling stimulus (e.g. a pulse), when such a stimulus is briefly preceded by a stimulus of subthreshold intensity (prepulse). Disruptions of sensorimotor gating are considered to reflect dysfunctions in the ability to filter out extraneous stimuli that might interfere with information processing and attention.24 Deficits in PPI are frequently observed in patients with schizophrenia.25–28 Interestingly, non-competetive NMDAR antagonist, e.g. PCP, ketamine or MK 801, disrupt PPI in rodents.29 These effects on PPI are also in line with the NMDAR hypofunction hypothesis of schizophrenia,30–32 based on the finding that NMDAR antagonists cause psychotic symptoms in healthy volunteers and worsen clinical symptoms in patients.33–36
The present study investigated which receptor(s) mediate the effects of KYNA on PPI. For this purpose we administered drugs selectively blocking the different receptor-sites known to be blocked by KYNA; Methyllycaconitine (MLA, Fig. 1b), a selective antagonist at the α7nAChR; SDZ 220–581 and CGS 19755 (Fig. 1c and 1d, respectively), selective blockers of the glutamate recognition-site of the NMDA-receptor; L-701,324 (Fig. 1e) and 4-Cl-KYN (in situ converted to 7-Cl-KYNA, Fig. 1f) was given to selectively block the glycine-site of the NMDAR. A putative role of the GPR35 receptor in this regard was not tested due to its limited expression in the brain.14
Materials and Methods
Animals
Experiments were performed on male Sprague-Dawley rats (B&K Universal AB, Sollentuna, Sweden; weighing between 200–330 g). The animals were housed in groups of five with free access to food and water. Environmental conditions were checked daily and maintained under constant temperature (25 °C) and 40%–60% humidity in a room with a regulated, reversed 12 h light/dark cycle (lights off at 07.00 AM, lights on at 07.00 PM). Animals were handled at least 2 days before testing to reduce any subsequent handling stress. Experiments were approved by and performed in accordance with the guidelines of the Ethical Committee of Northern Stockholm, Sweden and all efforts were made to minimize the number of animals used and their suffering.
Drugs
The following drugs were used: 4-Cl-KYN (kindly supplied by Vistagen Therapeutics, South San Francisco, CA, USA and dissolved in 7.5% (2-hydroxypropyl)-β-cyclodextrin, 7-Cl-KYNA, CGS 19755 and SDZ 220–581 (Tocris, Avonmouth, UK); KYNA, L-kynurenine sulfate salt, L-701,324 and MLA (Sigma, St. Louis, MO, USA). The chemicals used were: zinc acetate and acetic acid (Sigma, St. Louis, MO, USA); sodium acetate (Riedel-de Haen, Germany) and acetonitrile (Labasco, Partille, Sweden). 4-Cl-KYN, L-kynurenine, L-701,324 and MLA were administered intraperitoneally (i.p.). SDZ 220–581 and CGS 19755 were administered subcutaneously (s.c.). All doses are expressed as free base.
Apparatus
Two startle chambers were used for measuring the startle response (SR-LAB, San Diego Instruments, San Diego, California). Each chamber consisted of a Plexiglas cylinder (9-cm diameter) mounted on a frame, housed within a ventilated chamber (39 × 38 × 58 cm). Sudden movements within the cylinder were detected by a piezoelectric accelerometer attached below the cylinder. A loudspeaker (Super-tweeter; Radio Shack, Fort Worth, Texas) mounted 24 cm above the cylinder provided the broadband background noise and acoustic stimuli. Presentations of the acoustic stimuli were controlled by the SR-LAB software and interface system, which also rectified, digitized (0–4095), and recorded responses from the accelerometer. As described previously,37 sound levels [dB(A) scale] and accelerometer sensitivities within each chamber were calibrated regularly and found to remain constant over the test period.
