How did the study come about?
The rapid roll-out and subsequent scale-up of HIV and antiretroviral services in resource-limited countries has seen a >7-fold increase in the number of patients on antiretroviral therapy (ART) over the 4 years prior to 2007.1 ART in low- and middle-income countries has been shown to be similarly effective in terms of individual immunological and virological response as in high-resource settings.2,3 However, challenges remain relating to barriers to access,4 retention on treatment,5 early mortality2,6,7 and limited and less well-tolerated second-line regimens.8
In South Africa, home to 5.7 million HIV-infected persons,9 HIV incidence and prevalence remain high.10,11 With a large burden of disease constrained by limited resources, the roll-out of ART in rural areas illustrates the challenge, similarly faced by most resource-poor settings, of managing this chronic infectious disease. The national plan for HIV aims to more than triple the number of individuals on ART between 2007 and 2012,12 but if it is to be successful, should be guided by appropriate programmatic evidence to allow successful control of the epidemic.
The programme reported here was implemented as a joint initiative by the Department of Health (DoH) and the Africa Centre for Health and Population Studies (http://www.africacentre.ac.za) in response to the Comprehensive HIV and AIDS Care Management and Treatment Plan in 2004.13 HIV treatment and care are offered in 16 nurse-led primary health-care (PHC) clinics and analysis will add to the limited evidence of decentralized models of health-care delivery.14 From inception, the programme has adhered to South African and KwaZulu-Natal guidelines for ART initiation, drug regimens and follow-up.15,16 A secure database system was developed to capture clinical and demographic information on individuals initiated onto ART; this is regularly updated to contain follow-up status and laboratory results (Table 1). The database was established in 2007 when records from the start of the programme in late 2004 were retrospectively captured.
Table 1.
Data collected on those initiated on ART in the Hlabisa HIV Treatment and Care Programme, and on those in the Africa Centre Demographic Information System
| ART database | |
| Basic details | Age, sex, address, ID number, contact details, treatment clinic |
| Personal circumstance | Grants received, employment, school attendance, number of dependents |
| Tuberculosis (TB) record | Previous history of TB, TB treatment at initiation, new episodes of TB |
| ART record | WHO clinical stage, Previous ART and/or PMTCT, ART regimen at initiation, changes in ART regimen during treatment |
| Laboratory data | Baseline and 6-monthly CD4 and HIV viral load, baseline haematology and biochemistry |
| Clinic visits | Monthly attendance |
| Africa Centre Demographic Information System (ACDIS) | |
| Household demographics and socio-economic data | Owner and members of households, latitude and longitudinal position of homestead, household expenditure, asset ownership |
| Individual socio-economic data | Age, sex, membership of household, education, employment, receipt of grants |
| HIV status | VCT history, dried blood spot for HIV testing and instant VCT offered |
| Sexual behaviour | Pregnancy history, contraceptive use, sexual activity |
| Deaths | Location of death, description of circumstances, verbal autopsy data |
| Births | Pregnancy outcomes, delivery environment, birth weight |
| Migrations | Details of destination and reason for migration |
PMTCT, Prevention of Mother-to-Child Transmission; VCT, voluntary counselling and testing.
Study objectives and funding
The Hlabisa HIV Treatment and Care Programme is a South African DoH programme, with additional support from the Africa Centre, funded from 1 August 2004 to 31 December 2006 by the Elizabeth Glaser Paediatric AIDS Foundation (EGPAF) and since 1 May 2005 by the President’s Emergency Plan for AIDS Relief (PEPFAR) and United States Agency for International Development (USAID). The Wellcome Trust funded Africa Centre provides additional database management. PEPFAR sponsored staffing has increased from 63 full-time staff in December 2007 to 123 in December 2008, working alongside joint-trained DoH staff, and 540 home-based care volunteers. The programme supports the integration of HIV services into PHC, aiming to link treatment and care with prevention services. The database serves dual functions of providing feedback indicators to funders and allowing internal evaluation of the programme. The data collected allow examination of a wide range of clinical research questions including how best to deliver HIV treatment and care through PHC systems in rural South Africa and to assess how that treatment and care impacts on the community.
Where is the study area and who are in the sample?
