Abstract
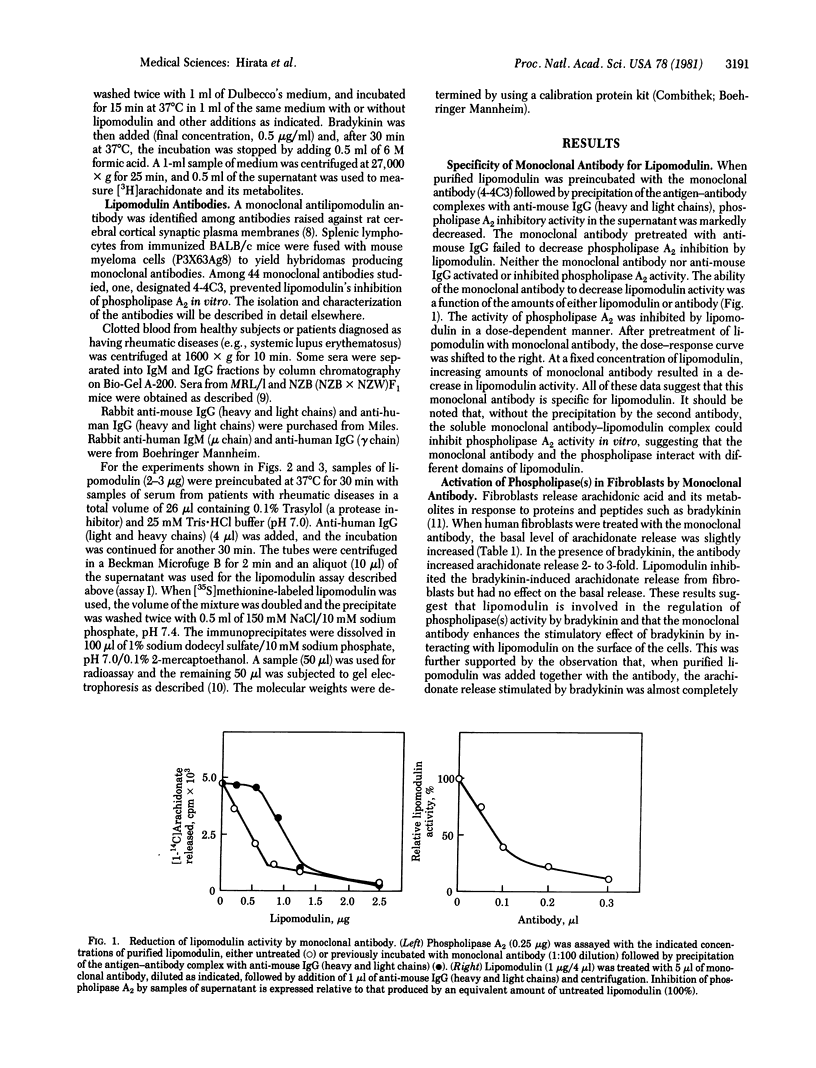
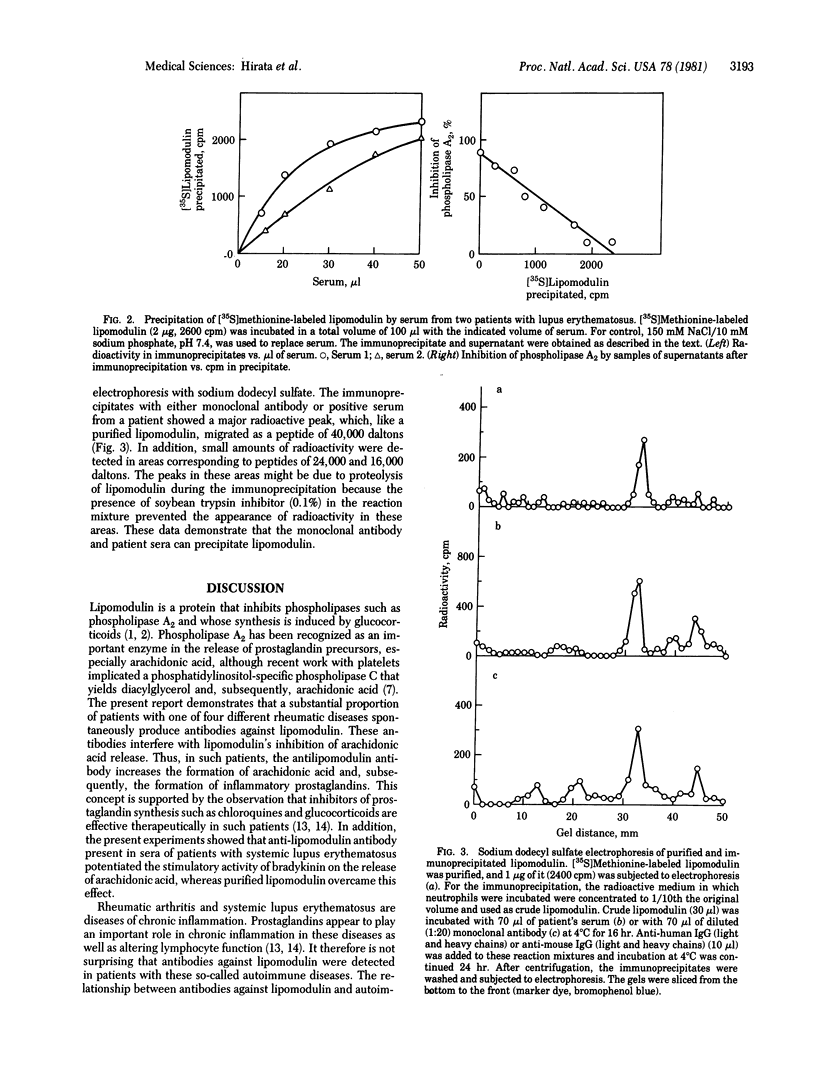
The activity of phospholipase inhibitory protein, lipomodulin, partially purified from rabbit neutrophils, was markedly decreased after treatment with sera from patients with rheumatic diseases such as systemic lupus erythematosus, rheumatoid arthritis, and dermatomyositis. The decrease of the protein's inhibitory activity on phospholipase A2 paralleled the amount of [35S]methionine-labeled lipomodulin precipitated by the sera. Absorption of patients' sera with anti-human IgM (mu chain) or protein A-agarose, but not with anti-human IgG (gamma chain), decreased their ability to decrease the activity of lipomodulin on phospholipase A2 or to precipitate the radioactive lipomodulin. The IgM fraction of patients' sera could precipitate [35S]methionine-labeled lipomodulin (40,000 daltons) which comigrated with highly purified lipomodulin on gel electrophoresis with sodium dodecyl sulfate. All of these observations suggest that the sera of many patients with rheumatic diseases contain autoantibody against lipomodulin. A monoclonal antibody against lipomodulin was also obtained. Stimulating human fibroblasts with bradykinin in the presence of monoclonal antilipomodulin antibody markedly enhanced arachidonic acid release due to the activation of phospholipase(s) in the intact cells, and this stimulatory effect was blocked by adding purified lipomodulin. These findings suggest that lipomodulin regulates the activity of phospholipase(s) on the cell surface and that autoantibodies against lipomodulin may play a role in certain symptoms of rheumatic diseases, especially by the formation of prostaglandins and other metabolites of arachidonic acid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell G. J., Carnuccio R., Di Rosa M., Flower R. J., Parente L., Persico P. Macrocortin: a polypeptide causing the anti-phospholipase effect of glucocorticoids. Nature. 1980 Sep 11;287(5778):147–149. doi: 10.1038/287147a0. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Kahn C. R., Roth J., Bar R. S. Antibodies that impair insulin receptor binding in an unusual diabetic syndrome with severe insulin resistance. Science. 1975 Oct 3;190(4209):63–65. doi: 10.1126/science.170678. [DOI] [PubMed] [Google Scholar]