Experimental protocols
To elevate levels of endogenous brain KYNA, rats (n = 14) were pretreated with kynurenine (200 mg/kg) i.p. 60 min before testing. Control rats (n = 13) received vehicle i.p. 60 min before testing for comparison with animals treated with kynurenine. In order to block the glutamate recognition-site of the NMDAR, rats were pretreated with SDZ 220–581 (2.5 mg/kg, n = 12) s.c. 30 min before testing or CGS 19755 (10 mg/kg, n = 12) s.c. 45 min before testing. For these experiments, rats receiving saline (n = 12) s.c. 30 min before testing, were used as controls. In a third experiment, rats were treated with drugs blocking the glycine-site of the NMDAR or the α7nAChR. In order to block the glycine-site of the NMDAR, in situ produced 7-Cl-KYNA or pretreatment with L-701,324 (1 mg/kg, n = 13 or 4 mg/kg, n = 17) i.p. 15 min before testing were used. To elevate 7-Cl-KYNA, rats were pretreated with 4-Cl-KYN (25 mg/kg, n = 15; 50 mg/kg, n = 14; or 100 mg/kg, n = 10) i.p. 60 min before testing. For selective blocking of the α7nAChR, rats were treated with methyllycaconitine (MLA, 6 mg/kg, n = 15) i.p. 10 min before testing. Controls in this study (n = 18) received saline i.p. 15 min before testing. Pre-treatment times were based on previous studies.18,38,39 All drug combinations were balanced across the two startle chambers. The experimental session consisted of a 5 min acclimatization period to a 65-dB background noise (continuous throughout the session), followed by a 20-min acoustic PPI test session. Seven days before any drug testing, animals were pre-exposed to the chambers and the testing session. The purpose of the preexposure was to acclimatize the animals to the testing chambers and startle/prepulse stimuli and to baseline-match the groups for subsequent testing (groups were matched for equivalent mean startle magnitude and percent PPI, as defined below). In the test session, a background noise (65 dB) was presented alone for 5 min and then continued throughout the remainder of the session. The test session used in all of the experiments contained five different trial types and had a duration of 20 min: a “pulse-alone” trial, in which a 40-msec 120-dB broadband burst was presented; three “prepulse-pulse” trials, in which 20-msec noises that were either 3, 6, or 12 dB above the background noise were presented 100 msec before the onset of the 120-dB pulse; and a “no stimulus” trial, which included only the background noise. All trial types were presented several times in a pseudorandom order for 60 trials (12 pulse-alone trials, 10 each of the remaining prepulse trial types, and eight no-stimulus trials). Five pulse-alone trials, which were not included in the calculation of PPI values, were presented at the beginning of the test session to achieve a relatively stable level of startle reactivity for the remainder of the session (based on the observation that the most rapid habituation of the startle reflex occurs within the first few presentations of the startling stimulus40). In addition, five pulse-alone trials occurred at the end of the session to assess startle habituation but were not included in the calculation of PPI. An average of 15 sec (range, 9–21 sec) separated consecutive trials. The whole session lasted approximately 24 min. A brief baseline session used to familiarize rats with the testing procedure and match groups for pharmacological studies consisted of 24 trials (18 120-dB pulse-alone and six prepulse-pulse trials with a 12-dB prepulse intensity).
Analysis of whole-brain kynurenic acid and 7-chloro-kynurenic acid
Immediately after the behavioral experiments, the rats were killed by decapitation. The brains were taken out rapidly and stored immediately at −70 °C for subsequent analysis of KYNA and 7-Cl-KYNA. The brains were sonicated with homogenization medium (perchloric acid 0.4 mol/L, Na2S2O5 0.1%, and ethylenediaminetetraacetic acid 0.05%), which was added in the same amount as the weight of the brain before sonication. The samples were centrifuged at 4000 g for 10 min, and 40 μL perchloric acid (70%) was added to the supernatant. Thereafter, the supernatant was centrifuged twice. For analysis of KYNA and 7-Cl-KYNA, an isocratic reversed phase high-performance liquid chromatography (HPLC) system was used, including a dual piston, high liquid delivery pump (Bischoff, Leonberg, Germany), a ReproSil-Pur C18 column (4 × 150 mm, Dr. Maisch GmbH, Ammerbuch, Germany) and a fluorescence detector (Jasco Ltd, Hachioji City, Japan) with an excitation and emission wavelength of 344 nm and 398 nm, respectively (18 nm bandwidth). A mobile phase of sodium acetate (50 mM, pH 6.20, adjusted with acetic acid) and acetonitrile (7% or 10%, for KYNA or 7-Cl-KYNA, respectively) was pumped through the reversed-phase column at a flow rate of 0.5 mL/min. Samples of 30 ml were manually injected (Rheodyne, Cotati, CA, USA). Zinc acetate (0.5 M, not pH adjusted) was delivered post column by a peristaltic pump (P-500, Pharmacia, Uppsala, Sweden) at a flow rate of 0.10 mL/min. The signals from the fluorescence detector were transferred to a computer for analysis utilizing Datalys Azur (Grenoble, France). The retention time of KYNA or 7-Cl-KYNA was about 7 or 16 min, respectively.