The cohort includes HIV-infected people enrolled at 16 PHC clinics located in the predominately rural Hlabisa sub-district of Umkhanyakude in northern KwaZulu-Natal (Figure 1) with a population of 228 000. The local district hospital (Hlabisa) has 296 beds. Six of the DoH clinics, and 40% of patients, fall within the Africa Centre Demographic Surveillance Area (DSA), which has a population of 85 000 people in a 438-km2 area.17 The DSA population is well characterized17—since 2000, data collection is based on twice-yearly interviews with key-household informants, annual household socio-economic surveys, and, since 2003, an annual individual HIV surveillance among adults. HIV prevalence in 2004 was 22% overall, peaking at 51% for females in the 25–29-year age group and at 44% for men in the 30–34-year age group.10 Locally, 62% of people have access to electricity and 78% to piped water (not necessarily in their homestead); 22% of children are orphans of one or both parents;17 and 33% of those regarded as household members in the surveillance area do not reside within it.17 Information on these individuals is collected within the Africa Centre Demographic Information System (ACDIS; Table 1) and a random 12.5% are tracked each year for the HIV surveillance, allowing a more complete understanding of the determinants of HIV infection.18
Figure 1.
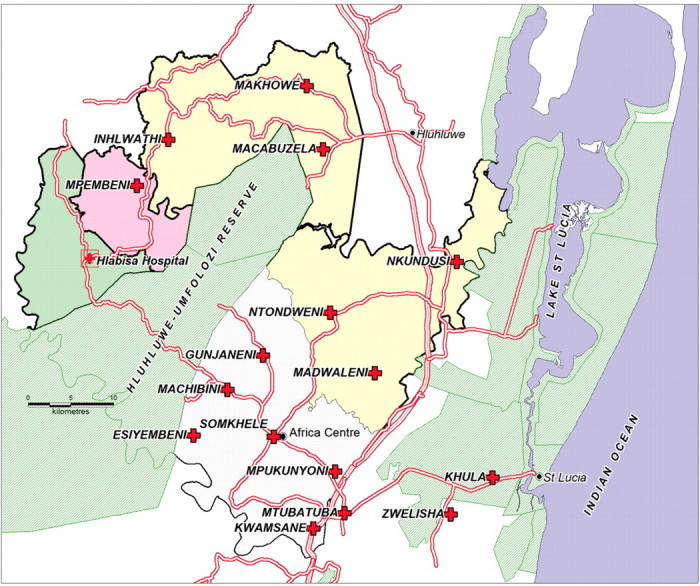
Hlabisa sub-district, northern KwaZulu-Natal, showing position of hospital with one on-site clinic and 15 peripheral clinics. The Hlabisa sub-district extends to the area at the bottom-right of the map to encompass Zwelenisha and Mtubatuba clinics. The clinic at Khula village was only established in late 2009; previously patients accessed Kwamsane or Zwelenisha clinics
After national roll-out in 2004, ART services were only available from the hospital clinic. Later the same year, services were expanded to include a peri-urban clinic, (KwaMsane) (Figure 1), now the largest ART clinic. By late 2006, six clinics were offering ART initiation; by late 2007, all 16 clinics offered ART initiation and follow-up.
Diagnosis and treatment
The programme adheres to South African DoH guidelines on HIV diagnosis, ART eligibility, screening, treatment regimens and follow-up.15 In adults and children >18 months of age, a first positive rapid HIV ELISA is confirmed by a second, and venous blood ELISA is requested if results are discordant. If the first rapid test is negative, repeat testing is recommended every 6 months. Children <18 months old are diagnosed by HIV DNA PCR after 6 weeks of age; if negative, repeat testing is performed 6 weeks after cessation of breastfeeding.16
Adults with WHO clinical stage 4 disease or CD4 count <200 cells/µl are eligible for ART.15,16 Provincial guidelines for children were updated in 2008 to include all four WHO disease stages and new eligibility criteria for treatment initiation, particularly for infants, in line with findings from South Africa that earlier treatment reduces childhood mortality.16,19
Prior to ART initiation, adults and children are screened for TB with symptom screen, sputum acid-fast bacilli smear with or without culture and chest X-ray.20 Other opportunistic and sexually transmitted infections are diagnosed and treated appropriately. Baseline blood tests include HIV viral load, full blood count, creatinine, liver function tests and hepatitis B surface antigen. Chemoprophylaxis with co-trimoxazole is used for adults and children eligible for ART and children awaiting PCR results. The ART regimen for adults and children >3 years of age and >10 kg in weight includes stavudine, lamivudine and either efavirenz or nevirapine. Children <3 years of age and <10 kg in weight are treated with stavudine, lamivudine and ritonavir-boosted lopinavir.15,16 Anti-retrovirals are not available as co-formulations, although liquids are available for paediatric use. Adverse drug reaction reports and requests for individual drug substitutions or regimen changes are submitted to the provincial pharmacy office. Switch to second-line therapy requires persistent viral load ≥5000 copies/ml in spite of documented adherence ≥95%; regimens are zidovudine, didanosine and ritonavir-boosted lopinavir for adults and children >3 years of age; and zidovudine, didanosine and nevirapine/efavirenz in children <3 years of age.15,16 Resistance testing is currently not available in the programme.
