Abstract
Pseudoxanthoma elasticum (PXE) is an autosomal recessive disorder in which calcification of connective tissue leads to pathology in skin, eye and blood vessels. PXE is caused by mutations in ABCC6. High expression of this transporter in the basolateral hepatocyte membrane suggests that it secretes an as-yet elusive factor into the circulation which prevents ectopic calcification. Utilizing our Abcc6 −/− mouse model for PXE, we tested the hypothesis that this factor is vitamin K (precursor) (Borst et al. 2008, Cell Cycle). For 3 months, Abcc6 −/− and wild-type mice were put on diets containing either the minimum dose of vitamin K required for normal blood coagulation or a dose that was 100 times higher. Vitamin K was supplied as menaquinone-7 (MK-7). Ectopic calcification was monitored in vivo by monthly micro-CT scans of the snout, as the PXE mouse model develops a characteristic connective tissue mineralization at the base of the whiskers. In addition, calcification of kidney arteries was measured by histology. Results show that supplemental MK-7 had no effect on ectopic calcification in Abcc6 −/− mice. MK-7 supplementation increased vitamin K levels (in skin, heart and brain) in wild-type and in Abcc6 −/− mice. Vitamin K tissue levels did not depend on Abcc6 genotype. In conclusion, dietary MK-7 supplementation increased vitamin K tissue levels in the PXE mouse model but failed to counteract ectopic calcification. Hence, we obtained no support for the hypothesis that Abcc6 transports vitamin K and that PXE can be cured by increasing tissue levels of vitamin K.
Keywords: Pseudoxanthoma elasticum, ABC transporter, Vitamin K, Ectopic calcification, Vascular calcification, Connective tissue, Cardiovascular, Vitamins, Calcium metabolism, Mouse models
Introduction
PXE is an autosomal recessive disease characterized by progressive mineralization of connective tissue [1–4]. Clinically important sites of calcification are elastic layers in skin, Bruch’s membrane of the eye and vascular arteries. The calcification leads to skin lesions, vision loss and various cardiovascular complications.
Positional cloning indicated that PXE is caused by mutations in ABCC6 [5, 6]. Inactivation of Abcc6 in the mouse resulted in the development of a PXE-like phenotype with calcification in eye, skin and blood vessels [7, 8]. This confirmed the role of ABCC6 in PXE and provided us with an animal model for PXE.
ABCC6 encodes a member of the ABCC family of organic anion transporters [9]. The protein is prominently expressed in liver and kidney but virtually absent from the affected sites [10–12]. In the liver, ABCC6 localizes to the basolateral membrane of the hepatocyte [7, 13], where it is supposed to transport its substrate from the cytoplasm into the circulation. Mutations that impair this transport function cause PXE [14]. These findings suggested that PXE is a metabolic disease brought about by changes in circulating factors that occur when the ABCC6 pump is not functioning properly [15]. Recent studies provide strong experimental evidence for this hypothesis. Serum from PXE patients caused abnormal elastic fibre formation in cultures of fibroblasts, irrespective of whether fibroblasts were derived from PXE patients or from unaffected individuals [16]. Jiang et al. [17] demonstrated that muzzle skin transplants of Abcc6 −/− mice grafted onto wild-type (wt) mice remained normal, whereas wt transplants grafted onto Abcc6 −/− mice developed ectopic calcification.
Several studies implicate vitamin K in the pathophysiology of PXE. An intriguing finding was that patients with a mutation in the gamma-glutamyl carboxylase (GGCX) gene develop PXE-like symptoms [18]. GGCX catalyzes the vitamin K-dependent carboxylation of glutamate residues to calcium-binding gamma-glutamate (Gla) residues. During this reaction, the hydroquinone form of vitamin K is oxidized to its epoxide form. A small number of proteins require γ-carboxylation for their activity. This group includes several clotting factors as well as proteins involved in calcification, such as matrix Gla-protein (MGP). MGP is an inhibitor of tissue calcification [19]. Interestingly, carboxylation of MGP was found to be reduced in PXE patients [20, 21] and in the Abcc6 −/− mouse [22]. Recently, Vanakker et al. [21] reported that PXE patients have reduced vitamin K serum levels.
These data suggest that PXE symptoms are caused by a local deficiency in vitamin K-dependent protein carboxylation. They support the “PXE-K hypothesis” [23], which postulates that vitamin K (or a precursor of vitamin K) is the substrate of the ABCC6 pump. It is known that vitamin K, once it is taken up by the digestive system and brought into the blood, is avidly taken up by the liver [24]. Here, it can be used in the production of clotting factors. According to the PXE-K hypothesis, ABCC6 protein exports vitamin K (precursor) from the hepatocyte back into the bloodstream. This will carry it to the periphery, where vitamin K dependent γ-carboxylation of MGP prevents calcification. Inactivation of ABCC6 would thus lead to shortage of vitamin K in extra-hepatic tissues and failure to prevent spontaneous calcification in these tissues.
The PXE-K hypothesis implies that providing the periphery with functional vitamin K will prevent PXE. In the present study, we tested this hypothesis by supplementing vitamin K to the animal model for PXE, the Abcc6 −/− mouse [7]. A high dose of vitamin K was added to the food, aiming to achieve high blood levels, which would allow for a sufficient amount of vitamin K to bypass the liver and reach the periphery. Vitamin K is a group name for several structurally related molecules, including phylloquinone (vitamin K1) and menaquinones (K2 vitamins) [24]. Menaquinones (MK) are classified by the length of their aliphatic side chain (MK-n). We chose MK-7 for the dietary supplementation. While all forms of vitamin K serve as a co-factor in the γ-carboxylation of glutamate, MK-7 has a relatively long half-life (3 days) in the blood [25, 26] suggesting that this form of K is apt to escape rapid sequestration by the liver and reach extra-hepatic, peripheral tissues. As biomarker for PXE symptoms in the mouse, we chose the mineralization of the connective tissue capsule surrounding the blood sinus at the base of the whiskers [27]. Mineralization was monitored in vivo by X-ray micro-computed tomography (micro-CT). After a 3-month diet, mice were sacrificed, and vitamin K content and tissue distribution were determined.