Data and statistical analysis
For each pulse-alone and prepulse-pulse trial, the startle response to the 120-dB burst was recorded. Two measures were then calculated from these data for each animal. First, startle magnitudes were calculated as the average response to the pulse-alone trials within each of the four blocks and analyzed with mixed-design analyses of variance (ANOVAs), with block as the repeated measure and pretreatment and/or treatment as between-subject factors. Data from the first and last blocks of five pulse-alone trials are not presented, because the startle data from the middle two blocks when PPI was assessed were representative of the treatment effects, and no reliable effects on startle habituation were observed. Second, the amount of PPI was calculated as a percentage score for each prepulse + pulse trial type: %PPI = 100—([(startle response for prepulse + pulse trial)/(startle response for pulse-alone trial)] × 100). All data were first analyzed in a three-factor ANOVA with blocks (first and second halves of the session) and prepulse as within subject factors and treatment as a between subject factor. When the block factor did not interact with another factor, only the two-factor ANOVA (treatment and prepulse intensity) are reported. The main effect of prepulse intensity was always significant and is not reported specifically. Post hoc comparisons of means were carried out with Tukey’s test. Each experiment was analyzed separately.
All data are presented as mean ± SEM. Statistically significant differences regarding concentrations of KYNA and 7-Cl-KYNA were established using Kruskal-Wallis analysis of variance followed by Mann-Whitney U-test. Alpha was set at 0.05.
Results
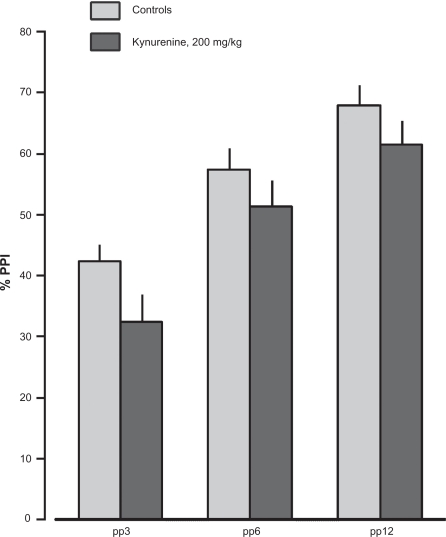
Rats administered kynurenine (200 mg/kg) displayed a 5-fold increase in whole brain KYNA levels (123.1 ± 18.8 nM, n = 14) compared to controls (23.3 ± 2.7 nM, n = 13; Table 1). This elevation of brain KYNA was associated with a tendency to decrease PPI at all pre-pulse intensities (F(1.25) = 2.56, p = 0.12; Fig. 3) and a trend toward decreasing in startle magnitude (F(1,25) = 3.37, p = 0.078).
Table 1.
Whole brain concentrations of KYNA or 7-Cl-KYNA in rats pretreated with kynurenine (i.p., 1.5 h, n = 14) or 4-Cl-KYN (i.p., 1.5 h, n = 10–15).1
| Treatment | KYNA, nM | 7-Cl-Kyna, nM |
|---|---|---|
| Control2 | 23.26 ± 2.67 | - |
| Kynurenine, 200 mg/kg | 123.10 ± 18.76*** | - |
| 4-Cl-Kynurenine, 25 mg/kg | – | 9.23 ± 1.60 |
| 4-Cl-Kynurenine, 50 mg/kg | – | 18.44 ± 3.14 |
| 4-Cl-Kynurenine, 100 mg/kg | – | 52.10 ± 8.84 |
Values represent mean ± SEM. Statistics:
p < 0.001 vs. control (Mann-Whitney U-test).
n = 13.
