Abstract
Of the major components of the kynurenine pathway for the oxidative metabolism of tryptophan, most attention has focussed on the N-methyl-D-aspartate (NMDA) receptor agonist quinolinic acid, and the glutamate receptor blocker kynurenic acid. However, there is increasing evidence that the redox-active compound 3-hydroxyanthranilic acid may also have potent actions on cell function in the nervous and immune systems, and recent clinical data show marked changes in the levels of this compound, associated with changes in anthranilic acid levels, in patients with a range of neurological and other disorders including osteoporosis, chronic brain injury, Huntington’s disease, coronary heart disease, thoracic disease, stroke and depression. In most cases, there is a decrease in 3-hydroxyanthranilic acid levels and an increase in anthranilic acid levels. In this paper, we summarise the range of data obtained to date, and hypothesise that the levels of 3-hydroxyanthranilic acid or the ratio of 3-hydroxyanthranilic acid to anthranilic acid levels, may contribute to disorders with an inflammatory component, and may represent a novel marker for the assessment of inflammation and its progression. Data are presented which suggest that the ratio between these two compounds is not a simple determinant of neuronal viability. Finally, a hypothesis is presented to account for the development of the observed changes in 3-hydroxyanthranilic acid and anthranilate levels in inflammation and it is suggested that the change of the 3HAA:AA ratio, particularly in the brain, could possibly be a protective response to limit primary and secondary damage.
Keywords: anthranilic acid, 3-hydroxyanthranilic acid, inflammation, Huntington’s disease, stroke, kynurenines
Introduction
In many organs and tissues, the major route for the metabolism of tryptophan is the kynurenine pathway. The initial enzymes for this pathway are indoleamine-2.3-dioxygenase (IDO), present in most organs and tissues except the liver, and tryptophan-2,3-dioxygenase (TDO). The latter is present almost exclusively in the liver, although low levels—usually associated with the presence of specific cell types such as leucocytes or endothelial cells—have been demonstrated in areas such as the CNS.1–3 This includes the demonstration using immunohistochemistry, of TDO occurrence in neurons.1
Although recognised for many years as an important route for the endogenous synthesis of nicotinic acid and, thus, of the vital co-factor nicotinamide adenine dinucleotide (NAD), it was only in 1981 that specific actions on cellular receptors were discovered, with the demonstration that quinolinic acid was an agonist at receptors for the neurotransmitter glutamate, specifically those sensitive to N-methyl-D-aspartate (NMDA).4 A more extensive screening of the kynurenine pathway components then led to the discovery that kynurenic acid was an antagonist at glutamate receptors,5 with later work showing a preference for blocking NMDA receptors at the co-agonist site for glycine (the glycineB or strychnine-resistant glycine receptors).6
Since that early work, these two tryptophan metabolites have been implicated in a wide range of neurological and psychiatric disorders,7–10 but it has also been recognised that other components of the kynurenine pathway, notable 3-hydroxykynurenine and 3-hydroxyanthranilic acid are highly redox-active, and might play a role in the regulation of oxidative stress.11,12 In this paper, a possible rationale for these latter compounds playing a significant role in disorders with an inflammatory component will be presented, together with a hypothesis of how changes in their endogenous levels arise.
The 3-hydroxyanthranilic Acid: Anthranilic Acid Ratio
Human studies
Osteoporosis
The first indication that 3-hydroxyanthranilic acid may be of significance in a disease process arose from a study of a peripheral disorder, osteoporosis. This disorder is most frequently observed in post-menopausal women and elderly men, and has been claimed to have a substantial inflammatory component. It is also a common consequence of treating patients with corticosteroid drugs.
In a series of 29 patients (compared with 10 control subjects), none of whom was consuming steroids at the time, blood samples were taken at the initial clinical presentation and formal diagnosis of osteoporosis, which included a Dual-Energy X-ray Absorptiometry (DEXA) scan to determine bone density. The concentrations in the plasma of the major components of the kynurenine pathway were subsequently measured by HPLC.13 Similar analyses were made of samples taken from the same patients after 2 years of standard pharmacological treatment with either the bisphosphonate drug etidronate, or the selective oestrogen receptor modulator (SERM) drug, raloxifene—currently accepted therapies at the time of this study.
One of the most striking changes noted was that at the time of diagnosis, those patients with osteoporosis exhibited much lower baseline levels of 3-hydroxyanthranilic acid (1.04 ± 0.10 nM) when compared with the healthy controls i.e. (7.89 ± 1.15 nM). In contrast, the levels of anthranilic acid were substantially increased (139.2 ± 14.7 nM) compared with controls (21.56 ± 2.25 nM) and the 3HAA: AA ratio had reversed.