Materials and methods
Mice
Abcc6 −/− mice were generated in a hybrid background of C57BL/6 and 129/Ola and then backcrossed to C57BL/6 [7]. In the present study, we used mice that had been backcrossed to C57BL/6 for ten generations. Abcc6 proficient (+/+) littermates and pure C57BL/6 mice were used as wt control mice. Mice were bred in our animal facilities and kept in 12-h light–dark cycle of 50–80 lx with water and food (Teklad 2918, Harlan Teklad, Madison, WI) ad libitum. All animal studies were approved by an independent animal ethical committee under Dutch law.
Vitamin K supplementation
We designed two diets. One diet contained vitamin K in the minimum dose required (MDR) (1×MK-7 diet), the other 100 times as much (100×MK-7 diet). The diets were made by mixing MK-7 (Kind gift of J-Oil Mills, Nakashinden, Fukuroi City, Japan; dissolved in sunflower oil) with a mouse food lacking vitamin K (diet #4165.00, in powder form, Arie Blok Diervoeding, Woerden, NL). The MDR for rodents is about 0.25–0.5 μg/g food [28], based on blood coagulation. Our diets contained 0.25 μg MK-7 (1×MDR) or 25 μg MK-7 (100×MDR) per gram food.
Mice were divided into two equal groups of five Abcc6 −/− and six wt mice (four Abcc6 +/+ and two C57BL/6). One group received the 1×MK-7 food, the other the 100×MK-7 food. The food was provided ad libitum. The diet started at 1 year of age and lasted for 3 months.
In vivo measurement of calcification in the muzzle
Abcc6 −/− mice develop calcification in the capsule surrounding the blood sinus at the base of the whiskers [8]. We in vivo monitored development and progression of this calcification by micro-CT scans of the snout using a Skyscan 1076 (SkyScan, Kontich, Belgium). Scans were made using a 1-mm Al filter, the source set at 40 kV with a current of 250 μA and a rotation step of 1.5°. During the scan, mice were anaesthetized by isoflurane inhalation, and the condition was monitored visually using a camera and by measuring the rhythm of breathing. A scan of the muzzle area took about 10 min. Scans were done once a month. The first one was done at the start of the diet. After 3 months, the experiment was stopped.
From the scans, a 3D reconstruction of the snout was made resulting in datasets with an isotropic voxel size of 35 μm using software supplied with the scanner. In these reconstructions, both normal calcifications of skull and teeth and ectopic calcification of blood sinuses were clearly visible. Using an in-house-made Matlab software tool (Matlab r2007b, The Mathworks, Natick, MA, USA), calcification of skull and teeth was removed automatically from the datasets. The remaining pathological calcification in sinus capsules was quantified by assessing total mineralized volume after applying a density threshold. The fixed threshold was chosen such that the dynamics in sinus calcification were optimally represented.
Ex vivo measurement of calcification in the kidney
Abcc6 −/− mice spontaneously develop calcification in the walls of arteries in the kidney cortex [7, 29]. This vascular calcification phenotype was quantified ex vivo, in paraformaldehyde-fixed and paraffin-embedded kidneys by two methods: micro-CT and histology.
Kidneys were scanned in a Skyscan 1076 micro-CT scanner (Skyscan, Kontich, Belgium) using a 0.5-mm Al filter, the source set at 55 kV and 181 μA and a rotation step of 0.5°, resulting in datasets with a 9-μm isotropic voxel size. After manually removing calcification in pelvis and tubules from the images, the total volume of the calcifications in the renal cortex was automatically determined using Matlab.
Next, sagittal sections of 6 μm were cut of the kidney, and five sections per mouse, each separated by 180 μm, were stained for calcification according to von Kossa. In these sections, we counted the number of von Kossa positive blood vessel profiles in the kidney cortex and calculated the average number per section [29]. Micrographs were taken with a Zeiss Axioplan 2 (Carl Zeiss AG, Germany) equipped with an Evolution MP camera (MediaCybernetics, Bethesda, USA) and Image-Pro Plus acquisition software (version 6, MediaCybernetics, Bethesda, MD, USA).
Tissue collection
Mice were anaesthetized and sacrificed by cervical dislocation. Several organs were collected and either fixed in 2% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, or frozen in liquid N2 and stored at −20°C. For histopathology, paraformaldehyde-fixed tissue was embedded in paraffin. Sections were stained with haematoxylin–eosin and von Kossa using standard procedures.
Vitamin K measurement
Concentrations of vitamin K (K1 and K2) were analyzed by HPLC as described previously [30]. Briefly, part of the tissue was weighed and homogenized in ethanol/water (v/v) at a ratio 1:2 using a blender (Ultra Turrax, Janke and Kunkel, Staufen, Germany). Homogenized samples were supplemented with vitamin K1 (25) (GLSynthesis, Worcester, USA) as an internal standard, extracted with four volumes of n-hexane and pre-purified on silica columns. Quantification of vitamin K was performed by RP-HPLC using fluorescence detection after post-column reduction on a zinc column (Riedel-DeHaën, Seelze, Germany) at 40°C. The mobile phase consisted of ethanol/acetonitrile/reduction buffer/water at a ratio (v/v) of 360:90:4:3 and was degassed continuously with helium. Reduction buffer contained: 1 M ZnCl2, 1 M NaOAc and 1 M AcOH in MeOH.
Statistics
In order to analyze dynamics of muzzle calcification during the diet period we performed regression analysis for each mouse with either the actual calcification value or the log-transformed calcification as dependent variable and diet duration as independent variable in SPSS (version 16). The R 2 adjusted of the linear and logarithmic regression was calculated to assess the quality of the fit. To formally describe the relation between calcification, diet and time (diet duration), we performed a linear mixed model analysis (in SPSS, version 16). The following parameters were included in the model: the log of the volume of ectopic calcification, measured by micro-CT, as dependent variable, diet as factor and time (diet duration) as covariate. Fixed effects in the analysis were diet, time and their interaction. The analysis was performed with a random intercept (to account for difference in start position) and random slope (to account for difference in slope) per mouse. Statistical analysis of the effect of MK-7 supplementation on kidney cortex calcification was done with Student’s t test.