How often have patients been followed up and what has been measured?
Patients attend three group education sessions followed by individual counselling sessions prior to initiation. Patients must have identified a treatment supporter and signed an agreement to attend clinic and adhere to treatment. Patients can also consent on the same form to their details being passed on to a tracker should they default treatment. Initial follow-up at 2 weeks assesses adherence and evidence of toxicity (with nurse and counsellor). If the patient is taking nevirapine, liver function tests are checked at 2, 4 and 8 weeks; for zidovudine, full blood counts are monitored at baseline, 4, 8 and 12 weeks. All patients are seen monthly by a nurse and counsellor for treatment collection, attending group followed by individual counselling session prior to receiving medication. No routine medical reviews are scheduled after initiation but physicians visit each clinic at least fortnightly and patients can be referred by ART nurses. CD4 and viral load monitoring are performed 6-monthly and were initially analysed at centralized provincial and district hospitals; with programme expansion this process has become decentralized. CD4 counts have been performed at Hlabisa hospital since June 2008 and viral loads from mid-to late 2009. CD4 counts are analysed using Beckman Coulter EPICS® XL flow cytometer (Beckman Coulter, Inc.); viral loads using Nuclisens EasyQ® HIV-1 assay (Biomérieux) with a lower detection limit of 25 copies/ml. All test results, including those from other laboratories, are collated weekly into a laboratory database and imported into the programme database, allowing immunological and virological follow-up information on all patients on ART, in addition to the clinical follow-up information (Table 1). This system also provides information on patients who have not yet met eligibility criteria for initiation onto ART who are advised to have CD4 blood tests and a nurse review for clinical staging every 6 months for adults, and more frequently for children depending on age and immunological/clinical status.
What has been found so far?
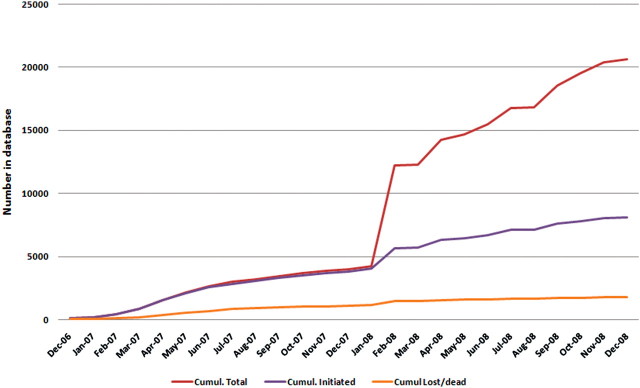
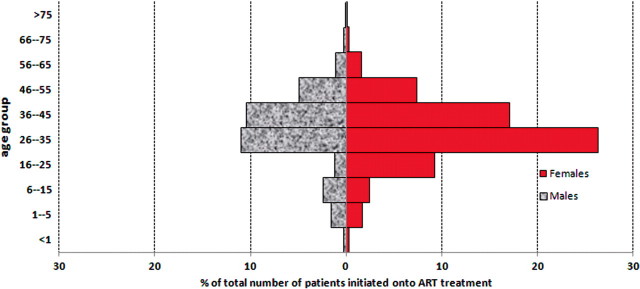
The programme has increased rapidly since inception from 1800 people on ART by 31 October 2006 (after 2 years of ART programme) to 7576 by 31 December 2008. Further, from January 2008, CD4 results of those accessing testing in the clinics have also been documented (Figure 2). At the time of data censoring (31 December 2008) the database contained results for 19 566 individuals, 14 280 (73.0%) of whom were female. The over-representation of females is a reflection of the local prevalence10 and continues to be illustrated in those initiated onto treatment (Figure 3). In 2004, in the ACDIS, overall, 27% of female and 13.5% of male residents were HIV infected.10 Aspects of equity and access to treatment are currently being examined, but preliminary findings suggest that males are only slightly less likely to access treatment in comparison to females (Personal Communication F Tanser, Africa Centre, 2009), contrary to recent reports that more attention needs to be paid to ensure men access HIV treatment and care.21,22
Figure 2.