Following the 2 year period of therapy, a repeated DEXA scan confirmed significant improvements in bone density in those patients receiving etidronate. The biochemical analyses revealed that the levels of 3-hydroxyanthranilic acid, anthranilic acid, and the 3HAA:AA ratio were all comparable with control values. In addition, the levels of tryptophan itself had increased significantly when compared with the original, baseline values (see Table 1).
Table 1.
Levels (mean ± s.e. mean) and ratios of 3-hydroxyanthranilic acid (3HAA) and anthranilic acid (AA) in control subjects and patients with various clinical conditions.
| Medical condition | Mean 3HAA level (patients) nmol/l | Mean 3HAA level (controls) nmol/l | Mean AA level (patients) nmol/l | Mean AA level (controls) nmol/l | Mean 3HAA: AA ratio (patients) | Mean 3HAA: AA ratio (controls) |
|---|---|---|---|---|---|---|
| Stroke (baseline) | ||||||
| T: total (n = 50) | T: 9.62 ± 1.22 | 23.74 ± 1.55 | T: 54.66 ± 9.15 | 28.59 ± 1.63 | T: 1:5.68 | 1:1.20 |
| I: ischaemic (n = 36) | I: 10.4 ± 1.45 | I: 55.2 ± 11.66 | I: 1:5.31 | |||
| H: haemorrhagic (9) | H: 5.44 ± 2.17 | H: 54.08 ± 6.15 | H: 1:9.94 | |||
| C: controls (n = 35) | ||||||
| Stroke (14 days) | T: 10.07 ± 1.32 | T: 40.89 ± 8.32 | T: 1:4.06 | 1:1.20 | ||
| I: 10.18 ± 1.69 | I: 35.05 ± 4.68 | I: 1:3.44 | ||||
| H: 9.52 ± 2.78 | H: 25.4 ± 4.92 | H: 1:2.67 | ||||
| Chronic brain | 1.52 ± 0.26 | 7.71 ± 1.21 | 105.65 ± 8.8 | 72.85 ± 5.72 | 1:69.51 | 1:9.45 |
| Injury (n = 15) | ||||||
| Controls (n = 15) | ||||||
| Huntington’s disease 1 | 1.29 ± 0.77 | 7.71 ± 1.21 | 108.06 ± 21.77 | 72.85 ± 5.72 | 1:83.77 | 1:9.45 |
| HD (n = 11) | ||||||
| Controls (n = 15) | ||||||
| Huntington’s disease 2* | ||||||
| group O–ve (n = 29) | 0–28.45 ± 1.88 | 26.26 ± 2.71 | 0–23.43 ± 1.78 | 23.06 ± 2.58 | 1:0.82 | 1:0.88 |
| group O+ve (n = 19) | 0 + 28.37 ± 3.06 | 0 + 20.94 ± 1.21 | 1:0.74 | |||
| group 1+ (n = 14) | 1 + 25.94 ± 2.73 | 1 + 21.94 ± 1.87 | 1:0.85 | |||
| group 2+ (n = 40) | 2 + 24.65 ± 1.8 | 2 + 23.42 ± 1.21 | 1:0.95 | |||
| Controls (n = 11) | ||||||
| Coronary bypass (n = 28) | 9.04 ± 0.91 | 33.25 ± 3.02 | 1:3.68 | |||
| Thoracic surgery (n = 28) | 6.28 ± 0.76 | 34.69 ± 2.74 | 1:5.54 | |||
| Depression | ||||||
| SSRI (n = 19) | 21.06 ± 1.64 | 23.20 ± 2.04 | 28.33 ± 4.8 | 24.64 ± 2.93 | 1:1.35 | |
| SSRI + T3 (n = 9) | 24.98 ± 3.88 | 27.39 ± 3.88 | 1:1.10 (on drugs) | |||
| Controls (n = 18) | 1:1.06 (not on drugs) | |||||
| Osteoporosis | 1.04 ± 0.13 | 7.89 ± 1.15 | 139.2 ± 14.7 | 21.56 ± 2.25 | 1:134 | 1:2.73 |
| Patients (n = 29) | ||||||
| Controls (n = 10) |
Huntington’s disease 2.
Group O–ve = Gene –ve asymptomatic HD family member.
Group O+ve = Gene +ve, asymptomatic HD family member.
Group 1+ = Gene +ve mildly affected HD family member.
Group 2+ = Gene +ve severely affected HD family member.