The effect of diet and genotype on tissue levels of various vitamin K forms was analyzed by ANOVA in SPSS (version 18) with vitamin K level as dependent variable and diet and genotype as fixed factors. Since Levene’s homogeneity test for equality of variances indicated that variances were often unequal between the groups, this ANOVA was performed on rank-transformed data. Missing measurement values (presumably below detection limit) were not included in the ranking. The variance in the rank-transformed data was homogeneous (Levene’s test).
Results
Dietary vitamin K supplementation
To examine the effect of supplemental vitamin K on the PXE phenotype, we prepared two diets differing in vitamin K content (1×MK-7, 100×MK-7), fed these for 3 months to Abcc6 −/− and wt mice and monitored calcification in the animals. Vitamin K was supplied as MK-7. The diets did not cause overt discomfort to the mice. One mouse died during the experiment, 1 month before the end of the diet period. This was an Abcc6 −/− mouse on the 100×MK-7 diet. The cause of death was not determined.
Effect of dietary vitamin K supplementation on calcification in the muzzle
A prominent feature of the pathology of Abcc6 −/− mice is spontaneous calcification of the capsule surrounding the blood sinus at base of the whiskers [8]. Mice have 25–27 large whiskers on each muzzle, arranged in five rows of 5–7 whiskers each [31]. We monitored the ectopic mineralization in this tissue in vivo by micro-CT of the snout.
Micro-CT scans of wt mice did not show any calcification of whisker sinus capsules at any time and dietary regime. In contrast, all Abcc6 −/− mice had calcifications already at the start of the diet (Fig. 1). These consisted of shell-shaped calcifications of the whisker sinus capsule (Fig. 2). Not all whisker sinus capsules were mineralized at this stage. Follow-up scans showed a clear progression of the calcification (Fig. 3). Both number of capsules affected and extent of calcification in each capsule increased during the diet period.
Fig. 1.
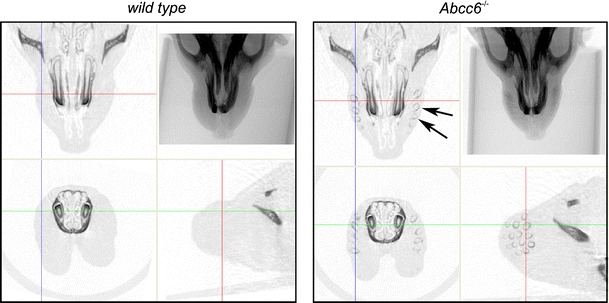
In vivo micro-CT imaging of calcification in the muzzle. X-ray image and cross sections of the 3D reconstructed micro-CT image of the rostral part of the head of a wt (left) and Abcc6 −/− (right) mouse. Skull and teeth are clearly visible, both in wt and Abcc6 −/− mouse. In addition, the Abcc6 −/− mouse has shell-shaped calcified profiles (arrows) in the muzzles as illustrated by the cross-sections. Coloured lines indicate positions of the cross-sectional planes
Fig. 2.
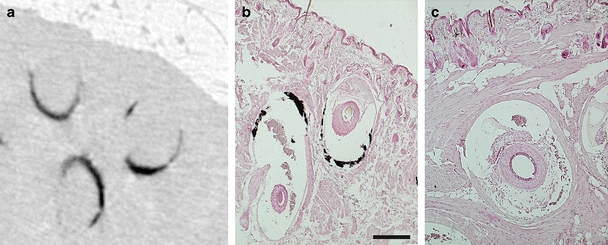
a Micro-CT scan of an Abcc6 −/− mouse muzzle showing shell-shaped calcifications. b Paraffin section of the muzzle of an Abcc6 −/− mouse (aged 9 months; 1×MK-7diet) stained with von Kossa localizes these calcifications (black) to the connective tissue capsule of the blood sinus of the whisker hair follicle. c Whisker follicle of a wt mouse (aged 8 months; 1×MK-7 diet) without calcification. Scale bar is 200 μm
Fig. 3.
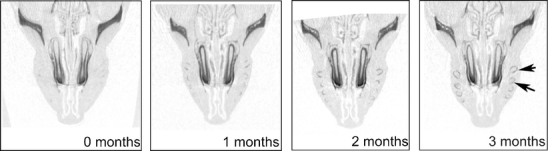
Cross sections from micro-CT scans of an Abcc6 −/− mouse (on 1×MK-7 diet) showing the in vivo progression in calcification of whisker sinuses, from the start of the diet (0 months) until the end (3 months). Arrows point at whisker sinuses that are calcified after 3 months, whereas they were not calcified at the start of the diet
To quantify the ectopic calcification, we used the 3D reconstructed micro-CT image of the snout, applied a density threshold for calcification, removed the dense signal of skull and teeth and calculated the volume of the remaining density as representing the ectopic calcification of the whisker capsule. Graphs of changes in calcification volume over time are shown in Fig. 4. The graphs for the individual mice illustrate the large individual differences. The mice which we had assigned to the 1×MK-7 diet group happened to have slightly more calcification at the start of the diet than the mice on the 100×MK-7 diet. Please note that calcification increased during the diet period in all mice, in both diet groups. Regression analysis indicated that progression of calcification during the diet period is well described by an exponential model (log calcification volume vs. time, average R 2 adjusted = 0.94) or a linear model (calcification volume vs. time, average R 2 adjusted = 0.93). Calcification volume increased considerably (3–4-fold) in both diet groups over the 3-month diet period.
Fig. 4.
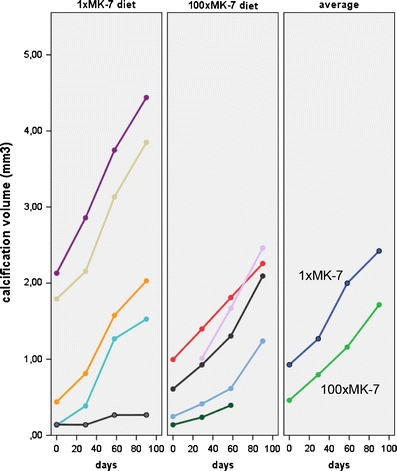
Progression of calcification in the muzzles of Abcc6 −/− mice from the start of the diet (time = 0) to the end, after 90 days. Coloured lines in the two graphs on the left depict the mineralization process in the individual mice. The graph on the right shows the averages per diet group (with missing values imputed). The 1×MK-7 diet group had, by chance, a slightly higher calcification at the start of the diet. Importantly, calcification progressed in all mice and no difference in the pace of calcification was noticed between the diets (for statistics, see Results)
To formally describe the relation between volume of ectopic calcification, diet and time (diet duration), we performed a linear mixed model analysis with the log of the calcification as dependent variable. The analysis showed that calcification increased with time (P = 0.001), but this progression was not dependent on diet (P interaction = 0.976).