Figures 3.
Effects of kynurenine (200 mg/kg, i.p., 60 min, n = 14) or vehicle (i.p. 60 min, n = 13) on prepulse inhibition (PPI). Values represent mean ± SEM for each group.
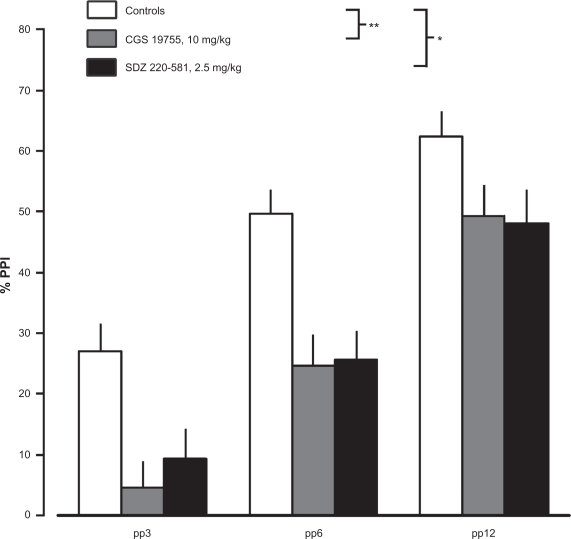
Treatment with drugs blocking the glutamate recognition site of the NMDA receptor, i.e SDZ 220–581 (2.5 mg/kg; F(1,22) = 12.33, p < 0.01) or CGS 19755 (10 mg/kg; F(1,22) = 16.47, p < 0.001), was found to clearly reduce PPI (Fig. 4). While SDZ 220–581 had no effect on startle magnitude, CGS 19755 significantly decreased startle magnitude (F(1,22) = 5.32, p < 0.05).
Figures 4.
Effects of CGS 19755 (10 mg/kg, s.c., 45 min, n = 12), SDZ 220–581 (2.5 mg/kg, s.c., 30 min, n = 12) or saline (n = 12) on PPI. Values represent mean ± SEM for each group. Statistics: *p < 0.01 vs. saline, **p < 0.001 vs. saline.
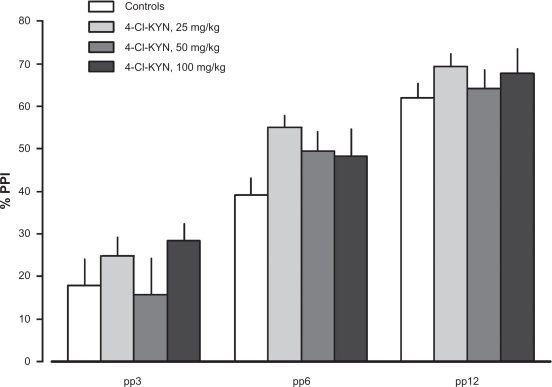
In order to investigate if blockade of the glycine-site of the NMDA receptor or blockade of the α7nAChR disrupts PPI, three different drugs were used, i.e. L-701,324, 4-Cl-KYN and MLA. Administration of L-701,324 (1 and 4 mg/kg; F(2,45) = 2.22, p = 0.12), 4-Cl-KYN (25; 50 and 100 mg/kg; NS), which in situ was converted to 7-Cl-KYNA (a potent and selective antagonist at the glycine-site of the NMDA receptor) or MLA (6 mg/kg) were not associated with disrupted PPI (F(1,31) = 2.84, p = 0.102; Figs. 5 and 6). MLA and 4-Cl-KYN did not affect startle magnitude; whereas L-701,324 (4 mg/kg) reduced startle (F(2,45) = 3.20, p = 0.0503).
Figures 5.
Effects of 4-Chloro-kynurenine (25, 50 or 100 mg/kg, i.p., 60 min; n = 15, n = 14 and n = 10, respectively; in situ converted to 7-Cl-KYNA) or saline (n = 18) on PPI. Values represent mean ± SEM for each group.
Figures 6.
Effects of L-701,324 (1 or 4 mg/kg, i.p., 15 min, n = 13 and n = 17, respectively), MLA (6 mg/kg, i.p., 10 min, n = 15) or saline (n = 18) on PPI. Values represent mean ± SEM for each group.