Huntington’s disease and brain injury patients
In parallel with this study, an analysis of the kynurenine pathway was also undertaken in patients with severe Huntington’s disease,14 the patients being sufficiently disabled that they were permanently hospitalised, and also in a group of patients with head injuries sustained at least one year before blood sampling.15 In both groups of patients, i.e end stage Huntington’s disease and chronic brain injury patients, one of the most consistent and marked changes in the kynurenine pathway proved to be in the pair of metabolites, 3-hydroxyanthranilic acid and anthranilic acid, with the ratio between these being reversed with AA levels being higher than 3-hydroxyanthranilic acid levels, which contrasts with the 3HAA:AA ratio in healthy control subjects (Table 1).
In another study of Huntington’s disease (HD) patients16 (including HD gene negative and HD gene positive patients, the latter with mild, moderate or severe disease), there was no change in the 3HAA: AA ratio, a finding which may attributable to the fact that many patients in this second study were at early stages of the disease progression. This would be consistent with the finding in the same study of highly significant correlations between tryptophan levels in the blood and disease severity as indicated by the number of CAG motif repeats (negative correlation, p = 0.0004), and between the kynurenine:tryptophan ratio and CAG repeat length (positive correlation, p = 0.0025).16
In neither of these two patient groups is there any available pharmacological strategy for significantly improving the long-term outcome of the brain injury or neurodegeneration, so therapeutic studies to assess responses to drug therapies as performed in osteoporosis patients (above) are not currently possible.
Stroke
In a later study of stroke injury, a series of patients experiencing a stroke (or relatives of the patients) consented to the taking of a series of blood samples during hospitalisation. The results indicated not only that the kynurenine pathway had indeed been activated by the development or occurrence of the infarct, since the kynurenine:tryptophan ratio was very significantly raised, but also that the ratio of 3-hydroxyanthranilic acid to anthranilic acid was again decreased to about 20% of control levels at 24 hours after the stroke, irrespective of whether the latter was ischaemic or haemorrhagic.17
Several additional features of the 3HAA:AA ratio were striking in these patients. Firstly, the 3HAA: AA ratio remained substantially lower than control subjects at 14 days after the stroke. Secondly, a subgroup analysis revealed that the most severely affected patients—those who died within 21 days of the stroke—exhibited values for the 3HAA:AA ratio which were significantly lower than those patients who survived beyond this time.17 It was also interesting to see that patients who did not survive the stroke had significantly higher levels of kynurenic acid than those who did survive, a fact which tempts speculation about the potentially protective role of kynurenic acid in acute stroke patients.17 Lastly, a comparison between the 3HAA:AA ratio and the size of the cerebral infarct, estimated from CT scans, indicated consistent, statistically significant negative correlations between 3-hydroxyanthranilic acid levels and infarct size up to 7 days following the stroke, with Spearman correlation coefficients of −0.466 (P < 0.05) at 24 hours, −0.433 (P < 0.01) at 48 hours, −0.359 (P < 0.05) at 72 hours, and −0.478 (P < 0.05) at 7 days.
Cardiac bypass or thoracic surgery
In a further clinical study, as yet unpublished, the 3HAA:AA ratio was also reversed in two groups of patients, those about to undergo coronary by-pass grafting and those about to have surgery for other intrathoracic pathologies, frequently neoplastic.
Depression
Finally, in a clinical study of non-hospitalised patients suffering from depression the 3HAA:AA ratio was again reversed before drug treatment or counselling had begun.18
Experimental study
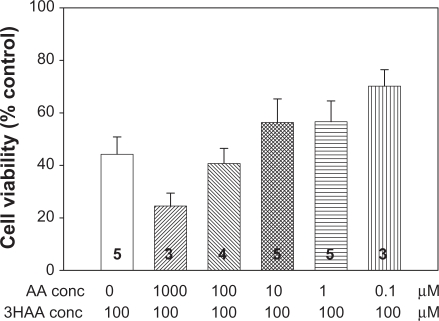
In order to determine whether the ratio between 3-hydroxyanthranilic acid and anthranilic acid had any effects on neuronal viability, cerebellar granule neurons were cultured from neonatal rats and maintained in culture for 9 days as described previously19 before different concentrations and ratios of 3-hydroxyanthranilic acid and anthranilic acid were added. These compounds were left in contact with the neurons for 5 hours, after which the viability of the neurons was examined using the Alamar Blue (Bio-source) assay. The results are summarised in Figure 1, which indicates that, although there was a tendency for viability to decrease after exposure to higher concentrations of the two compounds, there was no overall evidence for a significant deleterious effect of any concentration, or combination of concentrations, on viability. In particular, there was no indication that the combination of these two substances generated neuronal death at levels greater than either compound alone, nor was there any indication that the toxic effects of 3-hydroxyanthranilic acid which have been reported on some cell types20 were evident on cerebellar granule neurons and could be exacerbated or prevented by anthranilic acid.