In summary, our in vivo measurements showed that the 100-fold increase in dietary MK-7 had no significant effect on the pace of mineralization in whisker sinus capsules of Abcc6 −/− mice.
Effect of dietary vitamin K supplementation on calcification in the kidney
Calcification of walls of arteries is an important clinical feature of PXE. In the Abcc6 −/− mouse, this phenotype is particularly prominent in the kidney cortex. We visualized this calcification ex vivo by two methods: First, micro-CT scans were made of the isolated kidney. Next, the kidney was sectioned, and a sample of sections was stained for calcification by von Kossa.
While wt mice had no distinct renal cortex calcifications, all Abcc6 −/− mice showed mineralization of arteries in the cortex (Fig. 5). Calcification was quantified by measuring the total volume of calcified objects in the kidney cortex by micro-CT, and secondly, by counting the number of calcified profiles in the histological sections. The correlation between the outcomes of both methods was high: a correlation coefficient of 0.98 was found for calcification in the Abcc6 −/− mice. To determine the effect of diet on calcification in Abcc6 −/− mice, we applied Student’s t test: no significant difference was found (P values 0.47 and 0.31 for histology and micro-CT, respectively; Fig. 5).
Fig. 5.
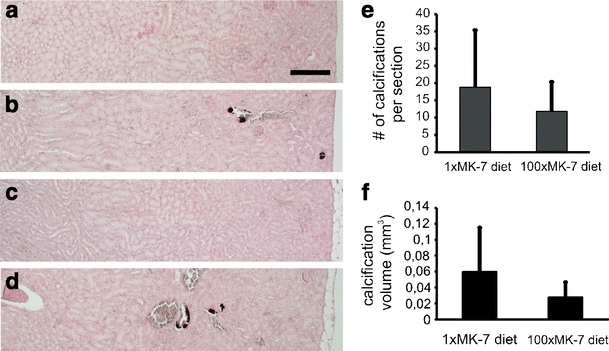
Effect of vitamin K supplementation on calcification in arteries in the kidney cortex. a–d Kidney sections of wt (a, c) and Abcc6 −/− mice (b, d) on 1×MK-7 (a, b) or 100×MK-7 diet (c, d). Von Kossa staining shows calcification (black deposits) in walls of arteries in the cortex of Abcc6 −/− mice on either diet. No calcifications were observed in wt mice. Cortex is on the right, medulla on the left. Bar is 200 μm. e Effect of diet on calcifications in arteries in the kidney cortex in Abcc6 −/− mice, assessed by histology. Histograms represent mean number ± standard deviation (s.d.) of calcified profiles per sagittal kidney section. Diet did not cause a significant difference in calcification (p = 0.47, Student’s t test). f Effect of diet on calcifications in arteries in the kidney cortex in Abcc6 −/− mice, assessed by micro-CT. Histograms represent average volume (±s.d.) of calcifications per kidney. Diet did not cause a significant difference in calcification (p = 0.31, Student’s t test)
Hepatic vitamin K levels
To determine whether Abcc6 genotype and dietary MK-7 concentration influence uptake and tissue distribution of vitamin K, we sacrificed the mice at the end of the 3-month diet period and measured vitamin K levels in liver and several extra-hepatic tissues. Please note that the mice had received standard rodent chow until the start of experimental diets. During the experiment, they received vitamin K only in the form of MK-7.
Liver tissue samples were measured for vitamin K1 and for MK-4, MK-6 and MK-7. Measurable levels of K-vitamers were found in liver samples of most wt and Abcc6 −/− mice on 100×MK-7 diet. However, analysis of liver samples of mice on the 1×MK-7 diet often gave no measurable outcome, possibly because vitamin K levels in these samples were below detection threshold. For example, in the 1×MK-7 diet group, we obtained measurable MK-7 levels in only one out of six wt mice and in one out of five Abcc6 −/− mice.
Figure 6 shows the measured vitamin K levels in the liver as function of diet and Abcc6 genotype. In view of missing (presumably below detection limit) data points of the 1×MK-7 group, this plot underestimates the effect of supplementation. Nonetheless, it suggests that dietary MK-7 supplementation increased both MK-4 and MK-7 levels in liver. ANOVA indicated that MK-7 and MK-4 levels depended on supplementation (P = 0.028 and 0.025, respectively) but not on genotype (Table 1). Analysis of the effect of supplementation in Abcc6 −/− mice separately did not yield significance, possibly because of the missing data points in the 1×MK-7 diet group. For MK-6 and K1, no effect of supplementation was apparent.
Fig. 6.
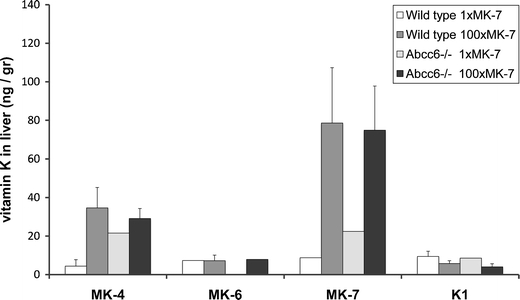
Vitamin K levels (±s.d.) in the liver as function of diet and Abcc6 genotype. Please note that some data points (mainly in the 1×Mk-7 diet group) were missing (as indicated by an absence of an error bar) presumably because the levels were below detection threshold
Table 1.