Cumulative total of persons who have accessed the Hlabisa HIV Treatment and Care Programme, showing those initiated onto ART, those with CD4 tests but not yet initiated and those lost to follow-up or deceased
Figure 3.
Distribution by age and sex of those initiated on ART in the HIV Treatment and Care Programme
There were 8832 adults with CD4 counts >200 cells/µl, thus not yet eligible for treatment, and 2689 adults with eligible CD4 counts but not yet started on ART within our programme where there is no waiting list for treatment (Table 2). There are multiple possible reasons why this latter group has not started ART; they may have moved out of the district, not returned to access their CD4 results, were in the process of being initiated, have started ART in the private sector or local NGO programmes or have died. Of the patients initiated, the median CD4 at initiation was 118 cells/µl and plasma viral load 4.58 Log; most people started on the efavirenz containing regimen (Table 2). Among children, the median age at initiation was relatively high, with half initiating treatment at ≥6 years of age. The majority of children were already in WHO stage 3 or 4 at the time of initiation. However, early results suggest success can be achieved with clinical, immunological and virological improvement in this rural paediatric cohort.23
Table 2.
Characteristics of individuals enrolled in the Hlabisa HIV Treatment and Care Programme
| Eligible and on ART (n = 6909) | Eligible but not on ARTa(n = 2689) | Not yet eligible for ARTb,c(n = 8832) | |
|---|---|---|---|
| No. or median | No. or median | No. or median | |
| Adults (>15 years old) | |||
| Age, years | 35 (29–42) | 33 (27–40) | 30 (24–38) |
| Missing data | 91 (3.4) | 201 (2.3) | |
| Sex, female | 4688 (67.9) | 1732 (64.4) | 7253 (82.1) |
| Missing data | 2 (0.07) | 11 (0.1) | |
| CD4 count at initiation (or at latest test), cells/μl | 118 (58–175) | 92 (36–148) | 401 (298–552) |
| Missing data | 528 (7.6) | ||
| Log10 viral load at initiation | 4.58 (3.87–5.18) | ||
| Missing data | 2890 (41.8) | ||
| WHO clinical stage at initiation | |||
| 1 or 2 | 625 (9.0) | ||
| 3 or 4 | 3068 (44.4) | ||
| Missing data | 3216 (46.6) | ||
| Initiation drug regimen | |||
| d4T/3TC/EFV | 5378 (77.8) | ||
| d4T/3TC/NVP | 1419 (20.5) | ||
| Otherd | 68 (1.0) | ||
| Missing data | 44 (0.6) | ||
| Status (as of 31 December 2008) | |||
| Active | 5759 (83.4) | ||
| Deceased | 620 (9.0) | ||
| Lost to follow-up | 348 (5.0) | ||
| Transferred out | 182 (2.6) | ||
| Eligible and on ART (n = 677) | Eligible but not on ARTa(n = 207) | Not yet eligible for ARTb,c(n = 262) | |
|---|---|---|---|
| No. or median | No. or median | No. or median | |
| Children (≤15 years old) | |||
| Age, years | |||
| <1 | 48 (7.2) | 40 (19.3) | 11 (4.2) |
| 1–5 | 253 (37.9) | 102 (49.3) | 98 (37.4) |
| 6–15 | 366 (54.9) | 65 (31.4) | 153 (58.4) |
| Sex, female | 337 (50.5) | 109 (52.7) | 161 (61.5) |
| CD4 percentage at initiation (or latest test) | |||
| <1 year | 17 (11–25) | 18 (8.5–26.5) | 42 (38–46) |
| 1–5 years | 17 (11–26) | 14 (9–18) | 28 (24–34) |
| 6–15 years | 16 (9–25) | 9 (5–12) | 24 (19–29) |
| Log10 viral load at initiation | 3.65 (1.39–4.66) | ||
| Missing data | 413 (61.9) | ||
| WHO clinical stage at initiation | |||
| 1 or 2 | 114 (17.1) | ||
| 3 or 4 | 479 (71.8) | ||
| Missing data | 74 (11.1) | ||
| Status (as of 31 December 2008) | |||
| Active | 595 (89.2) | ||
| Deceased | 39 (5.9) | ||
| Lost to follow-up | 18 (2.7) | ||
| Transferred out | 15 (2.2) | ||
Values are given as median (Interquartile range (IQR)) or n(%).