Figure 1.
Neurotoxic effects of 3-hydroxyanthranilic acid/anthranilic acid combinations. The effects on neuronal viability (cerebellar granule neurons) of different ratios of AA and 3-hydroxyanthranilic acid. In these selected examples of a wider series, 3-hydroxyanthranilic acid (3HAA) at a concentration of 100 μM is illustrated in combination with anthranilic acid (AA) at concentrations ranging from 0 to 1000 μM. Although there is a clear trend for the higher concentration to increase 3-hydroxyanthranilic acid—induced neurotoxicity, lower concentrations tend to reduce the damage, although none reached statistical significance in this series. Although the range of concentrations illustrated showed no significant interaction, lower concentrations which were closer to those measured in human blood, also showed no apparent interaction in this system. No clear, significant interactions were seen when the ratios of 3HAA:AA were reversed. The number of experiments is indicated within each column. (Adapted from A J Smith, PhD thesis, University of Glasgow, 2008).
Clearly these remain relatively preliminary data and several factors could account for these apparently negative results. Most obviously perhaps is the fact that there may be some degree of differential loss of the two compounds in the culture medium over the time course of this work. 3-hydroxyanthranilic acid, for example, is much less stable than anthranilic acid in aqueous solution (see below), with auto-oxidation producing a rapid loss of 3-hydroxyanthranilic acid compared with anthranilic acid.21,22 It would be of interest to assess the levels of both compounds at different stages of the cell culture experiments to address this question, and to ensure a frequent replenishment of the 3-hydroxyanthranilic acid so as to maintain a consistent balance with the level of anthranilic acid.
Biological importance of 3-hydroxyanthranilic acid: anthranilic acid ratio
The existence of such marked changes in 3-hydroxyanthranilic acid and anthranilic acid in a range of clinical disorders suggests a possible relevance to the disease process or recovery, especially since the changes were almost invariably reciprocal, with low 3-hydroxyanthranilic acid levels and raised AA levels. This degree of reciprocity suggests a consistent biochemical change, rather than purely random changes in the levels of one or other of these compounds.
There are two possible reasons why 3-hydroxyanthranilic acid in particular might be relevant to the disease process. Firstly, it is a highly reactive compound, which readily auto-oxidises to a dimeric analogue, cinnabarinic acid.21,22 The auto-oxidation process has been studied in some detail by Dykens et al21 and Iwahashi et al23 who have noted the ability of ambient redox conditions to affect the rate of transformation, especially in the face of changed levels of superoxide dismutase activity. It is probably the ability of 3-hydroxyanthranilic acid to modify the local redox environment which accounts for its toxic effects on some cell types.20 Whether 3-hydroxyanthranilic acid is pro- or anti-oxidant24 in any one situation will depend, as for any other compound, on the local redox conditions.
Secondly, 3-hydroxyanthranilic acid and anthranilic acid are known to interact at the level of 3-hydroxyanthranilic acid oxidase (3HAO). This enzyme is known to exist in central neurons and anthranilic acid is an effective inhibitor, reducing the conversion of 3-hydroxyanthranilic acid to quinolinic acid and picolinic acid.25 Using a concentration of 300 μM 3-hydroxyanthranilic acid as substrate for 3HAO, anthranilic acid is a competitive inhibitor with a Ki of 40 μM.26 The ratio of 300 μM substrate to 40 μM inhibitor Ki indicates that anthranilic acid is highly effective as an inhibitor at around 13% of the natural substrate. At the ratio seen in blood samples, therefore, in which anthranilic acid concentrations normally equal or exceed 3-hydroxyanthranilic acid levels by up to 5-fold (Table 1), there should be substantial overall inhibition of the enzyme. It is likely that the enzyme status in vivo will be determined by a plethora of factors and co-factors which make this conclusion more or less correct, but it remains probable that in normal humans the conversion of 3-hydroxyanthranilic acid to quinolinate will be limited by endogenous levels of anthranilate.
It is interesting to speculate, therefore, that in the patient groups, in whom the ratio of 3HAA:AA is invariably less than in control subjects (Table 1), this limitation of quinolinic acid formation may reflect a compensatory mechanism to reduce cell toxicity.