ANOVA of the effect of diet and Abcc6 genotype on vitamin K tissue levels
| All mice | Wild type | Abcc6 −/− | ||||
|---|---|---|---|---|---|---|
| Diet | Genotype | Interaction diet×genotype | Diet | Diet | ||
| Liver | MK-4 | 0.026 | 0.823 | 0.566 | 0.026 | 0.260 |
| MK-7 | 0.050 | 0.914 | 0.592 | 0.056 | 0.195 | |
| Kidney | MK-4 | 0.006 | 0.597 | 0.851 | 0.002 | 0.228 |
| MK-7 | –a | –a | –a | –a | –a | |
| Skin | MK-4 | 0.000 | 0.006 | 0.664 | 0.000 | 0.030 |
| MK-7 | 0.013 | 0.980 | 0.482 | 0.002 | –a | |
| Heart | MK-4 | 0.000 | 0.069 | 0.885 | 0.002 | 0.016 |
| MK-7 | 0.801 | 0.995 | 0.506 | 0.558 | 0.687 | |
| Brain | MK-4 | 0.000 | 0.204 | 0.805 | 0.003 | 0.001 |
| MK-7 | –a | –a | –a | –a | –a | |
The analysis compared two diets with 100-fold difference in MK-7 content (P values of ANOVA on rank-transformed data, see M&M. P values < 0.05 are italicized)
aNot enough data points
Extra-hepatic vitamin K levels
To determine the distribution of vitamin K to extra-hepatic tissues, we analyzed samples of kidney, heart, brain and skin for K1, MK-4, MK-5, MK-6 and MK-7. The vitamers K1, MK-5 and MK-6 were hardly detected in our samples, and no conclusion on effect of diet or genotype on their distribution was drawn.
The amount of MK-4 in the tissue samples generally sufficed for quantification. In wt mice, the100-fold increased dietary MK-7 resulted in a 5–10-fold increased level of MK-4 in all extra-hepatic tissues (Fig. 7). Abcc6 −/− mice had similar MK-4 tissue levels as wt mice. As in wt mice, the 100×MK-7 diet increased MK-4 levels in skin, heart and brain of Abcc6 −/− mice (Fig. 7). ANOVA (Table 1) indicated that MK-4 levels depended on diet, but not on Abcc6 genotype (except possibly for the skin, where MK-4 levels surprisingly tended to be higher in Abcc6 −/− mice than wt mice). Importantly, ANOVA showed that the effect of diet on vitamin K tissue levels was not dependent on functional Abcc6 (Table 1, P values of interaction diet × genotype).
Fig. 7.
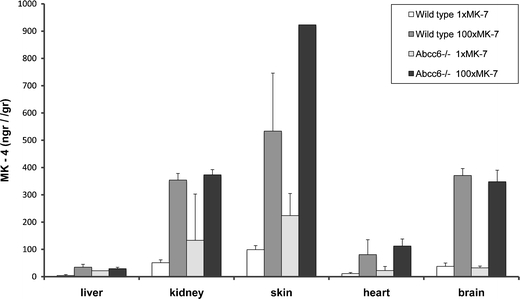
MK-4 levels (±s.d.) in liver and extra-hepatic tissues as function of diet and genotype. The 100×MK-7 diet increased MK-4 tissue levels both in wt and Abcc6 −/− mice (for statistics, see Table 1)
Since both calcification and vitamin K levels varied greatly between mice, we examined whether a correlation existed between muzzle calcification and vitamin K tissue level. No significant correlation was found (for example, for skin MK-4 level: Pearson’s r = −0.056, P = 0.928).
Measurements of extra-hepatic MK-7 levels were less clear (Fig. 8, Table 1). The amount of MK-7 was often below detection level or highly variable. A significant increase in MK-7 as a result of dietary supplementation was only found in skin (Table 1).
Fig. 8.
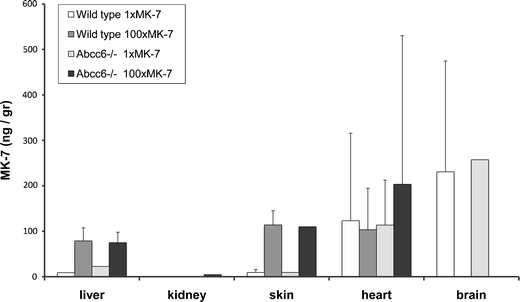
MK-7 levels (±s.d.) in liver and in extra-hepatic tissues as function of diet and genotype. MK-7 levels were highly variable. No differences were observed between the genotypes. A statistically significant effect of diet was found for skin and liver (for statistics, see Table 1)
Discussion
The PXE-K hypothesis [23] postulates that vitamin K (precursor) is the substrate of ABCC6. If true, this would provide a rationale for an easily applicable therapy, i.e. vitamin K supplementation. We performed a dietary supplementation experiment using the PXE mouse model to test hypothesis as well as potential therapy. Our results do not lend support to the PXE-K hypothesis: first, we did not see a reduction of pathology (calcification) by feeding Abcc6 −/− mice extra vitamin K. Secondly, vitamin K tissue levels did not depend on Abcc6 genotype. Thirdly, no correlation was found between ectopic calcification and vitamin K tissue levels.
Dietary MK-7 supplementation did not reduce ectopic calcification
To test the PXE-K hypothesis, we employed the Abcc6 −/− mouse model for PXE [7, 8]. As measure of PXE pathology, we analyzed calcification in the connective tissue capsule that surrounds the blood sinus at the base of the whiskers [8]. This feature of Abcc6 −/− mice has been used as a biomarker for ectopic mineralization in a number of studies [27]. An important advantage of this parameter is that it can be visualized and measured in vivo by micro-CT, as demonstrated in this study. This enabled us to monitor the mineralization process in individual mice during the diet period. This is particularly relevant, since inter-animal variation in the extent of calcification can be large, which can hamper statistical analysis when only endpoints are compared. In addition, this technique measures the entire muzzle calcification and does not rely on sectioning and sampling as histological techniques do.
Our in vivo measurements clearly showed that a 100-fold increased MK-7 concentration in the diet did not change the pace of calcification of whisker sinus capsules. To confirm and extend these findings, we measured ex vivo the calcification in arteries in the kidney cortex. This vascular calcification phenotype was not affected by MK-7 supplementation either.
It may be argued that we started the dietary intervention too late to obtain an effect of vitamin K. Supplementation of vitamin K may have effect only in initial stages and may not affect pathology once calcification has started. However, micro-CT scans taken before the start of the diet showed that not all whisker sinuses had begun to mineralize at that point. During our diet period, clearly both volume and number of calcified whiskers increased (Fig. 3). Additionally, in rats suffering from medial elastocalcinosis, 6 weeks of vitamin K treatment resulted in some 40% reduction of preformed calcifications [32]. Taken together, our conclusion of lack of an effect of vitamin K probably holds true for both ongoing mineralization and de novo mineralization of whiskers capsules in PXE.
