Abstract
Splenic lymphocytes of mice, immunized with membrane-enriched fractions of metastatic human mammary carcinoma tissues, were fused with the NS-1 non-immunoglobulin-secreting murine myeloma cell line. This resulted in the generation of hybridoma cultures secreting immunoglobulins reactive in solid-phase radioimmunoassays with extracts of metastatic mammary carcinoma cells from involved livers, but not with extracts of apparently normal human liver. As a result of further screening of immunoglobulin reactivities and double cloning of cultures, 11 monoclonal antibodies were chosen that demonstrated reactivities with human mammary tumor cells and not with apparently normal human tissues. These monoclonal antibodies could be placed into at least five major groups on the basis of their differential binding to the surface of various live human mammary tumor cells in culture, to extracts of mammary tumor tissues, or to tissue sections of mammary tumor cells studied by the immunoperoxidase technique. Whereas a spectrum of reactivities to mammary tumors was observed with the 11 monoclonal antibodies, no reactivity was observed to apparently normal cells of the following human tissues: breast, lymph node, lung, skin, testis, kidney, thymus, bone marrow, spleen, uterus, thyroid, intestine, liver, bladder, tonsils, stomach, prostate, and salivary gland. Several of the antibodies also demonstrated a "pancarcinoma" reactivity, showing binding to selected non-breast carcinomas. None of the monoclonal antibodies showed binding to purified ferritin or carcinoembryonic antigen. Monoclonal antibodies of all five major groups, however, demonstrated binding to human metastatic mammary carcinoma cells both in axillary lymph nodes and at distal sites.
Full text
PDF

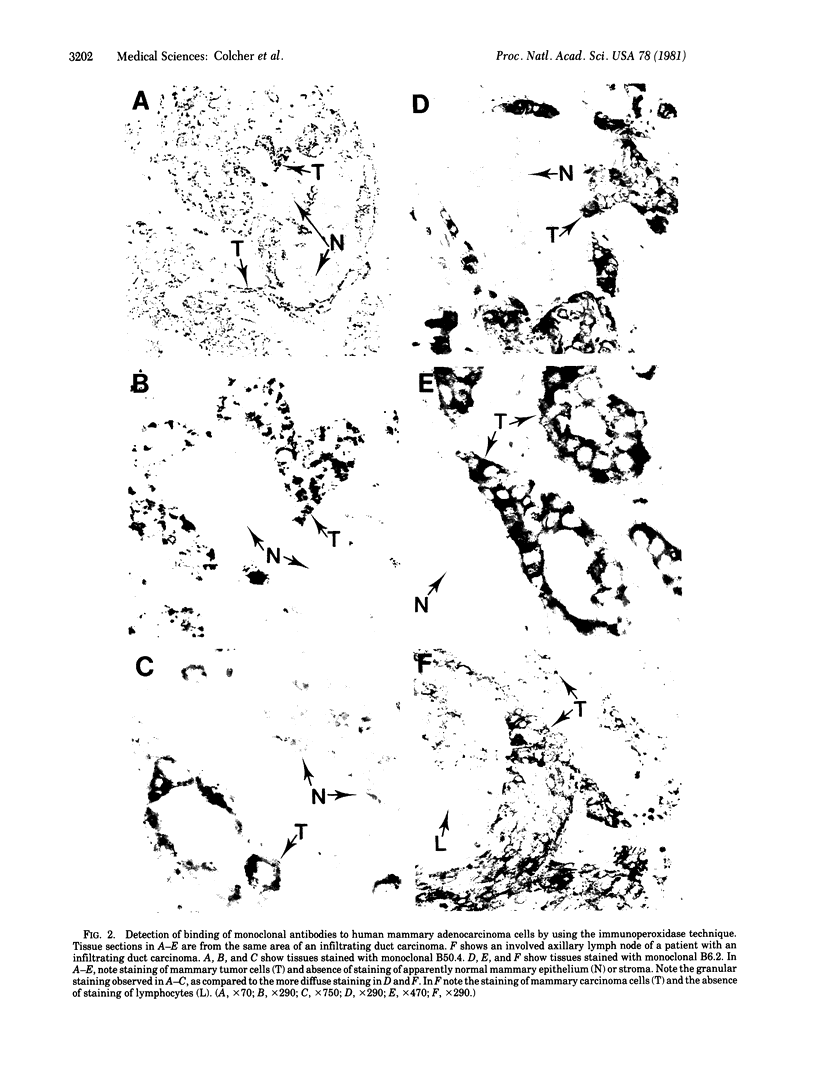

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avis F., Avis I., Newsome J. F., Haughton G. Antigenic cross-reactivity between adenocarcinoma of the breast and fibrocystic disease of the breast. J Natl Cancer Inst. 1976 Jan;56(1):17–25. doi: 10.1093/jnci/56.1.17. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Black M. M., Zachrau R. E., Shore B., Dion A. S., Leis H. P., Jr Cellular immunity to autologous breast cancer and RIII-murine mammary tumor virus preparations. Cancer Res. 1978 Jul;38(7):2068–2076. [PubMed] [Google Scholar]