The immune system
Thirdly, changes in the levels of 3-hydroxyanthranilic acid are increasingly recognised as being highly active in modulating the activity of T cells in the immune system. It has the ability to depress the release of cytokines from both T helper-1 (Th1) cells and Th2 cells, although in early or mild cases of inflammation the major effect seems to be on Th1, whereas more advanced, chronic inflammation is usually associated with a preferential action on Th2 cells.27,28 In addition, 3-hydroxyanthranilic acid can have a direct anti-proliferative effect on Th1 and Th2 cells under the same—acute or chronic—inflammatory conditions.28–30 At the molecular level it has also been demonstrated that 3-hydroxyanthranilic acid can suppress the activation of the pro-inflammatory transcription factor Nuclear Factor kappa-B (NFκB),28,31 as well as inhibiting nitric oxide synthase.31,32 There is also a highly specific ability to inhibit the stimulation of T cell activity by dendritic cells.33,34 The secretion of monocyte chemoattractant protein-1 (MCP-1) is depressed by 3-hydroxyanthranilic acid, an effect probably mediated via the induction of haem oxygenase-1.35 Changing levels of 3-hydroxyanthranilic acid could, therefore, exert a profound effect on the overall activity of T cells and the balance of Th1 and Th2 cell activity.33,34
Mechanisms of changing levels
At present there is no clear indication of the biochemical mechanism which leads to the change in levels of 3-hydroxyanthranilic acid and anthranilic acid. Baran and Schwarcz36 were able to demonstrate that, when added to brain homogenates, anthranilic acid could be readily converted into 3-hydroxyanthranilic acid, a transformation which they attributed to a hypothetical anthranilate oxidase activity. In principle, a loss of such activity would lead to the fall in 3-hydroxyanthranilic acid levels and the accumulation of anthranilic acid.
In addition, the ability of anthranilic acid to inhibit 3HAO as discussed above, means that any change in the level of the former will have a direct influence not only as described in the previous paragraph, on 3-hydroxyanthranilic acid levels but also on the production of quinolinic acid from 3-hydroxyanthranilic acid, with secondary effects on nicotinic acid and NAD levels as well as the potential for quinolinic acid to produce activation and excitotoxicity of neurones in the central and peripheral systems.
It is possible that the levels and availability of iron may also play a substantial role in determining both the absolute and relative levels of 3-hydroxyanthranilic acid and anthranilic acid. Several metal ions known to inhibit 3HAO, iron (Fe3+) being one of the most potent. This ion produces a non-competitive inhibition with a Km for iron of 6.3 μM. It is relevant then that anthranilic acid is able to chelate metal ions, especially Fe3+, and is able to promote the reduction of Fe3+ to Fe2+ in aqueous solution.37 This may also be relevant to the cell culture results described earlier, since the higher concentrations of anthranilic acid may have chelated a significant fraction of the iron required for neuronal survival in vitro. Indeed, the high levels of anthranilic acid generated by the inhibition of kynurenine-3-monoxygenase in an in vivo model of cerebral malaria38 could have chelated enough circulating iron to contribute to the anaemia reported in that study. Similar metal interactions have been proposed as having important physiological relevance in the mechanism of transmembrane transport of those ions39 so that a range of metabolic consequences might result from a general disturbance of metal ion absorption or availability to the intracellular milieu.
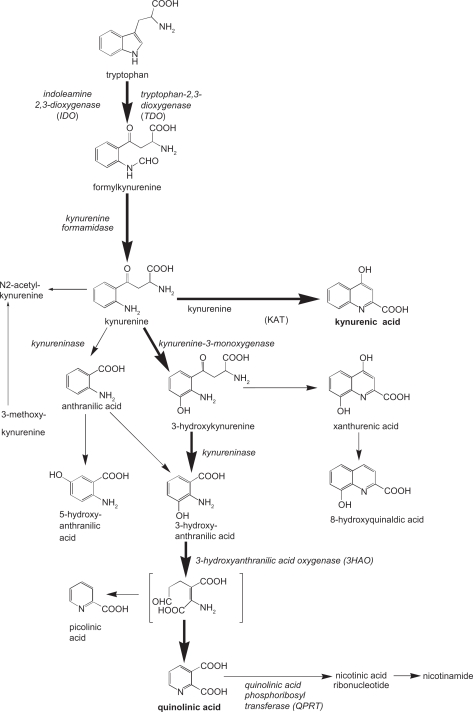
Another—not necessarily contradictory—hypothesis might be based around the role of riboflavin (vitamin B2) as a co-factor for the enzyme kynurenine-3-monoxygenase (KMO).40,41 There is an existing literature on the fact that infection or inflammation is associated with a marked loss of intracellular stores of riboflavin.42 Indeed, an abundant supply of intracellular riboflavin is required for effective resistance to infection,43 with riboflavin depletion affording a loss of that resistance44 It is, therefore, tempting to hypothesise that the onset of infection or inflammation is associated with a loss of intracellular riboflavin, which results in a decreased activity of KMO. This would reduce the conversion of kynurenine to 3-hydroxykynurenine (and thus to 3-hydroxyanthranilic acid), and drive kynurenine along the accepted alternative route to anthranilic acid (Fig. 2).