In addition, the objection may be raised that the diet was not given long enough to take effect. We consider this to be unlikely. The diet clearly increased tissue levels of vitamin K, measured at the end of the diet. When we compare the progress in calcification in the three intervals between the micro-CT scans, we did not notice a slower pace of calcification in the 100×MK-7 diet group in the last interval (Fig. 4). In addition, in a previous animal study, it was shown that a 4-week period of high vitamin K2 intake was sufficient to inhibit medial calcifications induced by coumarins [30].
MK-7 supplementation increased MK-4 tissue levels, irrespective of Abcc6 genotype
In wt mice, the 100×MK-7 diet caused higher levels of MK-7 in liver and skin. In other tissues, no increased MK-7 levels were found. Interestingly, MK-7 supplementation also raised MK-4 levels in the liver and in all extra-hepatic tissues investigated (skin, kidney, heart and brain). These MK-4 measurements indicate that at least part of the supplied MK-7 is converted into MK-4. Thijssen et al. [33] previously demonstrated that all K vitamins can be converted into MK-4 via the intermediate product menadione (K3). The side-chain removal most likely takes place in the enterocytes, after which the circulating K3 can be taken up and prenylated to MK-4 locally. In addition, our observation that the 100×MK-7 diet increased MK-7 (and not MK-6 or MK-5) in skin and liver suggests that MK-7 is also taken up without being converted.
According to the PXE-K hypothesis, Abcc6 transports vitamin K from the hepatocyte cytoplasm into the bloodstream. Since the liver efficiently clears vitamin K from the circulation, this transport is essential for the supply of extra-hepatic tissues with vitamin K. On the basis of the PXE-K hypothesis, we expected Abcc6 −/− mice to have lower extra-hepatic vitamin K levels than wt mice. However, we found that vitamin K levels were not reduced in Abcc6 −/− mouse tissues. In the Abcc6 −/− skin, MK-4 level even appeared to be increased. Whether this particular finding occurred by chance or has biological significance is not known. In a pilot experiment preceding this study, we compared vitamin K levels between five wt and five Abcc6 −/− male mice of 7 months old. No difference was found between the skin levels of K vitamins, including MK-4 (data not shown). Overall, the data suggest that Abcc6 genotype has no influence on MK-4 tissue levels. We also found that it had no influence on the increase in MK-4 levels that occurred by the dietary MK-7 supplementation. These findings suggest that, contrary to the PXE-K hypothesis, Abcc6 has no role in transport of vitamin K to extra-hepatic tissues.
Increased vitamin K tissue levels did not slow down mineralization
Vitamin K is needed as co-factor in the γ-glutamyl carboxylation-mediated activation of various proteins [34]. The calcification inhibitor MGP was found to be uncarboxylated and thus inactive in PXE patients [20]. Even though we failed to demonstrate a role of Abcc6 in transport of vitamin K, it is still possible that vitamin K supplementation would increase MGP carboxylation and, in this way, alleviate PXE symptoms. Our dietary regime significantly (5–10-fold) increased MK-4 levels in extra-hepatic tissue, both in wt and in Abcc6 −/− mice. Yet, the increased MK-4 concentrations did not reduce ectopic calcification. This suggests that in our Abcc6 −/− mice, local vitamin K concentration is not the limiting factor in preventing mineralization of connective tissue. This conclusion is supported by the fact that no correlation was found between MK-4 content and muzzle calcification in individual mice. In addition, the findings suggest that other forms of administration such as parental or intravenous administration may equally fail to halt this pathology.
In order to investigate directly whether vitamin K supplementation had changed the local status of MGP, we performed immunohistochemistry with conformation-specific antibodies for either ucMGP or cMGP on sections of aorta and muzzle. However, no clear immunostaining could be observed in wt or in Abcc6 −/− tissue. Apparently, our material was not suited for this technique (e.g. caused by poor antigen preservation). The local carboxylation status of MGP during our dietary regime thus remained undetermined.
Aetiology of PXE?
Previous studies strongly suggested that PXE symptoms are caused by local tissue deficiency in vitamin K-dependent protein carboxylation. These indications included (1) PXE-like features in patients with GGCX mutations [18], (2) reduced γ-carboxylation of MGP in PXE patients [20] and (3) reduced serum levels of vitamin K in PXE patients [21]. The hypothesis that Abcc6 transports vitamin K and thereby distributes vitamin K to extra-hepatic tissues offers an elegant explanation for these data.
Yet, in the present study, we found no indications that Abcc6 plays a role in vitamin K transport and distribution. Certainly, our study has limitations. Some of these were already discussed above. We can add here that our study was done in the mouse model for PXE and that there may be (yet unknown) differences between mouse and man in vitamin K trafficking. Nonetheless, with the present knowledge, the Abcc6 −/− mouse appears to be a valid PXE model for our current studies: It resembles PXE in genotype and phenotype [7] including the undercarboxylation of MGP [22]. Moreover, as for vitamin K trafficking in mice, vitamin K tissue levels increased as a result of MK-7 supplementation, both in wt and in Abcc6 −/− mice. Therefore, our conclusions obtained with the PXE mouse model likely have validity for the aetiology of PXE. They do not support the hypothesis of deficient vitamin K transport as a cause of PXE. Furthermore, while this study was under review, Li et al. [35] and Brampton et al. [36] reported that neither intravenous administration of a vitamin K conjugate (menadione gluthatione conjugate) nor dietary supplementation with MK-4 or K1 counteracted ectopic mineralization in the Abcc6 −/− mouse.
Other explanations must now be considered for the reduced vitamin K-dependent protein carboxylation in PXE tissues. Instead of vitamin K, Abcc6 may transport another substance that directly or indirectly affects γ-carboxylation. For example, since it has been reported that increased oxidative stress occurs in PXE [37–40], it could be that Abcc6 transports a substance that affects oxidative stress levels. Increased numbers of free radicals will scavenge the reduced form of vitamin K and thereby prevent the γ-carboxylation. In this way, not amount but rather redox status of vitamin K determines PXE. Differential measurement of the redox forms of vitamin K in Abcc6 −/− mouse tissue may solve this issue.