- Hirata F., Schiffmann E., Venkatasubramanian K., Salomon D., Axelrod J. A phospholipase A2 inhibitory protein in rabbit neutrophils induced by glucocorticoids. Proc Natl Acad Sci U S A. 1980 May;77(5):2533–2536. doi: 10.1073/pnas.77.5.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. L., Levine L. Stimulation of prostaglandin synthesis by bradykinin and thrombin and their mechanisms of action on MC5-5 fibroblasts. J Biol Chem. 1976 Sep 25;251(18):5814–5816. [PubMed] [Google Scholar]
- Huston D. P., Steinberg A. D. Animal models of human systemic lupus erythematosus. Yale J Biol Med. 1979 May-Jun;52(3):289–305. [PMC free article] [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Egan R. W. Prostaglandins, arachidonic acid, and inflammation. Science. 1980 Nov 28;210(4473):978–984. doi: 10.1126/science.6254151. [DOI] [PubMed] [Google Scholar]
- Patrick J., Lindstrom J., Culp B., McMillan J. Studies on purified eel acetylcholine receptor and anti-acetylcholine receptor antibody. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3334–3338. doi: 10.1073/pnas.70.12.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. R., Hall R. Thyroid-stimulating immunoglobulins in Graves' disease. Lancet. 1974 Aug 24;2(7878):427–431. doi: 10.1016/s0140-6736(74)91815-7. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Sadasivan R., Saito-Taki T., Stechschulte D. J., Balentine L., Helmkamp G. M., Jr Studies of Fc gamma receptors of human B lymphocytes: phospholipase A2 activity of Fc gamma receptors. Biochemistry. 1980 Dec 23;19(26):6037–6044. doi: 10.1021/bi00567a014. [DOI] [PubMed] [Google Scholar]
- Venter J. C., Fraser C. M., Harrison L. C. Autoantibodies to beta 2-adrenergic receptors: a possible cause of adrenergic hyporesponsiveness in allergic rhinitis and asthma. Science. 1980 Mar 21;207(4437):1361–1363. doi: 10.1126/science.6153472. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]