Figure 2.
Key elements of the kynurenine pathway.
This proposal is entirely consistent with existing experimental data, since it has been shown that the administration of a compound which inhibits KMO (Ro61-8048), does generate a massive 40-fold increase of brain anthranilic acid levels (from 2.0 ± 0.3 to 76.9 ± 23.3 fmols/mg protein), which is in turn increased a further 6-fold (to 487.0 ± 96 fmols/mg protein) in animals infected with malaria parasites.38
Implications for therapy
If this hypothesis is correct, then the administration of riboflavin might correct the cellular loss of KMO activity, restoring 3-hydroxyanthranilic acid levels to normal, correcting any induced changes in T cell function, and reducing overall levels of inflammation.
Of course, the changed 3-hydroxyanthranilic acid: anthranilic acid ratio may simply be an epiphenomenon, reflecting other, more important cellular changes which occur as a result of riboflavin depletion but without being critical to the overall degree of inflammatory activity. However, the therapeutic implication may still be correct, that riboflavin could be an effective corrective therapy, or adjunctive therapy, for some inflammatory disorders whatever the primary cellular machinery which is involved.
Summary
We present data to show that in a wide range of clinical diseases—chronic brain injury, Huntington’s disease, stroke, depression, coronary heart disease, intrathoracic disease including neoplasia and osteoporosis the normal ratio between 3-hydroxyanthranilic acid and anthranilic acid is changed, with lower levels of 3-hydroxyanthranilic acid and higher levels of anthranilic acid than normal.
The exact reasons for this reversed ratio are uncertain but a decreased 3HAA:AA ratio should produce a ‘cleaning up’ effect after insult or injury, antagonism of quinolinic acid toxicity, reduction in oxidative stress, protection against immunostimulation, immunosuppression and a reduced inflammatory response. All of these actions could protect against primary and secondary damage after an insult, and would be especially relevant in the brain.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors report no conflicts of interest.
References
- 1.Miller CL, Llenos IC, Dulay JR, Weiss S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073:25–37. doi: 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 2.Guillemin GJ, Cullen KM, Lim CK, et al. Characterization of the kynurenine pathway in human neurons. J Neurosci. 2007;27:12884–92. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanai M, Nakamura T, Funakoshi H. Identification and characterization of novel variants of the tryptophan-2,3-dioxygenase gene: Differential regulation in the mouse nervous system during development. Neurosci Res. 2009;64:111–7. doi: 10.1016/j.neures.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Stone TW, Perkins MN. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Europ. J Pharmacol. 1981;72:411–2. doi: 10.1016/0014-2999(81)90587-2. [DOI] [PubMed] [Google Scholar]
- 5.Perkins MN, Stone TW. An iontophoretic investigation of the action of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Research. 1982;247:184–7. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- 6.Birch PJ, Grossman CJ, Hayes AG. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine site. Europ J Pharmacol. 1988;154:85–7. doi: 10.1016/0014-2999(88)90367-6. [DOI] [PubMed] [Google Scholar]
- 7.Stone TW. Kynurenines in the CNS—from obscurity to therapeutic importance. Progr in Neurobiol. 2001;64:185–218. doi: 10.1016/s0301-0082(00)00032-0. [DOI] [PubMed] [Google Scholar]
- 8.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nature Reviews Drug Discovery. 2002;1:609–20. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 9.Moroni F. Tryptophan metabolism and brain function: focus on kynurenine and other indole metabolites. Europ J Pharmacol. 1999;375:87–100. doi: 10.1016/s0014-2999(99)00196-x. [DOI] [PubMed] [Google Scholar]