Acknowledgements and disclosure statement
The authors thank Drs P. Borst, K. van de Wetering, S. Kaing and W. Verweij for discussion and comments. Financial support of the MD Fonds, Landelijke Stichting voor Blinden en Slechtzienden, Oogfonds Nederland, Stichting Blinden-Penning, Algemene Nederlandse Vereniging ter Voorkoming van Blindheid and Retina Nederland is gratefully acknowledged. The authors declare no conflict of interests related to this study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Goodman RM, Smith EW, Paton D, Bergman RA, Siegel CL, Ottesen OE, Shelley WM, Push AL, McKusick VA. Pseudoxanthoma elasticum: a clinical and histopathological study. Medicine (Baltimore) 1963;42:297–334. [PubMed] [Google Scholar]
- 2.Hu X, Plomp AS, van Soest S, Wijnholds J, de Jong PT, Bergen AA. Pseudoxanthoma elasticum: a clinical, histopathological, and molecular update. Surv Ophthalmol. 2003;48:424–438. doi: 10.1016/S0039-6257(03)00053-5. [DOI] [PubMed] [Google Scholar]
- 3.Plomp AS, Hu X, de Jong PT, Bergen AA. Does autosomal dominant pseudoxanthoma elasticum exist? Am J Med Genet A. 2004;126A:403–412. doi: 10.1002/ajmg.a.20632. [DOI] [PubMed] [Google Scholar]
- 4.Finger RP, Charbel IP, Ladewig MS, Gotting C, Szliska C, Scholl HP, Holz FG. Pseudoxanthoma elasticum: genetics, clinical manifestations and therapeutic approaches. Surv Ophthalmol. 2009;54:272–285. doi: 10.1016/j.survophthal.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, Swart J, Kool M, van Soest S, Baas F, ten Brink JB, de Jong PT. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet. 2000;25:228–231. doi: 10.1038/76109. [DOI] [PubMed] [Google Scholar]
- 6.Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B, Quaglino D, Pasquali-Ronchetti I, Pope FM, Richards A, Terry S, Bercovitch L, De Paepe A, Boyd CD. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet. 2000;25:223–227. doi: 10.1038/76102. [DOI] [PubMed] [Google Scholar]
- 7.Gorgels TG, Hu X, Scheffer GL, van der Wal AC, Toonstra J, de Jong PT, van Kuppevelt TH, Levelt CN, de Wolf A, Loves WJ, Scheper RJ, Peek R, Bergen AA. Disruption of Abcc6 in the mouse: novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum Mol Genet. 2005;14:1763–1773. doi: 10.1093/hmg/ddi183. [DOI] [PubMed] [Google Scholar]
- 8.Klement JF, Matsuzaki Y, Jiang QJ, Terlizzi J, Choi HY, Fujimoto N, Li K, Pulkkinen L, Birk DE, Sundberg JP, Uitto J. Targeted ablation of the abcc6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol. 2005;25:8299–8310. doi: 10.1128/MCB.25.18.8299-8310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- 10.Bergen AA, Plomp AS, Hu X, de Jong PT, Gorgels TG. ABCC6 and pseudoxanthoma elasticum. Pflugers Arch. 2007;453:685–691. doi: 10.1007/s00424-005-0039-0. [DOI] [PubMed] [Google Scholar]
- 11.Hu X, Peek R, Plomp A, ten Brink J, Scheffer G, van Soest S, Leys A, de Jong PT, Bergen AA. Analysis of the frequent R1141X mutation in the ABCC6 gene in pseudoxanthoma elasticum. Invest Ophthalmol Vis Sci. 2003;44:1824–1829. doi: 10.1167/iovs.02-0981. [DOI] [PubMed] [Google Scholar]
- 12.Hendig D, Langmann T, Kocken S, Zarbock R, Szliska C, Schmitz G, Kleesiek K, Gotting C. Gene expression profiling of ABC transporters in dermal fibroblasts of pseudoxanthoma elasticum patients identifies new candidates involved in PXE pathogenesis. Lab Invest. 2008;88:1303–1315. doi: 10.1038/labinvest.2008.96. [DOI] [PubMed] [Google Scholar]
- 13.Scheffer GL, Hu X, Pijnenborg AC, Wijnholds J, Bergen AA, Scheper RJ. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest. 2002;82:515–518. doi: 10.1038/labinvest.3780444. [DOI] [PubMed] [Google Scholar]
- 14.Ilias A, Urban Z, Seidl TL, Le Saux O, Sinko E, Boyd CD, Sarkadi B, Varadi A. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6) J Biol Chem. 2002;277:16860–16867. doi: 10.1074/jbc.M110918200. [DOI] [PubMed] [Google Scholar]
- 15.Uitto J, Pulkkinen L, Ringpfeil F. Molecular genetics of pseudoxanthoma elasticum: a metabolic disorder at the environment-genome interface? Trends Mol Med. 2001;7:13–17. doi: 10.1016/S1471-4914(00)01869-4. [DOI] [PubMed] [Google Scholar]
- 16.Le Saux O, Bunda S, VanWart CM, Douet V, Got L, Martin L, Hinek A. Serum factors from pseudoxanthoma elasticum patients alter elastic fiber formation in vitro. J Invest Dermatol. 2006;126:1497–1505. doi: 10.1038/sj.jid.5700201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Q, Endo M, Dibra F, Wang K, Uitto J. Pseudoxanthoma elasticum is a metabolic disease. J Invest Dermatol. 2009;129:348–354. doi: 10.1038/jid.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanakker OM, Martin L, Gheduzzi D, Leroy BP, Loeys BL, Guerci VI, Matthys D, Terry SF, Coucke PJ, Pasquali-Ronchetti I, De PA. Pseudoxanthoma elasticum-like phenotype with cutis laxa and multiple coagulation factor deficiency represents a separate genetic entity. J Invest Dermatol. 2007;127:581–587. doi: 10.1038/sj.jid.5700610. [DOI] [PubMed] [Google Scholar]
- 19.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 20.Gheduzzi D, Boraldi F, Annovi G, DeVincenzi CP, Schurgers LJ, Vermeer C, Quaglino D, Ronchetti IP. Matrix Gla protein is involved in elastic fiber calcification in the dermis of pseudoxanthoma elasticum patients. Lab Invest. 2007;87:998–1008. doi: 10.1038/labinvest.3700667. [DOI] [PubMed] [Google Scholar]