aDefined as CD4 count <200 cells/μl (adults) or CD4 eligibility criteria by age (children) prior to 1 October 2008 with no record of initiation by 31 December (excludes 812 adults and 154 children with CD4 count results between 1 October and 31 December 2008).
bDefined only on CD4 criterion (≥200 cells/μl) without clinical staging information.
cBased on latest CD4 count taken between 1 January 2007 and 31 December 2008.
dNon-standard regimens in patients transferred in from other programmes.
In 2008, an average of 253 adults and 27 children were initiated every month. In the paediatric cohort, 667 children (≤15 years old) had been initiated onto ART by 31 December 2008 (8.8% of the total initiated). Only 48 (7.2%) infants (≤1 year old) were initiated on ART illustrating the enormous challenges of identifying and treating young children. Strategies have recently been implemented to increase the overall number of children on treatment, concentrating on infants, including strengthening the follow-up of mothers and infants in the local PMTCT programme, ensuring PCR testing of infants at 6 weeks of age and fast tracking of infected infants for treatment.
The home-based care (HBC) branch of the programme has increased significantly since October 2007, when service level agreements were formalized between existing local care organizations and the Hlabisa HIV Treatment and Care Programme. By February 2009, 24 HBC organizations had been recruited, with 540 volunteers covering 5800 patients.24 Carers visit community members after referral from multiple sources including community members, hospitals or clinics and local NGOs. Two full-time professional nurses and a dedicated social worker are also employed in the team.
Three interventions have been implemented in the sub-district to increase access and uptake of HIV testing. In the 16 fixed PHC clinics, between 1000 and 1500 people are tested and counselled for HIV each month; and, from early 2009, the programme has offered a Home and Mobile Testing Service employing seven counsellors, counsellor supervisors and one full-time coordinator. It operates in areas outside the DSA, offering rapid HIV testing in one of two modalities; home-based VCT in individual houses or VCT in a mobile unit. Secondly, the use of Provider-initiated Testing and Counselling (PITC) has been piloted in the sub-district.25 The pilot resulted in a 3-fold increase in HIV testing rates in the month after implementation.26 Currently, the model has been expanded to a further five clinics before roll-out to all 16. Finally, TB and HIV services in the sub-district have been integrated so that there is a physical proximity between the two services at each clinic; all TB patients are offered an HIV test and almost 90% accept.27,28
What analyses are planned for the future?
The primary aim of the Africa Centre’s clinical research on a programmatic level is to investigate how best to deliver ART in this rural setting. On a larger public health level, it is to relate the impact of the programme in terms of impact on overall population mortality. The impact of rapid expansion must be quantified initially, and a series of analyses are being conducted to examine clinical and virological outcomes of adults and children on ART, time to treatment failure and retention in the programme. Approximately 40% of HIV-infected patients on treatment in the sub-district live inside the Africa Centre’s DSA and therefore have been linked (after obtaining ethical approval) between databases allowing matching of clinical, virological and immunological progress and outcome with socio-economic, demographic and household information.
There is a specific focus on co-infections, particularly TB with calculations of incidence rate of new TB infection in those on ART,29 as well as hepatitis B co-infection.30 The research agenda aims to determine the impact of these co-infections and the best way to integrate their management within the HIV programme. A further database of all people started on TB treatment in the sub-district has been established for the Hlabisa TB control programme of the DoH: this could also be linked to the ACDIS and ART databases, with appropriate ethical approval, to characterize the evolving TB epidemic within rural South Africa, a country with the second highest incidence of TB worldwide.31 The detailed data on the local population from the ACDIS afford the opportunity to explore mortality at the community level and the impact of the ART programme on patterns of deaths in adults and children.32 With the exponential rise in numbers of people on ART, the sustainability of ART programmes using current approaches is debated. We intend to model different approaches to follow-up of patients on treatment, and explore these in terms of sustainability of the programme and cost benefits. We also aim to evaluate the success or otherwise of the local PMTCT programme, and to use data from the ART database to estimate access to services and impact of treatment on HIV prevalence and incidence in the population.