Discussion
Present results show that pharmacological elevation of brain KYNA is associated with a tendency to disrupt PPI in the rat. The reason for not reaching statistical significance may reflect the fact that kynurenine does not selectively increase KYNA but also increases other neuroactive metabolites of the kynurenine pathway, e.g. 3-hydroxykynurenine and quinolinic acid.41 In a previous study we showed that both kynurenine as well as PNU 156561A, a drug blocking kynurenine 3-hydroxylase and thereby shunting the synthesis towards KYNA, significantly reduce PPI in Sprague Dawley rats.23 A limitation with the present study is the fact that pharmacological tools, aiming at selectively increase KYNA, are lacking, simply because such tools are not available.
The results of the present study also show that administration of CGS 19755 or the highest dose of L-701,324 reduce startle. Such an effect was previously observed in rats with a 60-fold increase in brain KYNA levels23 and thought to be related to sedation. In the present study no signs of sedation was observed in rats treated with CGS 19755 or L-701,324. The reduced PPI following administration of SDZ 220-581 or CGS 19755 confirms that blockade of the glutamate recognition site of the NMDA receptor is associated with disrupted PPI.38,42 In contrast, blockade of either the α7nAChR or the NMDAR/glycine-site had no effect on PPI. These effects are in line with previous studies showing that systemic administration of antagonists of the NMDAR/glycine-site do not affect PPI.42,43 However, local administration of 7-Cl-KYNA, intracerebroventricularely, or into the nucleus accumbens, has been found to reduce PPI.39,44,45 A benefit of using systemic administration of 4-Cl-KYN is the in situ production of 7-Cl-KYNA. 4-Cl-KYN utilizes the same enzymatic machinery as kynurenine and hence 7-Cl-KYNA will be produced in the same regions and micro-compartments in which KYNA is produced. The effects of locally produced 7-Cl-KYNA, derived from systemic administration of 4-Cl-KYN, should thus better correspond to the effects seen by increased levels of KYNA. A recent study supports this view, since it has been shown that the increased firing of midbrain dopamine cells, following in situ produced 7-Cl-KYNA, are almost identical to the enhanced dopaminergic firing following pharmacologically elevated levels of KYNA. Thus, the abscence of an effect on PPI following elevated levels of 7-Cl-KYNA reliably suggest that the glycine-site of the NMDAR is not primarily involved in the modulation of PPI. Of note, 4-Cl-KYN is developed for the treatment of neurological pain and the absence of a disruptive effect on PPI following administration of this agent suggest a lower risk of side effects related to cognition. Previous studies analyzing a putative involvement of the α7nAChR on PPI are conflicting as it has been shown that administration of α-bungarotoxin, another α7nAChR antagonist, or removal of hippocampal cholinergic afferents, disrupts PPI.46–48 However, the tendency to an increased PPI in the present study following administration of MLA, an antagonist of α7nAChR, is more consistent with a previous study by Schreiber et al,49 and by the finding that PPI is normal in α7* null mutant mice.50
KYNA plays a significant physiological role in the brain (c.f. Introduction). In addition, elevation of brain KYNA is associated with cognitive dysfunctions in rodents,51–53 deficits also present in psychiatric disorders, i.e. schizophrenia, bipolar disorder and major depressive disorder. In order to design novel therapeutic drugs, specifically aiming at preventing the effects of KYNA, the specific receptor involved in the variety of effects induced by KYNA must be ascertained. The present study suggests that neither antagonism of the glycine-site of the NMDAR nor antagonism of the α7nAChR disrupts PPI. Rather, with regard to the effects of KYNA, blockade of the glutamate recognition-site is necessary to reduce PPI.
Table 2.