- Colcher D., Horan Hand P., Teramoto Y. A., Wunderlich D., Schlom J. Use of monoclonal antibodies to define the diversity of mammary tumor viral gene products in virions and mammary tumors of the genus Mus. Cancer Res. 1981 Apr;41(4):1451–1459. [PubMed] [Google Scholar]
- Hollinshead A. C., Jaffurs W. T., Alpert L. K., Harris J. E., Herberman R. B. Isolation and identification of soluble skin-reactive membrane antigens of malignant and normal human breast cells. Cancer Res. 1974 Nov;34(11):2961–2968. [PubMed] [Google Scholar]
- Howard D. R., Taylor C. R. A method for distinguishing benign from malignant breast lesions utilizing antibody present in normal human sera. Cancer. 1979 Jun;43(6):2279–2287. doi: 10.1002/1097-0142(197906)43:6<2279::aid-cncr2820430618>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Leung J. P., Bordin G. M., Nakamura R. M., DeHeer D. H., Edgington T. S. Frequency of association of mammary tumor glycoprotein antigen and other markers with human breast tumors. Cancer Res. 1979 Jun;39(6 Pt 1):2057–2061. [PubMed] [Google Scholar]
- Mesa-Tejada R., Keydar I., Ramanarayanan M., Ohno T., Fenoglio C., Spiegelman S. Detection in human breast carcinomas of an antigen immunologically related to a group-specific antigen of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1529–1533. doi: 10.1073/pnas.75.3.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F. R., Heppner G. H. Immunologic heterogeneity of tumor cell subpopulations from a single mouse mammary tumor. J Natl Cancer Inst. 1979 Dec;63(6):1457–1463. [PubMed] [Google Scholar]
- Schlom J., Wunderlich D., Teramoto Y. A. Generation of human monoclonal antibodies reactive with human mammary carcinoma cells. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6841–6845. doi: 10.1073/pnas.77.11.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh K. M., Quismorio F. P., Friou G. J., Lee Y. T. Ductular carcinoma of the breast: serum antibodies to tumor-associated antigens. Cancer. 1979 Dec;44(6):2083–2089. doi: 10.1002/1097-0142(197912)44:6<2083::aid-cncr2820440619>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Springer G. F., Desai P. R., Murthy M. S., Scanlon E. F. Human carcinoma-associated precursor antigens of the NM blood group system. J Surg Oncol. 1979;11(2):95–106. doi: 10.1002/jso.2930110204. [DOI] [PubMed] [Google Scholar]
- Yu G. S., Kadish A. S., Johnson A. B., Marcus D. M. Breast carcinoma-associated antigen. An immunocytochemical study. Am J Clin Pathol. 1980 Oct;74(4):453–457. doi: 10.1093/ajcp/74.4.453. [DOI] [PubMed] [Google Scholar]