Quality of care and equity of access to treatment are critical issues.33 Work is investigating clinic-level determinants of quality of care and influences on outcome, such as loss to follow-up and time from eligibility for ART to initiation of treatment. A further study examining equity to health care in three main services—maternal health, TB and ART—is also in progress; Researching Equitable Access to Healthcare (REACH), and will use relevant data from the clinical database.
What are the strengths and weaknesses of the cohort?
The strengths of this cohort include its size, the quality and quantity of data to describe it and the ability to model the impact of the programme on the population due to the detailed, longitudinal information available about the community and the linkage between clinical and surveillance data.32 Accurate characterization of the cohort will allow understanding of the determinants of outcomes, and implications for service delivery in a rural population, which is likely to be applicable in similar poor-resource settings. Quantifying outcomes in a decentralized, mainly nurse- and counsellor-driven service is different from many other reports, which have described cohorts from specialist clinics in urban settings. As the sustainability of programmes is debated, ‘task shifting’ and decentralization of care are likely to become increasingly common in HIV programmes to cope with increased numbers of people on treatment.2,34,35
One of the main challenges common to all HIV treatment cohorts is attrition rates;6,36 this is especially so in this area with high migration rates. Using our linked datasets, we have another opportunity to examine the outcomes of a large proportion of people in the ART cohort and, through sensitivity analysis, can provide more informed estimates of coverage and outcomes including insights into the outcome of those lost to follow-up in terms of mortality, differentiating HIV-associated mortality from accidental mortality, non-HIV associated illness mortality or simple migration of the household.
As this is primarily an HIV treatment cohort, we have limited data on people who are eligible for, but have not yet accessed, ART. Delayed ART initiation has been reported to be a major cause of death in South Africa,37 but, as mortality data on this group are unavailable, we are unable to determine the proportion of people who have died while awaiting treatment.
In rural peripheral clinics it is not possible to maintain computerized records, and therefore data are entered centrally. It is challenging to update records at the central database from 16 peripheral clinics and although new systems are in place, such as database-generated monthly lists of patients who should be attending clinic with check boxes for new developments (TB treatment, pregnancy, for example), it is still possible that some details relating to people who were initiated in the clinic may escape data entry. On the other hand, the database provides a tool to improve patient care and retention and is increasingly used for that purpose.
In summary, this rural ART programme is well placed to offer complex and wide-ranging analyses on several aspects of ART in sub-Saharan Africa. The support offered by the Africa Centre both through external funding, database management, population data from the demographic surveillance and local scientific expertise allows it to be an effective and comprehensive treatment and care programme as well as a cohort for analysis. In an era when the consequences of rapid ART roll-out are starting to be felt, and the challenges of late presentation with HIV persist, along with high and increasing rates of infection such as TB, large rural decentralized cohorts are needed to describe these challenges, and the effect of interventions to address them.
Can I get hold of the data? Where can I find out more?
Further information about the data can be obtained from the Africa Centre website (www.africacentre.ac.za) or by contacting Ruth Bland at rbland@africacentre.ac.za . The cohort is an ongoing treatment cohort in partnership with the DoH, and relevant ethical approval is sought for all collaborative work. We are currently in the process of obtaining ethical permission to contribute data to the International epidemiologic Databases to Evaluate AIDS (IeDEA) Southern Africa (IeDEA-SA) collaboration (http://www.iedea-hiv.org/).
Funding
The Africa Centre is funded by the Wellcome Trust (grant #050534). The Hlabisa HIV Treatment and Care Programme is made possible by the generous support of the American people through the USAID and the PEPFAR under the terms of Award No. 674-A-00-08-00001-00. The opinions expressed herein are those of the authors and do not necessarily reflect the view of the USAID, or the United States Government, or the Department of Health, KwaZulu-Natal.
Conflict of interest: None declared.
Acknowledgements
The authors would like to acknowledge the contribution of Colin Newell, senior database scientist, in the development and support of the database, and thank Graham Cooke for his input into the programme from October 2006 to March 2008 and Clemens Roll for his commitment and hard work as Programme leader from March 2007 to December 2008. They are grateful to all the DoH and Africa Centre staff working in the clinics and at the Centre—their dedication is humbling. Finally, they thank the community of Hlabisa sub-district for hosting their initiatives.