Effects on startle magnitude.
| Vehicle | Kynurenine, 200 mg/kg | ||
| 281.82 ± 41.68 | 203.75 ± 17.95 | ||
| Saline | CGS 19755, 10 mg/kg | SDZ 220–581, 2.5 mg/kg | |
| 347.16 ± 43.07 | 235.87 ± 26.20* | 358.42 ± 62.10 | |
| Saline | 4-Cl-KYN, 25 mg/kg | 4-Cl-KYN, 50 mg/kg | 4-Cl-KYN, 100 mg/kg |
| 284.80 ± 31.54 | 342.44 ± 81.18 | 294.48 ± 49.01 | 288.82 ± 44.57 |
| L-701, 324, 1 mg/kg | L-701, 324, 4 mg/kg | ||
| 290.15 ± 50.24 | 189.21 ± 18.75 | ||
| MLA, 6 mg/kg | |||
| 264.59 ± 26.20 |
Values represent mean ± SEM.
p < 0.05 vs. control, F(1,22) = 5.32.
Acknowledgments
This study was supported by Hållstens Forskningsstiftelse, Swedish Brain Foundation, Swedish Research Council (No. 2009–3068, 2008–3822, 2009–4046), Åhlénsstiftelsen, Svenska Läkaresäll-skapet, Karolinska Institute, Torsten och Ragnar Söderbergs stiftelse, and National Institute of Health grant (MH052885). We thank Ms Kerstin Larsson, Department of Physiology and Pharmacology, Karolinska Institute, for technical assistance.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors report no conflicts of interest: Klas Linderholm, none; Susan Powell, none; Elin Olsson, none; Maria Holtze, none; Sophie Erhardt, none; R. Snodgrass, employee and stockholder of VistaGen Therapeutics, Inc.
References
- 1.Guillemin GJ, Kerr SJ, Smythe GA, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem. 2001 Aug;78(4):842–53. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 2.Kiss C, Ceresoli-Borroni G, Guidetti P, Zielke CL, Zielke HR, Schwarcz R. Kynurenate production by cultured human astrocytes. J Neural Transm. 2003 Jan;110(1):1–14. doi: 10.1007/s00702-002-0770-z. [DOI] [PubMed] [Google Scholar]
- 3.Guidetti P, Hoffman GE, Melendez-Ferro M, Albuquerque EX, Schwarcz R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia. 2007 Jan 1;55(1):78–92. doi: 10.1002/glia.20432. [DOI] [PubMed] [Google Scholar]
- 4.Erhardt S, Olsson SK, Engberg G. Pharmacological manipulation of kynurenic acid: potential in the treatment of psychiatric disorders. CNS Drugs. 2009;23(2):91–101. doi: 10.2165/00023210-200923020-00001. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy states. Int J Trypt Res. 2009;2:1–19. doi: 10.4137/ijtr.s2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erhardt S, Blennow K, Nordin C, Skogh E, Lindström LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001 Nov 2;(1–2):313. 96–8. doi: 10.1016/s0304-3940(01)02242-x. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson LK, Linderholm KR, Engberg G, et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res. 2005 Dec 15;(2–3):80. 315–22. doi: 10.1016/j.schres.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Linderholm KR, Erhardt S, Lindqvist D, et al. Kynurenic acid in the cerebrospinal fluid of male suicide attempters—Increased levels in patients with MDD and correlations with cytokines. Program No. 305.5. Society for Neuroscience; 2009 Neuroscience Meeting Planner; Chicago, IL. 2009. [Google Scholar]
- 9.Olsson SK, Söderlund J, Walther Jallow L, et al. Aberrant cytokine profile in the CSF of individuals with bipolar disorder—Correlation to symptoms. Program No. 305.2. Society for Neuroscience; 2009 Neuroscience Meeting Planner; Chicago, IL. 2009. [Google Scholar]
- 10.Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989 Apr;52(4):1319–28. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 11.Parsons CG, Danysz W, Quack G, et al. Novel systemically active antagonists of the glycine site of the N-methyl-D-aspartate receptor: electrophysiological, biochemical and behavioral characterization. J Pharmacol Exp Ther. 1997 Dec;283(3):1264–75. [PubMed] [Google Scholar]
- 12.Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001 Oct 1;21(19):7463–73. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993 Sep;45(3):309–79. [PubMed] [Google Scholar]
- 14.Wang J, Simonavicius N, Wu X, et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem. 2006 Aug 4;281(31):22021–8. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- 15.Erhardt S, Oberg H, Mathé JM, Engberg G. Pharmacological elevation of endogenous kynurenic acid levels activates nigral dopamine neurons. Amino Acids. 2001;20(4):353–62. doi: 10.1007/s007260170032. [DOI] [PubMed] [Google Scholar]