- 10.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: Glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Therap. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 11.Giles GI, Collins CA, Stone TW, Jacob C. Electrochemical and in vitro evaluation of the redox properties of kynurenine species. Biochem Biophys Res Comm. 2003;300:719–24. doi: 10.1016/s0006-291x(02)02917-0. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein LE, Leopold MC, Huang X, et al. 3-hydroxykynurenine and 3-hydroxyanthranilic acid generate hydrogen peroxide and promote α-crystallin cross-linking by metal ion reduction. Biochemistry. 2000;39:7266–75. doi: 10.1021/bi992997s. [DOI] [PubMed] [Google Scholar]
- 13.Forrest CM, Mackay GM, Oxford L, Stoy N, Stone TW, Darlington LG. Kynurenine pathway metabolism in patients with osteoporosis after two years of drug treatment. Clin Exp Pharmacol Physiol. 2006;33:1078–87. doi: 10.1111/j.1440-1681.2006.04490.x. [DOI] [PubMed] [Google Scholar]
- 14.Stoy N, Mackay GM, Forrest CM, et al. Tryptophan metabolism and oxidative stress in patients with Huntington’s disease. J Neurochem. 2005;93:611–23. doi: 10.1111/j.1471-4159.2005.03070.x. [DOI] [PubMed] [Google Scholar]
- 15.Mackay GM, Forrest CM, Stoy N, et al. Tryptophan metabolism and oxidative stress in patients with chronic brain injury. Europ J Neurol. 2006;13:30–42. doi: 10.1111/j.1468-1331.2006.01220.x. [DOI] [PubMed] [Google Scholar]
- 16.Forrest CM, Mackay GM, Stoy N, et al. Blood levels of kynurenines, interleukin IL-23 and sHLA-G at different stages of Huntington’s disease. J Neurochem. 2009;112:112–22. doi: 10.1111/j.1471-4159.2009.06442.x. [DOI] [PubMed] [Google Scholar]
- 17.Darlington LG, Mackay GM, Forrest CM, Stoy N, George C, Stone TW. Altered kynurenine metabolism correlates with infarct volume in stroke. Europ J Neurosci. 2007;26:2211–21. doi: 10.1111/j.1460-9568.2007.05838.x. [DOI] [PubMed] [Google Scholar]
- 18.Mackay GM, Forrest CM, Christofides J, et al. Kynurenine metabolites and inflammation markers in depressed patients treated with fluoxetine or counselling. Clin Exp Pharmacol Physiol. 2008;36:425–35. doi: 10.1111/j.1440-1681.2008.05077.x. [DOI] [PubMed] [Google Scholar]
- 19.Smith AJ, Smith RA, Stone TW. 5-Hydroxyanthranilic Acid, a tryptophan metabolite, generates oxidative stress and neuronal death via p38 activation in cultured cerebellar granule neurons. Neurotox Res. 2009;15:303–10. doi: 10.1007/s12640-009-9034-0. [DOI] [PubMed] [Google Scholar]
- 20.Okuda S, Nishiyama N, Saito H, Katsuki H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J Neurochem. 1998;70:299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [DOI] [PubMed] [Google Scholar]
- 21.Dykens JA, Sullivan SG, Stern A. Oxidative reactivity of the tryptophan metabolites 3-hydroxyanthranilate, quinolinate and picolinate. Biochem Pharmacol. 1987;36:211–7. doi: 10.1016/0006-2952(87)90691-5. [DOI] [PubMed] [Google Scholar]
- 22.Ishii T, Iwahashi H, Sugata R, Kido R, Fridovich I. Superoxide disumutases enhance the rat of autoxidation of 3-hydroxyanthranilic acid. Arch Biochem Biophys. 1990;276:248–50. doi: 10.1016/0003-9861(90)90034-v. [DOI] [PubMed] [Google Scholar]
- 23.Iwahashi H, Ishii T, Sugata R, Kido R. Superoxide dismutase enhances the formation of hydroxyl radicals in the reaction of 3-hydroxyanthranilic acid with molecular oxygen. Biochem J. 1988;251:893–9. doi: 10.1042/bj2510893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leipnitz G, Schumacher C, Dalcin KB, et al. In vitro evidence for an anti-oxidant role of 3-hydroxykynurenine and 3-hydroxyanthranilic acid in the brain. Neurochem Intern. 2006;50:83–94. doi: 10.1016/j.neuint.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Guillemin GJ, Cullen KM, Lim CK, et al. Characterisation of the kynurenine pathway in humans. J Neurosci. 2007;27:12884–92. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vescia A, de Prisco G. Studies on purified 3-hydroxyanthranilic acid oxidase. J Biol Chem. 1962;237:2318–24. [PubMed] [Google Scholar]