References
- 1.WHO. 2008 June. [(12 January 2010, date last accessed)]. Towards Universal Access: Scaling up priority HIV/AIDS interventions in the Health Sector. WHO, Geneva. http://www.who.int/hiv/pub/2008progressreport/en/ [Google Scholar]
- 2.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 3.Keiser O, Orrell C, Egger M, et al. Public-health and individual approaches to antiretroviral therapy: township South Africa and Switzerland compared. PLoS Med. 2008;5:e148. doi: 10.1371/journal.pmed.0050148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Posse M, Meheus F, van Asten H, van der Ven A, Baltussen R. Barriers to access to antiretroviral treatment in developing countries: a review. Trop Med Int Health. 2008;13:904–13. doi: 10.1111/j.1365-3156.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 5.Brinkhof MW, Dabis F, Myer L, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86:559–67. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–48. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 8.Boyd MA, Cooper DA. Second-line combination antiretroviral therapy in resource-limited settings: facing the challenges through clinical research. AIDS. 2007;21(Suppl 4):S55–63. doi: 10.1097/01.aids.0000279707.01557.b2. [DOI] [PubMed] [Google Scholar]
- 9.2007. [(12 January 2010, date last accessed)]. Joint United Nations Programme on HIV/AIDS and World Health Organization. http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf. [Google Scholar]
- 10.Welz T, Hosegood V, Jaffar S, Batzing-Feigenbaum J, Herbst K, Newell ML. Continued very high prevalence of HIV infection in rural KwaZulu-Natal, South Africa: a population-based longitudinal study. AIDS. 2007;21:1467–72. doi: 10.1097/QAD.0b013e3280ef6af2. [DOI] [PubMed] [Google Scholar]
- 11.Barnighausen T, Tanser F, Gqwede Z, Mbizana C, Herbst K, Newell ML. High HIV incidence in a community with high HIV prevalence in rural South Africa: findings from a prospective population-based study. AIDS. 2008;22:139–44. doi: 10.1097/QAD.0b013e3282f2ef43. [DOI] [PubMed] [Google Scholar]
- 12. [(12 January 2010, date last accessed)]. South African National AIDS Council. HIV and AIDS and STI National Strategic Plan 2007–2011. http://www.doh.gov.za/docs/misc/stratplan-f.html. [Google Scholar]
- 13.Operational Plan for Comprehensive HIV and AIDS Care, Management and Treatment. 2003. [(12 January 2010, date last accessed)]. South African Government. http://www.info.gov.za/otherdocs/2003/notused/aidsplan.pdf. [Google Scholar]
- 14.Briggs CJ, Garner P. Strategies for integrating primary health services in middle- and low-income countries at the point of delivery. Cochrane Database Syst Rev. 2006;2:CD003318. doi: 10.1002/14651858.CD003318.pub2. [DOI] [PubMed] [Google Scholar]
- 15.National Antiretroviral Treatment Guidelines. 1st edn. 2004. [(12 January 2010, date last accessed)]. South Africa National Department of Health. http://www.hst.org.za/uploads/files/sa_ART_Guidelines1.pdf. [Google Scholar]
- 16.McKerrow NH, Naidoo KL, Reddy R, Stephen CR. Step-by-Step Guideline for the Management of Children on ART. 3rd. March 2008. Department of Paediatrics, Pietermaritzburg Metropolitan Hospitals Complex, KwaZulu-Natal. [Google Scholar]
- 17.Tanser F, Hosegood V, Barnighausen T, et al. Cohort Profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int J Epidemiol. 2008;37:956–62. doi: 10.1093/ije/dym211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnighausen T, Hosegood V, Timaeus IM, Newell ML. The socioeconomic determinants of HIV incidence: evidence from a longitudinal, population-based study in rural South Africa. AIDS. 2007;21(Suppl 7):S29–38. doi: 10.1097/01.aids.0000300533.59483.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Practical Guidelines. 2004. [(12 January 2010, date last accessed)]. The South Africa National Tuberculosis Control Programme. http://www.kznhealth.gov.za/chrp/documents/Guidelines/Guidelines%20National/Tuberculosis/SA%20TB%20Guidelines%202004.pdf. [Google Scholar]