- 16.Erhardt S, Engberg G. Increased phasic activity of dopaminergic neurones in the rat ventral tegmental area following pharmacologically elevated levels of endogenous kynurenic acid. Acta Physiol Scand. 2002 May;175(1):45–53. doi: 10.1046/j.1365-201X.2002.00962.x. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson LK, Linderholm KR, Erhardt S. Subchronic treatment with kynurenine and probenecid: effects on prepulse inhibition and firing of midbrain dopamine neurons. J Neural Transm. 2006 May;113(5):557–71. doi: 10.1007/s00702-005-0343-z. [DOI] [PubMed] [Google Scholar]
- 18.Linderholm KR, Andersson A, Olsson S, et al. Activation of rat ventral tegmental area dopamine neurons by endogenous kynurenic acid: a pharmacological analysis. Neuropharmacology. 2007 Dec;53(8):918–24. doi: 10.1016/j.neuropharm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Schwieler L, Erhardt S, Nilsson L, Linderholm K, Engberg G. Effects of COX-1 and COX-2 inhibitors on the firing of rat midbrain dopaminergic neurons—possible involvement of endogenous kynurenic acid. Synapse. 2006 Apr;59(5):290–8. doi: 10.1002/syn.20241. [DOI] [PubMed] [Google Scholar]
- 20.Schwieler L, Linderholm KR, Nilsson-Todd LK, Erhardt S, Engberg G. Clozapine interacts with the glycine site of the NMDA receptor: electrophysiological studies of dopamine neurons in the rat ventral tegmental area. Life Sci. 2008 Aug 1;(5–6):83. 170–5. doi: 10.1016/j.lfs.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Olsson SK, Andersson AS, Linderholm KR, et al. Elevated levels of kynurenic acid change the dopaminergic response to amphetamine: implications for schizophrenia. Int J Neuropsychopharmacol. 2009 May;12(4):501–12. doi: 10.1017/S1461145708009383. [DOI] [PubMed] [Google Scholar]
- 22.Rassoulpour A, Wu HQ, Ferre S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J Neurochem. 2005 May;93(3):762–5. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- 23.Erhardt S, Schwieler L, Emanuelsson C, Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol Psychiatry. 2004 Aug 15;56(4):255–60. doi: 10.1016/j.biopsych.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 24.McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–16. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 25.Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978 Jul;15(4):339–43. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 26.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001 Jul;(2–3):156. 234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 27.Geyer MA. The family of sensorimotor gating disorders: comorbidities or diagnostic overlaps? Neurotox Res. 2006 Dec;(3–4):10. 211–20. doi: 10.1007/BF03033358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008 Aug;199(3):331–88. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001 Jul;(2–3):156. 117–54. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 30.Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–60. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- 31.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991 Oct;148(10):1301–8. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 32.Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999 Mar;20(3):201–25. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 33.Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry. 1959 Mar;81(3):363–9. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- 34.Itil T, Keskiner A, Kiremitci N, Holden JM. Effect of phencyclidine in chronic schizophrenics. Can Psychiatr Assoc J. 1967 Apr;12(2):209–12. doi: 10.1177/070674376701200217. [DOI] [PubMed] [Google Scholar]