- 21.Vanakker OM, Martin L, Schurgers LJ, Quaglino D, Costrop L, Vermeer C, Pasquali-Ronchetti I, Coucke PJ, De PA (2010) Low serum vitamin K in PXE results in defective carboxylation of mineralization inhibitors similar to the GGCX mutations in the PXE-like syndrome. Lab Invest. doi:10.1038/labinvest.2010.68 [DOI] [PubMed]
- 22.Li Q, Jiang Q, Schurgers LJ, Uitto J. Pseudoxanthoma elasticum: reduced gamma-glutamyl carboxylation of matrix gla protein in a mouse model (Abcc6−/−) Biochem Biophys Res Commun. 2007;364:208–213. doi: 10.1016/j.bbrc.2007.09.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borst P, van de Wetering K, Schlingemann R. Does the absence of ABCC6 (multidrug resistance protein 6) in patients with Pseudoxanthoma elasticum prevent the liver from providing sufficient vitamin K to the periphery? Cell Cycle. 2008;7:1575–1579. doi: 10.4161/cc.7.11.6005. [DOI] [PubMed] [Google Scholar]
- 24.Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thromb Haemost. 2008;100:530–547. [PubMed] [Google Scholar]
- 25.Schurgers LJ, Teunissen KJ, Hamulyak K, Knapen MH, Vik H, Vermeer C. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. 2007;109:3279–3283. doi: 10.1182/blood-2006-08-040709. [DOI] [PubMed] [Google Scholar]
- 26.Schurgers LJ, Vermeer C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis. 2000;30:298–307. doi: 10.1159/000054147. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Q, Li Q, Uitto J. Aberrant mineralization of connective tissues in a mouse model of pseudoxanthoma elasticum: systemic and local regulatory factors. J Invest Dermatol. 2007;127:1392–1402. doi: 10.1038/sj.jid.5700729. [DOI] [PubMed] [Google Scholar]
- 28.Ronden JE, Thijssen HH, Vermeer C. Tissue distribution of K-vitamers under different nutritional regimens in the rat. Biochim Biophys Acta. 1998;1379:16–22. doi: 10.1016/s0304-4165(97)00075-5. [DOI] [PubMed] [Google Scholar]
- 29.Gorgels TG, Waarsing JH, De WA, ten Brink JB, Loves WJ, Bergen AA (2010) Dietary magnesium, not calcium, prevents vascular calcification in a mouse model for pseudoxanthoma elasticum. J Mol Med. doi:10.1007/s00109-010-0596-3 [DOI] [PMC free article] [PubMed]
- 30.Spronk HM, Soute BA, Schurgers LJ, Thijssen HH, De Mey JG, Vermeer C. Tissue-specific utilization of menaquinone-4 results in the prevention of arterial calcification in warfarin-treated rats. J Vasc Res. 2003;40:531–537. doi: 10.1159/000075344. [DOI] [PubMed] [Google Scholar]
- 31.Rice FL, Mance A, Munger BL. A comparative light microscopic analysis of the sensory innervation of the mystacial pad. I. Innervation of vibrissal follicle-sinus complexes. J Comp Neurol. 1986;252:154–174. doi: 10.1002/cne.902520203. [DOI] [PubMed] [Google Scholar]
- 32.Schurgers LJ, Spronk HM, Soute BA, Schiffers PM, DeMey JG, Vermeer C. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood. 2007;109:2823–2831. doi: 10.1182/blood-2006-08-040709. [DOI] [PubMed] [Google Scholar]
- 33.Thijssen HH, Vervoort LM, Schurgers LJ, Shearer MJ. Menadione is a metabolite of oral vitamin K. Br J Nutr. 2006;95:260–266. doi: 10.1079/BJN20051630. [DOI] [PubMed] [Google Scholar]
- 34.Vermeer C. Gamma-carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase. Biochem J. 1990;266:625–636. doi: 10.1042/bj2660625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Q, Li Q, Grand-Pierre AE, Schurgers LJ, Uitto J. Administration of vitamin K does not counteract the ectopic mineralization of connective tissues in Abcc6 (−/−) mice, a model for pseudoxanthoma elasticum. Cell Cycle. 2011;10:701–707. doi: 10.4161/cc.10.4.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brampton C, Yamaguchi Y, Vanakker O, Van LL, Chen LH, Thakore M, De PA, Pomozi V, Szabo PT, Martin L, Varadi A, Le Saux O (2011) Vitamin K does not prevent soft tissue mineralization in a mouse model of pseudoxanthoma elasticum. Cell Cycle 10:1810–1820 [DOI] [PMC free article] [PubMed]
- 37.Pasquali-Ronchetti I, Garcia-Fernandez MI, Boraldi F, Quaglino D, Gheduzzi D, De Vincenzi PC, Tiozzo R, Bergamini S, Ceccarelli D, Muscatello U. Oxidative stress in fibroblasts from patients with pseudoxanthoma elasticum: possible role in the pathogenesis of clinical manifestations. J Pathol. 2006;208:54–61. doi: 10.1002/path.1867. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Fernandez MI, Gheduzzi D, Boraldi F, Paolinelli CD, Sanchez P, Valdivielso P, Morilla MJ, Quaglino D, Guerra D, Casolari S, Bercovitch L, Pasquali-Ronchetti I. Parameters of oxidative stress are present in the circulation of PXE patients. Biochim Biophys Acta. 2008;1782:474–481. doi: 10.1016/j.bbadis.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Li Q, Jiang Q, Uitto J. Pseudoxanthoma elasticum: oxidative stress and antioxidant diet in a mouse model (Abcc6−/−) J Invest Dermatol. 2008;128:1160–1164. doi: 10.1038/sj.jid.5701145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zarbock R, Hendig D, Szliska C, Kleesiek K, Gotting C. Pseudoxanthoma elasticum: genetic variations in antioxidant genes are risk factors for early disease onset. Clin Chem. 2007;53:1734–1740. doi: 10.1373/clinchem.2007.088211. [DOI] [PubMed] [Google Scholar]