- 21.Braitstein P, Boulle A, Nash D, et al. Gender and the use of antiretroviral treatment in resource-constrained settings: findings from a multicenter collaboration. J Womens Health (Larchmt) 2008;17:47–55. doi: 10.1089/jwh.2007.0353. [DOI] [PubMed] [Google Scholar]
- 22.Mills EJ, Ford N, Mugyenyi P. Expanding HIV care in Africa: making men matter. Lancet. 2009;374:275–76. doi: 10.1016/S0140-6736(09)61348-9. [DOI] [PubMed] [Google Scholar]
- 23.Janssen N, Ndirangu J, Newell ML, Bland RM. Successful paediatric HIV treatment in rural primary care in Africa. Arch Dis Child. 2009 doi: 10.1136/adc.2009.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labuschagne E, Buthelezi M. Southern African AIDS Conference. South Africa: Durban; 2009. Facilitating and effective home based care programme in a resource-limited rural setting. [Google Scholar]
- 25.WHO. 2007. [(12 January 2010, date last accessed)]. Guidance on Provider-Initiated HIV Testing and Counselling in Health Facilities, Geneva. http://www.who.int/hiv/pub/vct/pitc/en/index.html. [Google Scholar]
- 26.Houlihan C, Maheswaran H, Thulare H, Heller T, Newell M. Southern African AIDS Conference. South Africa: Durban; 2009. Pilot of provider initiated testing and counselling in a rural primary health care clinic. [Google Scholar]
- 27.Wallrauch C, Heller T, Kekane D, et al. Southern African AIDS Conference. South Africa: Durban; 2009. Practical TB/HIV integration: Experience from Hlabisa sub-district in northern KwaZulu-Natal. [Google Scholar]
- 28.Coovadia H, Bland R. From Alma-Ata to Agincourt: primary health care in AIDS. Lancet. 2008;372:866–8. doi: 10.1016/S0140-6736(08)61373-2. [DOI] [PubMed] [Google Scholar]
- 29.Houlihan C, Mutevedzi P, Cooke GS, Newell ML. Southern African AIDS Conference. South Africa: Durban; 2009. The challenge posed by tuberculosis to a rural HIV programme with a high prevalence of both tuberculosis and HIV. Abstract 263. [Google Scholar]
- 30.Lessells RJ, Mutevedzi P, Cooke GS, Newell ML. Southern African AIDS Conference. South Africa: Durban; 2009. HIV and hepatitis B virus (HBV) co-infection in rural KwaZulu-Natal: baseline characteristics and outcomes in first year of antiretroviral therapy. Abstract 277. [Google Scholar]
- 31.WHO. Global Tuberculosis Control Report. 2009. [(12 January 2010, date last accessed)]. Surveillance Planning Financing. Global tuberculosis report. A short update to the 2009 report. WHO, Geneva http://www.who.int/tb/publications/global_report/2009/update/en/index.html. [Google Scholar]
- 32.Herbst AJ, Cooke GS, Bärnighausen T, KanyKany A, Tanser F, Newell ML. Adult mortality and antiretroviral treatment roll-out in rural KwaZulu-Natal, South Africa. Bull WHO. 2009;87:733–804. doi: 10.2471/BLT.08.058982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landon BE, Wilson IB, McInnes K, et al. Effects of a quality improvement collaborative on the outcome of care of patients with HIV infection: the EQHIV study. Ann Intern Med. 2004;140:887–96. doi: 10.7326/0003-4819-140-11-200406010-00010. [DOI] [PubMed] [Google Scholar]
- 34.Djomand G, Roels T, Ellerbrock T, et al. Virologic and immunologic outcomes and programmatic challenges of an antiretroviral treatment pilot project in Abidjan, Cote d'I;voire. AIDS. 2003;17(Suppl 3):S5–15. doi: 10.1097/00002030-200317003-00002. [DOI] [PubMed] [Google Scholar]
- 35.Kamya MR, Mayanja-Kizza H, Kambugu A, et al. Predictors of long-term viral failure among ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;46:187–93. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 36.Low risk of death, but substantial program attrition, in pediatric HIV treatment cohorts in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2008;49:523–31. doi: 10.1097/QAI.0b013e31818aadce. [DOI] [PubMed] [Google Scholar]
- 37.May M, Ingle S, Timmerman V, Kotze E, Uebel K, Bachmann M. Proceedings of the 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention. Cape Town: Determinants of waiting times for ART in the Free State Province, South Africa: prospective cohort study wtih retrospective database linkage. July 19–22 Abstract LBPED05. 2009. http://www.ias2009.org/abstract.aspx?elementId=200722760 (12 January 2010, date last accessed) [Google Scholar]