- 27.Dai XC, Zhu BT. Suppression of T-cell response and prolongation of allograft survival in a rat model by tryptophan catabolites. Europ J Pharmacol. 2009;606:225–32. doi: 10.1016/j.ejphar.2008.12.053. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi T, Mo J-H, Gong X, et al. 3-hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Nat Acad Sci U S A. 2007;104:18619–24. doi: 10.1073/pnas.0709261104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morita T, Saito K, Takemura M, et al. 3-hydroxyanthranilic acid, an L-tryptophan metabolite, induces apoptosis in monocyte-derived cells stimulated by interferon-γ. Ann Clin Biochem. 2001;38:242–51. doi: 10.1258/0004563011900461. [DOI] [PubMed] [Google Scholar]
- 30.Fallarino I, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Diff. 2002;9:1069–77. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 31.Sekkai D, Guittet O, Lemaire G, Tenu J-P, Lepoivre M. Inhibition of nitric oxide synthase expression and activity in macrophages by 3-hydroxyanthranilic acid, a tryptophan metabolite. Arch Biochem Biophys. 1997;340:117–23. doi: 10.1006/abbi.1997.9913. [DOI] [PubMed] [Google Scholar]
- 32.Oh GS, Pae HO, Choi BM, et al. 3-hydroxyanthranilic acid, one of the metabolites of tryptophan via indolamine-2,3-dioxygenase pathway, suppresses inducible nitric oxide synthase expression by enhancing heme oxygenase-1 expression. Biochem Biophys Res Comm. 2004;320:1156–62. doi: 10.1016/j.bbrc.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 33.Lopez AS, Alegre E, Diaz-Lagares A, et al. Effect of 3-hydroxyanthranilic acid in the immunosuppressive molecules indoleamine dioxygenase and HLA-G in macrophages. Immunol Lett. 2008;117:91–5. doi: 10.1016/j.imlet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Lopez AS, Alegre E, LeMaoult J, et al. Regulatory role of tryptophan degradation pathway in HLA-G expression by human monocyte-derived dendritic cells. Molec Immunol. 2006;43:2151–60. doi: 10.1016/j.molimm.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Pae HO, Oh GS, Lee BS, Rim JS, Kim YM, Chung HT. 3-hydroxyanthranilic acid, one of the tryptophan metabolites, inhibits monocyte chemoattractant protein-1 secretion and vascular cell adhesion molecule-1 expression via heme oxygenase-1 induction in human umbilical vein endothelial cells. Atherosclerosis. 2006;187:274–84. doi: 10.1016/j.atherosclerosis.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Baran H, Schwarcz R. Evidence for the preferential production of 3-hydroxyanthranilic acid from anthranilic acid in the rat brain. Adv Exp Med Biol. 1991;294:485–8. doi: 10.1007/978-1-4684-5952-4_50. [DOI] [PubMed] [Google Scholar]
- 37.Kamneva AA, Kuzmann E. Mossbauer spectroscopic evidence for the reduction of iron(III) by anthranilic acid in aqueous solution. Polyhedron. 1997;16:3353–6. [Google Scholar]
- 38.Clark CJ, Mackay GM, Smythe GA, Bustamante S, Stone TW, Phillips RS. Prolonged survival of a murine model of cerebral malaria by kynurenine pathway inhibition. Infection and Immunity. 2005;73:5249–51. doi: 10.1128/IAI.73.8.5249-5251.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LeVier K, Day DA, Guerinot ML. Iron uptake by symbiosomes from soybean root nodules. Plant Physiol. 1996;111:893–900. doi: 10.1104/pp.111.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charconnet-Harding F, Dalgliesh CE, Neuberger A. The relation between riboflavin and tryptophan metabolism, studied in the rat. Biochem J. 1953;53:513–21. doi: 10.1042/bj0530513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevens CO, Henderson LM. Riboflavin and hepatic kynurenine hydroxylase. J Biol Chem. 1959;234:1191–4. [PubMed] [Google Scholar]
- 42.Brijal S, Lakshmi AV. Tissue distribution and turnover of [3H]riboflavin during respiratory infection in mice. Metab Clin Exp. 1999;48:1608–11. doi: 10.1016/s0026-0495(99)90253-6. [DOI] [PubMed] [Google Scholar]
- 43.Verdrengh M, Tarkowski A. Riboflavin in innate and acquired immune responses. Inflamm Res. 2005;54:390–3. doi: 10.1007/s00011-005-1372-7. [DOI] [PubMed] [Google Scholar]
- 44.Pinkerton H, Bessey OA. The loss of resistance to murine typhus infection resulting from riboflavin deficiency in rats. Science. 1939;89:368–70. doi: 10.1126/science.89.2312.368. [DOI] [PubMed] [Google Scholar]