- 35.Malhotra AK, Pinals DA, Weingartner H, et al. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996 May;14(5):301–7. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- 36.Malhotra AK, Pinals DA, Adler CM, et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997 Sep;17(3):141–50. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- 37.Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology (Berl) 1988;94(4):507–14. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- 38.Bakshi VP, Tricklebank M, Neijt HC, Lehmann-Masten V, Geyer MA. Disruption of prepulse inhibition and increases in locomotor activity by competitive N-methyl-D-aspartate receptor antagonists in rats. J Pharmacol Exp Ther. 1999 Feb;288(2):643–52. [PubMed] [Google Scholar]
- 39.Furuya Y, Ogura H. Competitive NMDA and strychnine-insensitive glycine-site antagonists disrupt prepulse inhibition. Pharmacol Biochem Behav. 1997 Aug;57(4):909–13. doi: 10.1016/s0091-3057(96)00452-2. [DOI] [PubMed] [Google Scholar]
- 40.Geyer MA, Swerdlow NR, Mansbach RS, Braff DL. Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Res Bull. 1990 Sep;25(3):485–98. doi: 10.1016/0361-9230(90)90241-q. [DOI] [PubMed] [Google Scholar]
- 41.Guidetti P, Eastman CL, Schwarcz R. Metabolism of [5–3H] kynurenine in the rat brain in vivo: evidence for the existence of a functional kynurenine pathway. J Neurochem. 1995 Dec;65(6):2621–32. doi: 10.1046/j.1471-4159.1995.65062621.x. [DOI] [PubMed] [Google Scholar]
- 42.Depoortere R, Perrault G, Sanger DJ. Prepulse inhibition of the startle reflex in rats: effects of compounds acting at various sites on the NMDA receptor complex. Behav Pharmacol. 1999 Feb;10(1):51–62. doi: 10.1097/00008877-199902000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Bristow LJ, Landon L, Saywell KL, Tricklebank MD. The glycine/NMDA receptor antagonist, L-701,324 reverses isolation-induced deficits in prepulse inhibition in the rat. Psychopharmacology (Berl) 1995 Mar;118(2):230–2. doi: 10.1007/BF02245847. [DOI] [PubMed] [Google Scholar]
- 44.Kretschmer BD, Koch M. Role of the strychnine-insensitive glycine binding site in the nucleus accumbens and anterodorsal striatum in sensorimotor gating: a behavioral and microdialysis study. Psychopharmacology (Berl) 1997 Mar;130(2):131–8. doi: 10.1007/s002130050220. [DOI] [PubMed] [Google Scholar]
- 45.Kretschmer BD, Koch M. The ventral pallidum mediates disruption of pre-pulse inhibition of the acoustic startle response induced by dopamine agonists, but not by NMDA antagonists. Brain Res. 1998 Jul 6;(1–2):798. 204–10. doi: 10.1016/s0006-8993(98)00424-7. [DOI] [PubMed] [Google Scholar]
- 46.Bickford PC, Wear KD. Restoration of sensory gating of auditory evoked response by nicotine in fimbria-fornix lesioned rats. Brain Res. 1995 Dec 24;(1–2):705. 235–40. doi: 10.1016/0006-8993(95)01157-9. [DOI] [PubMed] [Google Scholar]
- 47.Luntz-Leybman V, Bickford PC, Freedman R. Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res. 1992 Jul 31;587(1):130–6. doi: 10.1016/0006-8993(92)91437-j. [DOI] [PubMed] [Google Scholar]
- 48.Stevens KE, Bullock AE, Collins AC. Chronic corticosterone treatment alters sensory gating in C3H mice. Pharmacol Biochem Behav. 2001 Jul-Aug;69(3–4):359–66. doi: 10.1016/s0091-3057(01)00523-8. [DOI] [PubMed] [Google Scholar]
- 49.Schreiber R, Dalmus M, De Vry J. Effects of alpha 4/beta 2- and alpha 7-nicotine acetylcholine receptor agonists on prepulse inhibition of the acoustic startle response in rats and mice. Psychopharmacology (Berl) 2002 Jan;159(3):248–57. doi: 10.1007/s00213-001-0927-8. [DOI] [PubMed] [Google Scholar]
- 50.Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learn Mem. 1998 Sep-Oct;5(4–5):302–16. [PMC free article] [PubMed] [Google Scholar]
- 51.Chess AC, Bucci DJ. Increased concentration of cerebral kynurenic acid alters stimulus processing and conditioned responding. Behav Brain Res. 2006 Jun 30;170(2):326–32. doi: 10.1016/j.bbr.2006.03.006. Epub 2006 Apr 18. [DOI] [PubMed] [Google Scholar]
- 52.Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull. 2007 May;33(3):797–804. doi: 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chess AC, Landers AM, Bucci DJ. L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav Brain Res. 2009 Aug 12;201(2):325–31. doi: 10.1016/j.bbr.2009.03.013. [DOI] [PubMed] [Google Scholar]